Abstract
Abstract 4386
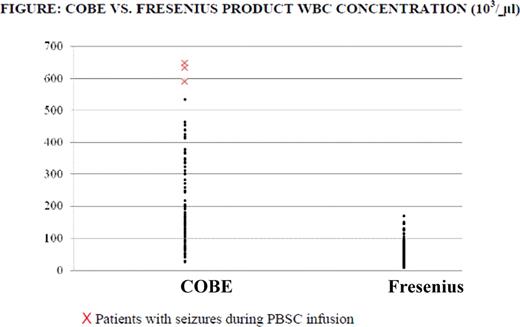
Seizures are rare during infusion of autologous peripheral blood stem cells (PBSC). We retrospectively analyzed 159 adult patients (pts.) collected consecutively between January 2006 and July 2009. Pts. were collected on either COBE Spectra (COBE) (n=85) or Fresenius AS 104 (Fresenius) (n=74) cell separators and mobilized with granulocyte colony stimulating factor (G-CSF) alone (n=47), G-CSF and Plerixafor (n=26), or G-CSF and chemotherapy (n=66). Pts. characteristics did not differ between the COBE and Fresenius cohorts, but there were differences in PBSC product (Table). Pts. collected with COBE had higher white blood cell (WBC) and total nucleated count (TNC) but lower mononuclear cell (MNC) percentage and cell viability than pts. collected with the Fresenius. Absolute CD34+ cells in the PBSC product, CD34+ cells / kg and total CD34+ cells / kg infused at transplant were not significantly different. CD34+ yields (calculated as the ratio of CD34+ cells /μl of the PBSC product to the patient's peripheral blood CD34+ cells / μl taken on the day of collection) were significantly higher on the COBE than Fresenius. No serious adverse events occurred during PBSC infusion except 3 of 159 pts. developed seizures during infusion of PBSC; all collected on the COBE and all three had product WBC > 590 × 103/μl (compared to a median of 163.3 × 103/ μl for all other products)(Figure). Evaluation of pts. did not identify abnormalities in imaging studies, cerebrospinal fluid analysis, electrolytes, or past history which might explain etiology of seizures. No significant difference in WBC or platelet engraftment was observed in pts. collected with COBE or Fresenius.
TIENT AND PRODUCT CHARACTERISTICS
| . | COBE (±SD) . | Fresenius (±SD) . |
|---|---|---|
| Number of Products | 165 | 180 |
| Number of Patients | 85 | 74 |
| Age at collection | 56 ± 14 | 56 ± 15 |
| Weight at Collection (kg) | 82.7 ± 17.9 | 79.5 ± 15.9 |
| Collections / Patient | 2 ± 1 | 2 ± 1 |
| Blood Volume Processed at end of Collection (L) | 18.0 ± 2.4 | 18.1 ± 2.7 |
| (*)Product Volume (ml) | 241 ± 56.8 | 402 ± 72.0 |
| Peripheral WBC (103/ μl) | 36.6 ± 18.9 | 33.3 ± 24.5 |
| (*)Product WBC(103/ μl) | 163.3 ± 136.0 | 55.8 ± 29.3 |
| (*)TNC (1010) | 3.51 ± 1.86 | 1.95 ± 1.19 |
| (*)MNC (1010) | 2.36 ± 1.19 | 1.60 ± 0.09 |
| (*)MNC (%) | 75.0 ± 23.3 | 85.0 ± 10.8 |
| Volume prior to freezing(ml) | 100 ± 54 | 100 ± 32 |
| (*)Post Freeze Viability (%) | 70 ± 14 | 75 ± 10 |
| Peripheral CD34+/ μl | 24.0 ± 43.8 | 25.3 ± 79.1 |
| (*)Product CD34+/μl | 726.7 ± 1325.9 | 264.63 ± 781.0 |
| (*)Product / Peripheral CD34+ | 24.87 ± 10.90 | 10.91 ± 6.64 |
| Absolute Product CD34+ cells (108) | 1.77 ± 3.52 | 1.14 ± 3.35 |
| Product CD34+/kg (106) | 2.02 ± 4.67 | 1.39 ± 4.15 |
| Total CD34+ cells infused (106 / kg) | 3.85 ± 3.20 | 3.85 ± 2.24 |
| (*) = p values < 0.05 |
| . | COBE (±SD) . | Fresenius (±SD) . |
|---|---|---|
| Number of Products | 165 | 180 |
| Number of Patients | 85 | 74 |
| Age at collection | 56 ± 14 | 56 ± 15 |
| Weight at Collection (kg) | 82.7 ± 17.9 | 79.5 ± 15.9 |
| Collections / Patient | 2 ± 1 | 2 ± 1 |
| Blood Volume Processed at end of Collection (L) | 18.0 ± 2.4 | 18.1 ± 2.7 |
| (*)Product Volume (ml) | 241 ± 56.8 | 402 ± 72.0 |
| Peripheral WBC (103/ μl) | 36.6 ± 18.9 | 33.3 ± 24.5 |
| (*)Product WBC(103/ μl) | 163.3 ± 136.0 | 55.8 ± 29.3 |
| (*)TNC (1010) | 3.51 ± 1.86 | 1.95 ± 1.19 |
| (*)MNC (1010) | 2.36 ± 1.19 | 1.60 ± 0.09 |
| (*)MNC (%) | 75.0 ± 23.3 | 85.0 ± 10.8 |
| Volume prior to freezing(ml) | 100 ± 54 | 100 ± 32 |
| (*)Post Freeze Viability (%) | 70 ± 14 | 75 ± 10 |
| Peripheral CD34+/ μl | 24.0 ± 43.8 | 25.3 ± 79.1 |
| (*)Product CD34+/μl | 726.7 ± 1325.9 | 264.63 ± 781.0 |
| (*)Product / Peripheral CD34+ | 24.87 ± 10.90 | 10.91 ± 6.64 |
| Absolute Product CD34+ cells (108) | 1.77 ± 3.52 | 1.14 ± 3.35 |
| Product CD34+/kg (106) | 2.02 ± 4.67 | 1.39 ± 4.15 |
| Total CD34+ cells infused (106 / kg) | 3.85 ± 3.20 | 3.85 ± 2.24 |
| (*) = p values < 0.05 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.