So, another cytokine called “interleukin” followed by a high number is important in immunology! Before you look away, this is no ordinary “new” cytokine system; in fact, the 4 proteins of the IL-36 system (sometimes collectively nicknamed the “IL-1Fs”) have spent the past decade grossly underperforming in vitro despite looking frustratingly like their vigorous cousin IL-1.1
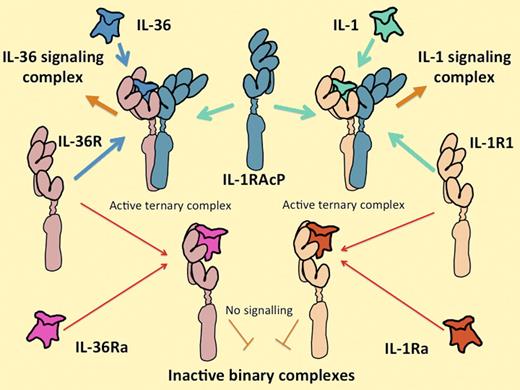
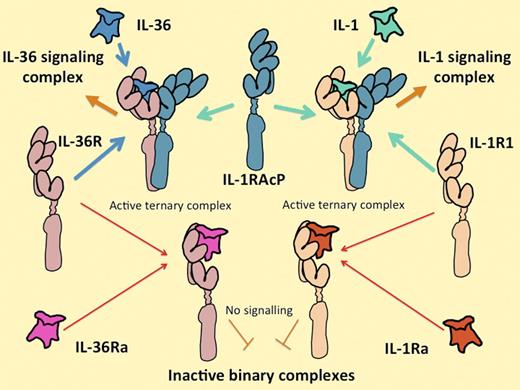
The IL-1 and IL-36 cytokine systems are extremely similar. IL-36 includes 3 functionally equivalent agonist cytokines, IL-36α, IL-36β, and IL-36γ, that interact with their own unique receptor IL-36 (formerly known as IL-1Rrp2), in the same way that the IL-1 agonists IL-1α and IL-1β interact with their unique signal-transducing receptor (IL-1R1). Alternatively, IL-36R can interact with a homologous antagonist molecule IL-36Ra in the same way that IL-1R1 interacts with the IL-1Ra. All of the agonist-receptor complexes interact with the same second receptor component, IL-1RAcP. Both receptor and antagonist complexes fail to associate with IL-1RAcP. Control of the expression of the ligands, their receptors, and their antagonists on different cells and at different times is likely to explain their differential functions.
The IL-1 and IL-36 cytokine systems are extremely similar. IL-36 includes 3 functionally equivalent agonist cytokines, IL-36α, IL-36β, and IL-36γ, that interact with their own unique receptor IL-36 (formerly known as IL-1Rrp2), in the same way that the IL-1 agonists IL-1α and IL-1β interact with their unique signal-transducing receptor (IL-1R1). Alternatively, IL-36R can interact with a homologous antagonist molecule IL-36Ra in the same way that IL-1R1 interacts with the IL-1Ra. All of the agonist-receptor complexes interact with the same second receptor component, IL-1RAcP. Both receptor and antagonist complexes fail to associate with IL-1RAcP. Control of the expression of the ligands, their receptors, and their antagonists on different cells and at different times is likely to explain their differential functions.
In this issue of Blood, Vigne and colleagues are the first to obtain results in a biologically relevant system with plausible concentrations of IL-36.2 They show that the IL-36 system interacts powerfully with both dendritic and T cells and is likely to be fundamental to the regulation of the immune response in the mammalian skin.
The 3 agonistic cytokines IL-36α, IL-36β, and IL-36γ are products of separate genes that (in humans) sit inside the 400 000 bp of the IL-1 gene cluster.3 A fourth gene in the IL-36 group encodes a competitive antagonist for the IL-36 receptor, IL-36 receptor antagonist (IL-36Ra), which is approximately 50% identical to IL-1Ra (which has the same function on the IL-1 receptor). The genes encoding the receptors for IL-36 and IL-1 are next-door neighbors too, both physically and in evolutionary space. When bound to an agonist ligand, the IL-36 receptor associates with its signaling partner, IL-1 receptor accessory protein (IL-1RAcP), which is exactly the same molecule that associates with the IL-1 receptor as illustrated above. The association is required to recruit the signaling complex4,5 (see figure). Like IL-1, IL-36 tends to generate an unequivocal and vigorous response in any of the rare cell lines that have constitutive receptors for it, without requiring the influence of other cytokines.4,5 It may seem startling, therefore, that the entire set of IL-36 cytokines was reported so long ago yet results like those of Vigne et al have been so slow to arrive.
Most cells have constitutive receptors for IL-1 but not IL-36. When added to almost any cell in tissue culture at concentrations of 100 picomolar, the response to IL-1 is virtually saturated and inflammatory cytokines, chemokines, and small molecule mediators are secreted abundantly. Distressingly, the recombinant full-length protein products of all of the IL-36 system have gram-for-gram efficacies, compared with IL-1, of around 1/100 000,4,5 and it has been very difficult to countenance (or obtain funding to research) a cytokine that needs to be added in the micromolar concentration range! A piece was missing from the puzzle.
IL-1 enthusiasts are not startled by very specialized pathways of proteolytic processing that can lead to activation because they are seen in IL-1β and IL-18 and maybe others. It was not obvious, though, how IL-36 could be processed. All 4 IL-36 group members are more or less the same size as fully processed IL-1α and IL-1β (and the unprocessed intracellular form of IL-1Ra) and the extra portions of the N- and C-terminus of the full-length IL-36 proteins do not easily suggest a consensus cleavage site. The logjam was broken when Towne and colleagues showed that all 3 IL-36 agonists and IL-36Ra need to be processed at one particular position (with apparently zero tolerance), close to the N-terminus of the precursor protein.6 Once supplied to cells that have IL-36 receptors in this “polished” form, the vast functional discrepancy disappears between IL-36 and IL-1. What we still do not know is which proteases in nature can “polish” IL-36. Knowing this will probably reveal much about the biologic function of IL-36.
In their paper, Vigne et al argue that responses to IL-1–like cytokines have more to do with the cell types that express them than to intrinsic differences surrounding the receptors2 ; a very sensible position given that the receptors themselves are so similar and that IL-1RacP is reused for IL-1, IL-33, and IL-36. They show that both dendritic cells and CD4+ T lymphocytes from mice have IL-36 receptors and that both cell types respond to “polished” IL-36. Dendritic cells responded more strongly to IL-36 than to IL-1 and the response was a barrage of cytokines and the up-regulation of cell-activation and antigen-presentation markers, suggesting that IL-36 should be better than IL-1 at promoting antigen presentation. In other experiments, Vigne and colleagues showed that IL-36 functions as an adjuvant in vivo.
There are differences between the response to IL-36 and IL-1 in CD4+ T cells too. It is not IL-1–like that IL-36 strongly induces interferon-γ and induces IL-4 significantly. These are activities that would be expected from IL-18 and IL-33, respectively. In isolation too, in these authors' hands, IL-1 induced IL-17 production strongly while IL-36 did not. Vigne and colleagues come to the conclusion that stimulation by IL-36 might favor the differentiation of the TH1 subset of T-helper cells rather than the TH17 subset, which are heavily implicated in autoimmune diseases. IL-1, by contrast, is essential for the TH17 response in vivo and drives the production of substantial amounts of the highly pro-inflammatory cytokine IL-17 from cultured spleen T cells.7 However, the story might not end this simply because strong IL-17 production in this type of assay in response to IL-1 usually requires IL-23, and Vigne et al have not yet reported the effect of co-stimulation of T cells with IL-36 and IL-23.
Complementing the work in the current paper, there is now strong genetic evidence that proper regulation of IL-36 in the skin is vital in humans.8 Individuals who have no functional IL-36Ra gene develop life-threatening episodic pustular inflammation; a clear case of immune dysregulation in the skin as well as more generalized episodic fever.
Now that we have the reagents, there will be plenty to investigate about the IL-36 system. We can now do so in the faith that it is actually functional!
Conflict-of-interest disclosure: The author declares no competing financial interests. ■