Abstract
Abstract 4465
Solid organ transplant recipients are profoundly immune suppressed and are at risk for post-transplant lymphoproliferative disorder (PTLD). PTLD is associated with substantial morbidity and mortality. With limited treatment options, earlier detection of disease and earlier initiation of therapy may be essential for optimizing therapeutic outcomes. Thus, identifying at risk groups is critical.
We conducted an IRB-approved retrospective study of 1,496 solid organ transplant recipients treated at our institution to identify risk factors for the development of PTLD. We identified 104 cases of PTLD going back over the past 20 years, through 1991. A cross-sectional sample of solid organ transplants evaluated at our institution was used as controls (N = 1,392). Charts were reviewed for relevant demographic variables, reason for transplantation, Epstein-Barr virus (EBV) serostatus pre-transplant, and Human Leukocyte Antigen (HLA)-type. Information on outcomes, such as organ rejection over follow up and death was also recorded. In individuals with PTLD, date of diagnosis, tumor characteristics (i.e., EBV status), tumor pathology, and PTLD treatment was recorded. Survival analysis (Kaplan-Meier plots and Cox proportional hazards regression) was conducted on collected data, to determine risk factors for the development of PTLD.
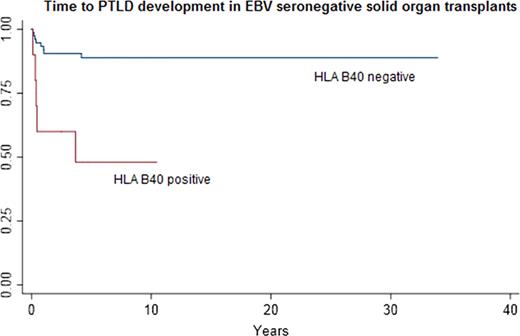
In the cohort as a whole, 86/1,496 (5.8%) of individuals were EBV seronegative at baseline, and 20.9% (312/1496) did not have an available documented baseline EBV serology. EBV serostatus was the major risk factor for EBV-associated PTLD (N=74, or 71% of PTLD cases): EBV-seronegative individuals had a Hazard Ratio (HR) =7.70 [95% CI = 3.8 – 15.5, p < 0.001] for EBV-associated PTLD compared to seropositive individuals. Thus, further analyses were stratified by EBV serostatus, to examine the effect of HLA-type on risk of development of PTLD. In an exploratory data analysis, we found that HLA-B40 was a major risk factor for the development of EBV-associated PTLD in EBV-seronegative individuals, while HLA-B8 was a risk factor for the development of EBV-associated PTLD in EBV-seropositive individuals. In baseline seronegative individuals HLA-B40 was associated with a HR=5.99 [95% CI = 1.95 – 18.4, p=0.002] for PTLD (see figure below for Kaplan Meier curves). In baseline seropositive individuals HLA-B8 was associated with a HR = 3.65 [95% CI = 1.52 – 8.76, p= 0.004] for PTLD. In EBV seropositive individuals, receipt of either a liver, cardiac, lung or pancreas transplant (compared to a kidney transplant alone) was associated with an increased risk of PTLD as well, HR = 6.72 [95% CI = 2.78 – 16.24, p < 0.001.
While the relation of HLA-type and EBV-associated PTLD seen in the study are novel, we recommend future studies to test these observations. These results demonstrate that risk factors for EBV-associated PTLD may verify substantially with baseline EBV-serostatus. We recommend future studies consider stratifying analyses by baseline EBV-serostatus, as EBV-associated PTLD resulting from primary EBV-infection may represent a distinct pathology than EBV-associated PTLD arising in individuals who are known to be EBV-seropositive.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.