Abstract
Abstract 4566
Busulfan (Bu) has been used for almost 3 decades in the conditioning of hematopoietic stem cell transplant. Given orally, Bu exhibits highly variable bioavailability and diverse inter-individual systemic exposure, which prompted various therapeutic drug monitoring approaches. An intravenous (i.v.) formulation was developed to overcome these issues; however 10 to 30% of the adult patients receiving i.v. Bu will not have an AUC within the therapeutic window. Clearly, a need for therapeutic i.v. dose adjustment still persists. Instead of looking into AUC after the 1st therapeutic dose and adjusting the subsequent doses, a safer and potentially more successful approach is to use a sub-therapeutic test dose prior to the conditioning administration and determine a personalized therapeutic dose, maximizing the possibility of achieving an adequate exposure. Beri et al. [1] used a 0.8 mg/kg i.v. test dose (1/4 of the standard daily dose). Without the test dose, 23% of the 17 patients would have fallen below and 12% above the therapeutic range. With the test dose, no patients had a sub-therapeutic AUC and only 12% had an AUC above the therapeutic window.
We investigated the bioequivalence of a very low sub-therapeutic test dose (1/10 of the standard daily dose) of i.v. Bu (Busulfex®) in order to reduce the unnecessary exposure and adverse effects of the test dose process. For this purpose we developed a sensitive and fast liquid chromatography tandem-mass spectrometry (LC-MS/MS) method which allowed us to measure Bu in a wide concentration range required in the study.
Utility of the new LC-MS/MS assay and bioequivalence of the test vs therapeutic regimens were investigated in the “Myeloid Cohort” (patients with a high chance of progressive myeloid diseases, marrow failure syndromes or myeloproliferative disorders) of the ongoing “Efficacy of T-Cell Depleted Allogeneic Non-Myeloablative Stem Cell Transplantation” study. The treatment flow chart is presented in Table 1. During both test and therapeutic Bu regimens, plasma was collected at pre-dose, 15 min, 1, 2, 4, and 24 hrs. To 100 μL of plasma sample, 100 ng of busulfan-d8 (internal standard) was added. After extraction of analytes by ethylacetate, evaporation of the solvent and reconstitution in LC mobile phase, 50 μL of the sample was injected into the LC-MS/MS system. Calibration samples in 1.6 ng/mL – 5 μg/mL range of pure Bu in plasma were run along the study samples. LLOQ of the method (80% accuracy) was 1.6 ng/mL. Pharmacokinetic (PK) parameters were calculated by non-compartmental approach using WinNonlin software.
Treatment flow chart
| DRUG/DOSE . | DAY -4 . | DAY -3 . | DAY -2 . | DAY -1 . | DAY 0-4 . |
|---|---|---|---|---|---|
| Alemtuzumab (in vivo) 20 mg/day | x | x | x | x | |
| Fludarabine 40 mg/m2/day | x | x | x | x | |
| Busulfan TEST dose 13 mg/m2 (18 min infusion) | x | ||||
| Busulfan 130 mg/m2/day (180 min infusion) | x | x | |||
| Allogeneic PBSC infusion | x |
| DRUG/DOSE . | DAY -4 . | DAY -3 . | DAY -2 . | DAY -1 . | DAY 0-4 . |
|---|---|---|---|---|---|
| Alemtuzumab (in vivo) 20 mg/day | x | x | x | x | |
| Fludarabine 40 mg/m2/day | x | x | x | x | |
| Busulfan TEST dose 13 mg/m2 (18 min infusion) | x | ||||
| Busulfan 130 mg/m2/day (180 min infusion) | x | x | |||
| Allogeneic PBSC infusion | x |
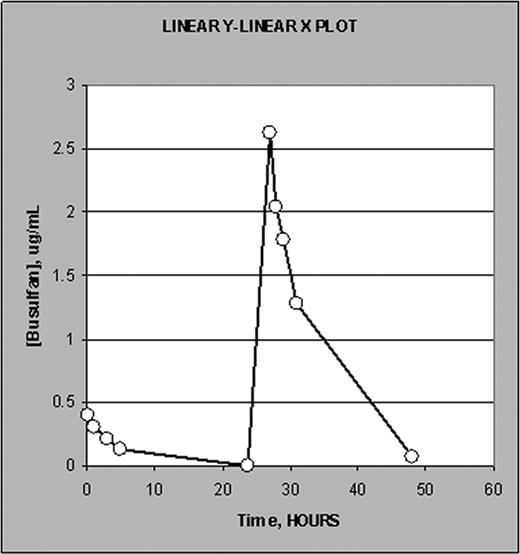
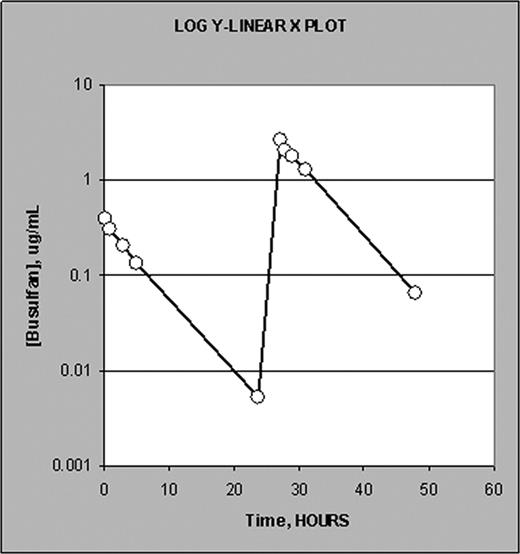
Presented here are results from 3 patients infused in consecutive days with 18 min long test dose and 180 min long therapeutic dose. Figure 1 shows a typical plasma Bu conc./time profile. Figure 2 is a semi-log (log conc. vs time) plot illustrating equal terminal slopes (elimination rate, clearance) for both test and therapeutic doses. Table 2 lists AUC values calculated within the boundaries of the PK sample collection time (24 hrs in case of both test and therapeutic regimens) as well as the AUCTEST/AUCTHERAP ratio.
Typical conc./time profile plot
Typical semi-log (log conc. vs time) plot
PK parameters
| PK parameter . | Pat. #1 . | Pat. #2 . | Pat. #3 . |
|---|---|---|---|
| AUCTEST (μM·min) | 447 | 376 | 403 |
| AUCTHERAP (μM·min) | 3367 | 3186 | 3106 |
| AUCTEST/AUCTHERAP | 7.5 | 8.5 | 7.7 |
| PK parameter . | Pat. #1 . | Pat. #2 . | Pat. #3 . |
|---|---|---|---|
| AUCTEST (μM·min) | 447 | 376 | 403 |
| AUCTHERAP (μM·min) | 3367 | 3186 | 3106 |
| AUCTEST/AUCTHERAP | 7.5 | 8.5 | 7.7 |
The newly developed LC-MS/MS assay proved to be optimal in terms of concentration range (1.6 ng/mL – 5 μg/mL), throughput (4 min per analytical run), and small sample size (100 μL plasma). PK calculations (AUC) show: 1) low inter-patient variability in both test and therapeutic doses and 2) promising value of the test dose in predicting exposure by the therapeutic dose (stable AUCTEST/AUCTHERAP ratio) although larger number of subjects is needed for accurate assessment.
Spasojevic:Otsuka America Pharmaceuticals, Inc.: Research Funding. Horwitz:Otsuka America Pharmaceuticals, Inc.: Honoraria. Rizzieri:Otsuka America Pharmaceuticals, Inc.: Research Funding.
1)
Author notes
Asterisk with author names denotes non-ASH members.