Abstract
Abstract 947
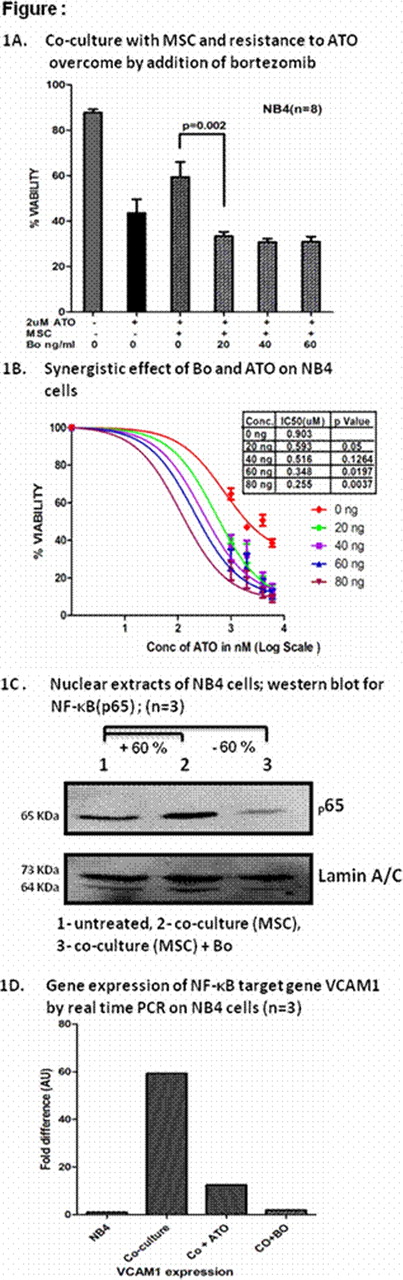
About 5–10% newly diagnosed and about 20–30% of relapsed acute promyelocytic leukaemia (APL) patients will have disease recurrence after receiving currently accepted standards of care. There are a limited number of drugs in the armamentarium for treatment of APL. Preliminary work from our laboratory suggests that stromal cell adhesion mediated drug resistance (CAM-DR) is probably significant with arsenic trioxide (ATO) and is seen both in APL cell lines (n=8; figure 1A) and primary APL cells (n=26; data not shown). Preliminary data suggests that bortezomib (Bo) has cytotoxic effect against promyelocytes and is also known to interfere with stromal and malignant cell interaction in other haematological malignancies.
Our in-vitro experiments suggest that Bo at pharmacologically relevant doses has a cytotoxic effect on APL (NB4) cell lines and primary APL cells (median IC50 = 9.8 ng/ml and 4.2 ng/ml, respectively) but has no effect at these doses on stromal cells (MSC) and mononuclear cells. We have also noted a significant synergistic cytotoxic effect when combined with ATO on NB4 cells (n = 4), P=0.05 (figure 1B).
In co-culture with MSC, a combination of ATO at 2 μmol and Bo over a range of pharmacologically relevant concentrations (20- 80ng/ml) significantly reduced the APL cell viability [NB4 cells (n=8; figure1A) and primary APL cells (n=26; data not shown), suggesting that Bo overcomes the CAM-DR to ATO in APL. Moreover, we noted increased activation of NF-κB (p65) and over expression of Vascular cell adhesion molecule 1 (VCAM1) in NB4 cells when they were co-cultured with MSC. These effects on NF-κB and VCAM1expression were inhibited by Bo (figure 1C and 1D) and ATO. Bo treatment, in comparison to untreated cells and cells treated with ATO alone, reduced the capacity for serial colony forming units (CFU) of NB4 cells in methyl cellulose medium (n=2).
A phase I clinical study was initiated combining ATO with weekly Bo at standard doses. A total of 3 patients have been treated and included a patient in relapse 1 and two in relapse 2. Median age was 31 years and there were 3 males. Two patients had previously under gone an autologous SCT for relapsed disease. Two patients were initially treated with a single agent ATO regimen while one patient had received a conventional ATRA plus chemotherapy regimen, this patient had subsequently had salvage chemotherapy, an autologous stem cell transplant and had relapsed; he had already exceeded the accepted safe cumulative dose of anthracycline at the time of his last relapse. All 3 patients achieved CR at a median of 59 days (range: 38–73) and all achieved a molecular remission at a median of 42 days (range: 39 – 56 days). Combination therapy was well tolerated; one second relapse patient had prolonged cytopenia while non hematological toxicity was mild and reversible and included one patient developing a transient grade II hepatotoxicity. None of the cases had evidence of significant neuropathy, worsening of coagulopathy or a differentiation syndrome.
Off Label Use: Bortezomib.
Author notes
Asterisk with author names denotes non-ASH members.