Abstract
Abstract 817
Strategies to suppress GVHD are often associated with broader suppression of the immune system leading to a compromised GVT effect. Using experimental models, we have demonstrated a novel strategy to enhance GVT effects and explicitly suppress GVHD using genetically engineered T lineage cells over-expressing TNF-Related Apoptosis Inducing Ligand (TRAIL). TRAIL can induce apoptotic signals through death receptor (DR) 4 and 5 molecules (only DR5 in mice) expressed on target cells. Expression of DR5 is higher on certain tumors and can be enhanced on others using small molecules rendering them susceptible to TRAIL mediated killing. TRAIL is therefore an attractive candidate for genetic engineering of donor T cells to enhance their GVT potential. We evaluated the effect of TRAIL over-expression (TRAIL+) in donor T cells (mature and precursor) on GVHD and GVT. Mature T cells derived from donor B6 splenocytes were transduced with a lentiviral TRAIL expression vector. The transduced TRAIL+ T cells were adoptively transferred on day 0 into lethally irradiated CBF1 recipients of T cell depleted allografts and LB27.4 tumor (B6 ^ CBF1+LB27.4) to assess their GVHD and GVT activity. TRAIL+ T cells displayed significantly enhanced antitumor immunity compared to T cells transduced with a control vector against LB27.4 tumor cell lines in vitro and upon transfer into tumor bearing allo-BMT recipients (p<0.01, 100% survival in TRAIL+ T cell group) (Fig 1A, also shown at the annual meeting last year). Precursor (pre)T cells have the benefit of regenerating the T cell compartment without causing GVHD and being available for “off the shelf” use. We generated TRAIL+ preT cells from transduced B6 hematopoietic stem cells and expanded them using the OP9-DL1 co-culture system. Adoptive transfer of B6 TRAIL+ preT cells into syngeneic-transplanted BALB/c mice could reconstitute the T cell compartment with TRAIL-expressing T cells and caused enhanced antitumor activity (p<0.05) compared to mock (GFP)-transduced controls.
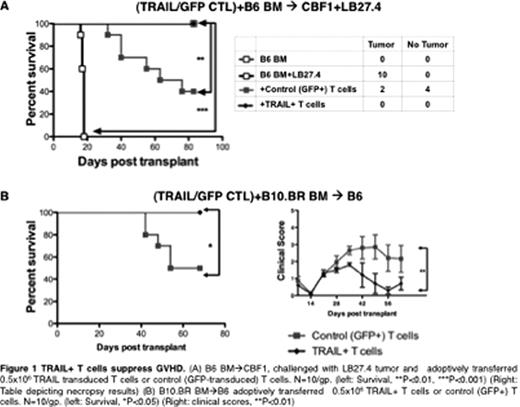
Interestingly, in addition to enhanced GVT, the recipients treated with TRAIL+ T cells had significantly less GVHD lethality and morbidity (Fig1B). This was observed across multiple GVHD models (B6 ^ CBF1, B6^ BALB/c and B10.BR^ B6). To explore the factors contributing to TRAIL-mediated suppression of GVHD, we used animals deficient in DR5 (DR5ko) in our models of GVHD. We found that GVHD suppressive effects of TRAIL were lost when hosts were DR5ko or when DR5ko TRAIL+ T cells were adoptively transferred indicating that TRAIL+ T cells suppress GVHD by targeting both host and donor compartments. We observed a higher DR5 expression in host MHC-IIhi antigen presenting cells (APC) following total body irradiation, suggesting that TRAIL+ donor T cells could potently eliminate host APC, resulting in less GVHD. Further, on transferring wild type T cells into irradiated hosts, we found that alloreactive CD25+ T cells had a significantly higher DR5 expression compared to CD25− T cells. This indicates that TRAIL+ T cells can specifically target the alloreactive CD25+ T cells in order to suppress GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.