Abstract
Abstract 5008
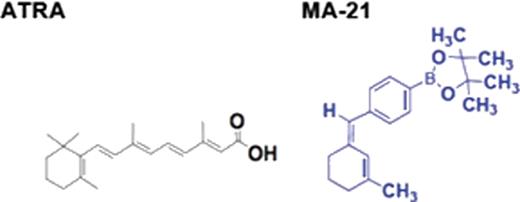
Cure rates of acute leukemia remain poor emphasizing the need to for novel therapies. All trans retinoic acid (ATRA) is an effective therapeutic agent in a subtype of acute myeloid leukemia and relieves transcriptional repression induced by the PML-RAR oncoprotein by binding to the retinoic acid receptor. Even though ATRA is effective, the treatment course is characterized by a high rate of toxicity and ATRA resistance is seen in some cases of acute promyelocytic leukemia. In an attempt to improve outcomes, we devised a methodology for creation of boronic acid and other newer retinoic acid analogues. Our lead compound, MA-21 was generated by replacing the terminal carboxyl group of ATRA with a boronic ester using Wittig reactions. Computation modeling revealed that MA-21 can fit in the RARa pocket and can form increased covalent bonding with cysteine residues within the receptor. As opposed to other synthetic retinoids, the addition of a boron atom resulted in significantly enhanced cytotoxicity in leukemic cell lines, even those that were resistant to ATRA. MA-21 at 1uM dose led to significant reduction in proliferation of ATRA sensitive NB4 APL cell line (1.8 fold decrease after 96hrs, p= 0.028) as well as in ATRA resistant cell lines NB4.007/6 (3.3 fold decrease after 72hrs and 2.1 decrease after 96hrs, p values of 0.018 and 0.046) and NB4.306 (2.6 fold decrease after 96hrs, p= 0.032). MA-21 was able to induce these effects by inducing significant G2/M cell cycle arrest and not by increased apoptosis or cellular differentiation. Cell cycle was assessed by Flow Cytometry after 96hrs of incubation and showed a significant increase in G2/M percentage in the ATRA sensitive and resistant cell lines compared to DMSO (NB4 cell line- 1.35 fold increase, p= 0.035; NB4.007/6- 1.35 fold increase, p= 0.015 and NB4.306- 2 fold increase, p= 0.023). Thus, we demonstrate novel synthetic methodology to synthesize boron containing novel retinoids and demonstrate the potential of these compounds as therapeutic agents in resistant leukemias.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011