Abstract
Abstract 490
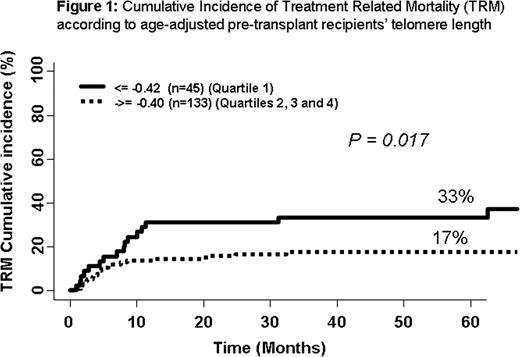
Telomeres are highly conserved protective terminal chromosomal structures consisting of hundreds of repeated TTAGGG hexamers and associated shelterin proteins. Telomeres shorten with every cell cycle, and telomere attrition has a fundamental role in cell senescence. Telomeres of leukocytes are shorter in transplant recipients than in their donors. Dyskeratosis congenita, a congenital aplastic anemia caused by mutations in the telomerase complex genes, is associated with treatment related mortality (TRM) after hematopoietic stem cell transplantation (HSCT). We hypothesized that age-adjusted pre-transplant telomere length might generally predict TRM after HSCT. Between 2000 and 2005, 178 consecutive patients underwent HSCT from HLA-identical sibling donor after myeloablative conditioning regimen (including TBI in 57 patients), mainly for hematological malignancies (n= 153) in our center. The stem cell source was bone marrow (BM) in 128 cases and peripheral blood (PB) in 50 cases. Median age at transplant was 32 years (range 3–65). Graft-versus-host disease (GvHD) prophylaxis mostly consisted of cyclosporine and methotrexate (n=149, 84%). Before HSCT, blood lymphocytes were obtained from each of the donor-recipient pair. Telomere length was assessed by real time quantitative PCR. We first determined the normal age distribution of telomere length using a group of 173 healthy French hematopoietic stem cell donors (f=-0.00833*age+1.522) as a control group. We then calculated the pre-transplant recipient age-adjusted telomere length in comparison to controls. After age adjustment, we categorized the population in quartiles (shortest telomeres for quartile 1) and analyzed the outcome post HSCT using competing risk in univariate and multivariate analyses (Fine and Gray). The mean telomere length in transplant recipients (1.05) was shorter than in the control group (1.23, p= 0.0001). After age-adjustment, patients' distribution was similar among all four quartiles except for disease severity (more high risk disease was present among patients with the shortest telomeres). The median follow-up was 51 months (range, 1 – 121 months). All patients engrafted. The median time to achieve absolute neutrophils count >500/ul was 18 days (range 4–45) and median time to platelet count >20.000/ul was 17 days (range 7–58). Cumulative incidence (CI) of acute GvHD grade II-IV was 45% (95% confidence interval [95CI] 37%–53%) and of chronic GvHD was 41% at 36 months (95CI 33%–49%). Thirty-four patients relapsed: CI: 22% at 5 years (95CI 16%–28%). There was no correlation between telomere length and engraftment, acute or chronic GvHD or relapse. The overall survival was 62% at 5 years (95CI 54%–70%). During the study, 37 patients died due to TRM. TRM rate inversely correlated with telomere length. In the first quartile, the 5-year CI of TRM was 33% (95CI 2%–22%), 20% (95CI, 8%–32%) in the second quartile; 20% (95CI, 8%–32%) in the third quartile; and 12% in the fourth quartile (95CI, 2%–22%) (p=0.06). When quartiles 2, 3 and 4 were pooled, the increased TRM in first quartile was statistically significant (p = 0.017) (Figure 1). In multivariate analysis using competing risk regression, (including age-adjusted telomeres length, disease stage, age, TBI and source of stem cells), age of the recipients (HR: 1.1, 95% CI [.0–1.1, p=0.0001] and age-adjusted telomeres [HR: 0.4, 95% CI [0.2–0.8, p=0.01]) were independently associated with TRM. The same two factors remained significant in subset analysis of patients with malignant diseases (n=154) (p= 0.0004, HR: 1.1 and p=0.018, HR: 0.43, respectively). No association was found between donor telomere length and outcome post HSCT. In conclusion, age-adjusted recipient pre-transplant telomere length is an independent biological marker of TRM after HSCT from related donors using a myeloablative conditioning regimen and cyclosporine-based GvHD prophylaxis.
Disclosures:
Peffault de Latour:Alexion: Consultancy, Research Funding.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011