Abstract
Abstract 4634
T-cell transcription factor NFAT1 (NFATc2) has been reported to regulate cell cycle regulatory proteins including cyclins, cyclin-dependent kinases (CDKs), and p21. MicroRNA-184 (miR-184) has been noted to have close sequence homology to the 3’ UTR of NFAT1 and to regulate NFAT1 mRNA translation in human adult and umbilical cord blood (UCB) CD3+CD4+ T-cells. In this study, we are characterizing the functional role of NFAT1 in T-ALL cells to determine whether its dysregulation may alter normal cell cycle regulation and thereby may contribute to T-ALL leukemogenesis.
In this study we used MOLT-4 cell line to investigate the role of NFAT1 in T-ALL, since MOLT-4 represents early stage of human T-ALL (CD4+CD8+ double positive) compared with other T-cell lines including Jurkat. We developed two distinct approaches to explore NFAT1 signalling, either by manipulating the expression level of NFAT1 protein by blocking miR-184, or by overexpressing a constitutively active form of NFAT1 (caNFAT1) holding a HA-tag (Addgene plasmid 11792, A. Rao, PhD, Harvard University). caNFAT1 was introduced into gateway system expression vector pEF-DEST51. MOLT-4 cells were maintained in complete RPMI 1640 medium according to ATCC guidelines. Electroporation transfection was performed on Amaxa Nucleofector I using program C-05. 200 nM of anti-miR-184 miRNA inhibitor (Dharmacon) or 2 μ g of plasmid containing caNFAT1 was transfected into 2×10e6 MOLT-4 cells in each sample. Transfection efficiency was tested using pmaxGFP (Lonza). Proteins were extracted at various time points after transfection using RIPA buffer (PIERCE) with protease inhibitor cocktail and tested for the expression of NFAT1 (BD Biosciences), phosphorylated NFAT1 (Santa Cruz Biotechnology), HA (Roche), and beta-actin (Sigma-Aldrich). Cell cycle was measured at various time points by pelleting down 0.5–1 X10e6 cells, followed by ethanol fixation, and incubation in propidium iodide (PI) (10 μ g/ml) staining solution and flow cytometric measurement for PI incorporation. Cell apoptosis was measured by PE-conjugated annexin V staining (BD Biosciences) in transfected MOLT-4 cells including empty plasmid controls.
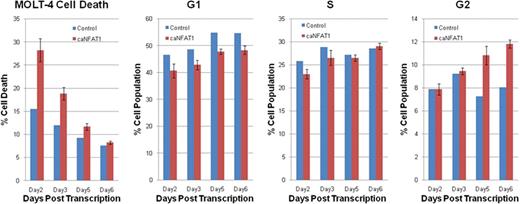
The over-expression of caNFAT1 in MOLT-4 led to increased proportion of cells in G2 phase than in controls (10.8% vs. 7.3% on day5, and 11.8% vs. 8.1% on day6). In addition to these cell cycle changes in MOLT-4 treated with caNFAT1 plasmid, higher rates of apoptosis were noted on day 2 (28.3% vs. 15.5%), which notably leveled off from day 3 to day 6 potentially attributed to compensatory cellular responses. Previous work by this group has shown higher expression of miR-184 in MOLT-4 by 56 fold compared with normal human CD4/8 double positive T-cells. Here, miR-184 knockdown was also notable for a larger proportion of MOLT-4 cells in G2 cell cycle phase and higher apoptosis, albeit modest changes compared with that exerted by caNFAT1.
In MOLT-4 cell line, this preliminary data suggests that increasing active non-phosphorylated NFAT1 is associated with enhanced proportion of cells in G2 phase as well as enhanced resultant apoptosis. Taken together, regulation of NFAT1 may be exploited as potential T-ALL targeted therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.