Abstract
Taliglucerase alfa (Protalix Biotherapeutics, Carmiel, Israel) is a novel plant cell–derived recombinant human β-glucocerebrosidase for Gaucher disease. A phase 3, double-blind, randomized, parallel-group, comparison-dose (30 vs 60 U/kg body weight/infusion) multinational clinical trial was undertaken. Institutional review board approvals were received. A 9-month, 20-infusion trial used inclusion/exclusion criteria in treatment-naive adult patients with splenomegaly and thrombocytopenia. Safety end points were drug-related adverse events: Ab formation and hypersensitivity reactions. Primary efficacy end point was reduction in splenic volume measured by magnetic resonance imaging. Secondary end points were: changes in hemoglobin, hepatic volume, and platelet counts. Exploratory parameters included biomarkers and bone imaging. Twenty-nine patients (11 centers) completed the protocol. There were no serious adverse events; drug-related adverse events were mild/moderate and transient. Two patients (6%) developed non-neutralizing IgG Abs; 2 other patients (6%) developed hypersensitivity reactions. Statistically significant spleen reduction was achieved at 9 months: 26.9% (95% confidence interval [CI]: −31.9, −21.8) in the 30-unit dose group and 38.0% (95% CI: −43.4, −32.8) in the 60-unit dose group (both P < .0001); and in all secondary efficacy end point measures, except platelet counts at the lower dose. These results support safety and efficacy of taliglucerase alfa for Gaucher disease. This study was registered at www.clinicaltrials.gov as NCT00376168.
Introduction
Gaucher disease is the first glycolipid storage disorder to be treated safely and effectively with IV β-glucocerebrosidase enzyme replacement therapy (ERT), initially with a placenta-extracted enzyme, alglucerase,1 and subsequently with a recombinant form, imiglucerase,2,3 expressed in mammalian CHO cells. Imiglucerase has been the standard of care for > 5000 patients worldwide for the past 15 years. Nevertheless, there are those rare patients who cannot tolerate the drug because of allergic and/or other adverse reactions,4 while other patients fail to achieve the same degree of improvement in all disease-specific therapeutic goals as described in registry cohorts.5,6 In addition, the optimal dosage has not yet been defined despite nearly 2 decades of ERT,7,8 partially because a well-controlled prospective study has not been performed,9 and therefore this can be investigated in a dose-comparison study. The high cost of the current treatment too has been a burden on patients, taxpayers, and health care providers.7 Finally, the problem of only a single therapeutic option commercially has been emphasized in 2009-2010 because of the global shortage of imiglucerase10,11 because of viral contamination at its production plant.12
Taliglucerase alfa (Protalix Biotherapeutics, Carmiel, Israel) is a carrot cell–expressed human recombinant β-glucocerebrosidase enzyme for IV treatment of Gaucher disease.13 Expression of taliglucerase alfa is targeted in the plant cell to the storage vacuoles using a plant-specific C-terminal sorting signal that dictates the formation of the desired mannose structure in vivo, and unlike imiglucerase, does not require subsequent exposure of mannose residues in vitro.14 Furthermore, taliglucerase alfa evinces biologic activity comparable with imiglucerase and its pharmacokinetic profile implies a prolonged half-life compared with imiglucerase,13 although this is currently of unclear clinical relevance. Taliglucerase alfa has the same core amino acid sequence as imiglucerase, including the single amino acid substitution of histidine to arginine at position 495; it also has a comparable homologous 3-dimensional structure to imiglucerase.13,14 These latter properties highlight the potential of taliglucerase alfa for enzyme replacement but also underlie its inability, like other enzymes, to traverse the blood-brain barrier. The plant cell platform (ProCellEx) is easy to scale-up and is not dependent on large-volume bioreactors; rather, reactors are custom-designed, entail low initial capital investment, and the protein expression system does not involve any mammalian or animal components, or transgenic plants. Thus, the unique features of the plant cell production platform may provide long-term safety with regard to exposure to mammalian viral infections as well as cost efficacy compared with mammalian cells bioreactors.15
The purpose of this study was to assess taliglucerase alfa at 2 doses for management of untreated adult patients with Gaucher disease as well as to provide proof of concept for its unique platform.
Methods
This 9-month, 20-infusion, phase 3 clinical trial (NCT number: 00376168) was designed as an interventional study for adult, treatment-naive patients, and was (American) Food and Drug Administration (FDA)–approved by a Special Protocol Assessment. Institutional review board approval was received at each site.
The study design was treatment-based, randomized, double-blind (including subject, caregiver, investigators, and outcomes assessors), dose-comparison with parallel assignment to subgroups: 30 U/kg body weight/infusion or 60 U/kg body weight/infusion. There was no placebo arm for ethical reasons related to availability of a commercial drug and this constraint was discussed with the FDA as well.
Patients were recruited from August 2007 through December 2008; enrollment was based on inclusion and exclusion criteria (see Figure 1 and/or clinicaltrials.gov NCT number: 00376168). Procedures to reduce bias (randomization, observer blinding, and inclusion of an intention-to-treat population who had received at least one dose and had a baseline evaluation) were incorporated. An effort was made not necessarily to enroll Ashkenazi Jews with the genotype N370S/N370S associated with milder disease,16 but rather to recruit patients from various ethnic/geographic backgrounds. Patient randomization after screening eligibility was performed by computer-generated coding.
CONSORT flowchart showing disposition of patients from screening to end of study.
CONSORT flowchart showing disposition of patients from screening to end of study.
Drug preparation was performed as previously described.13 Forty-four patients were screened in 11 centers, 32 patients entered the trial, and 31 patients received at least 1 dose of taliglucerase alfa. Safety measures such as drug-related and other adverse events using the WHO Common Toxicity gradations were continuously collected as were Ab assessments.13
Pharmacokinetic profiles13 were performed at baseline and at the end of study (month 9). Blood samples for analysis of taliglucerase alfa concentrations by ELISA were collected in all patients at 0, 45, 70, 110, 125, 135, 150, 175, 200, and 225 minutes after the start of the 2-hour infusion.
Assessment of antitaliglucerase Abs (IgG and IgE) in human serum samples was done using a validated ELISA.13 Confirmed positive samples were analyzed for detection of neutralizing Ab activity.13 The study was routinely monitored by an independent expert Data Monitoring Committee.
The primary efficacy endpoint was reduction in spleen volume based on magnetic resonance imaging (MRI) using a validated system with a highly standardized protocol (Bio-Clinica).17 Volumetric changes in spleen, the primary site of glucocerebroside storage, more closely track responsiveness to Gaucher-specific therapeutic intervention relative to changes in hematologic parameters. The use of MRI has classically been preferred to computed tomography when repeat evaluations are mandated and MRI does not expose patients to radiation. Both the new, validated system and readers trained in its protocol were in place before advent of the trial. Analysis of spleen and liver volumes was performed by 2 independent, blinded senior radiologists, using batches of de-identified scans; the mean of volumes calculated by both radiologists was the endpoint variable used for analysis.17
Major secondary end points included improvement in anemia, thrombocytopenia, and reduction in liver volume. Additional efficacy end points included levels of Gaucher-specific surrogate markers (chitotriosidase, CCL-18). Measures of bone involvement as exploratory end points in all patients included changes in bone mineral density (BMD) using Dual-Energy X-ray Absorptiometry (DEXA) and measurement of BM fat fraction by quantitative chemical shift imaging18 (QCSI; Academic Medical Center, Amsterdam, The Netherlands) in 4 patients from each dosing group, at baseline and at month 9.
Statistical analysis
A minimum of 12 patients per treatment group was required for 95% statistical power to detect 20% change in the primary endpoint (spleen volume). This number of patients was also sufficient for at least 90% power for the major secondary endpoints (hemoglobin, liver volume, and platelet count). All data obtained and recorded at each site were tabulated with descriptive statistics for continuous variables and categorical variables as appropriate. The primary and major secondary variables were analyzed with 2 one-sample t tests, 1 t test per dose group. A P value of .025 was prespecified as the α level of significance. Missing values at month 9 for the primary efficacy analysis were accounted for by using a multiple imputation approach, with 100 imputations performed for each dose group. Sensitivity analysis was also performed with analyses using Last Observation Carried Forward and no change from baseline for missing values at month 9. The 3 major secondary endpoints were selected to confirm efficacy and were examined in a sequential (step-down) approach. A mixed effects model that included dose and time, with subject as a random effect, was fit to examine whether there was a difference between dose groups for primary and major secondary outcomes.
A sensitivity analysis (although not a prespecified analysis) of the primary endpoint, that is, percent change in spleen volume, using a Wilcoxon signed-rank test to test the null hypothesis that percent change = 0, was performed and statistical significance at P < .0001 was still found.
Results
Safety
There were no serious adverse events reported in either group. All drug-related adverse events were mild/moderate and transient.
Overall, 23 patients (12 in the 30 U/kg/infusion group and 11 in the 60 U/kg/infusion group) experienced 137 adverse events. Of the nontreatment-related events, the most common in each group were nausea and headache. Eight of these patients (3 patients in the 30 U/kg/infusion group and 5 patients in the 60 U/kg/infusion subgroup) experienced 28 events which were considered by the respective investigators treatment related. Of the treatment-related events, none were seen in > 1 patient in each group (Table 2).
Two patients (6%) developed IgG Abs to taliglucerase alfa, one from each group; these Abs were non-neutralizing. Two other patients, also 1 from each group, developed hypersensitivity reactions (6%).
Regarding hypersensitivity, one patient experienced an immediate reaction after infusion of < 5 mL of drug and was excluded from the intention-to-treat efficacy population: predose serum IgE levels were elevated. This patient was subsequently identified as sensitive to imiglucerase as well. The inclusion criterion of a negative Ab test (to imiglucerase) was only for patients who had received imiglucerase in the past; therefore we did not test for presence of IgG Abs to imiglucerase in this patient at screening. Yet, even after exposure to both taliglucerase alfa and imiglucerase, this patient's IgG Ab tests were negative. A single patient withdrew after a hypersensitivity reaction which was mast cell mediated (also the only patient with elevated tryptase levels). A third patient was withdrawn because of pregnancy. Twenty-nine patients completed the 9-month protocol.
Efficacy
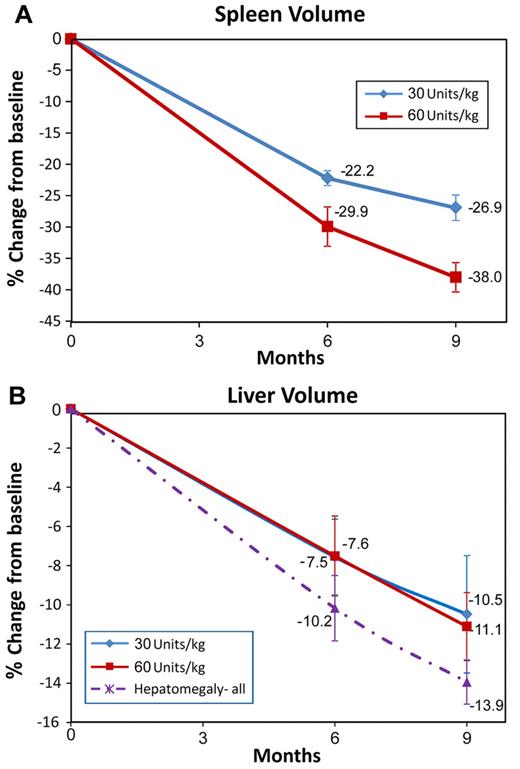
Individual patient data, including age, sex, and results of the primary and secondary end points, are displayed in Table 3. Figure 2 shows changes in spleen and liver volumes from baseline to month 9.
Results of efficacy parameters of spleen and liver organ volume changes measured by MRI. (A) Spleen volumes at screening were 8-54 multiple of normal (MN; normal spleen volume was calculated as 0.2% body weight): mean splenic MN was ∼ 15 MN in each group. Primary efficacy analysis at month 9 (n = 31) demonstrated significant reduction: 26.9% in the 30 U/kg/infusion subgroup and 38% in the 60 U/kg/infusion subgroup (P < .0001). At month 6, the percentage decreases were 22.2% and 29.9%, respectively (P < .0001). (B) Liver volume reduction at month 9 was 10.5% in the 30 U/kg/infusion subgroup (P = .004) 1 and 11.1% in the 60 U/kg/infusion subgroup (P < .0001). In patients with hepatomegaly (defined as liver volume > 1.5 times normal where normal volume was calculated as 2.5% body weight) reduction in liver volume was 13.9% at month 9 for both dose groups combined. The error bars represent SE.
Results of efficacy parameters of spleen and liver organ volume changes measured by MRI. (A) Spleen volumes at screening were 8-54 multiple of normal (MN; normal spleen volume was calculated as 0.2% body weight): mean splenic MN was ∼ 15 MN in each group. Primary efficacy analysis at month 9 (n = 31) demonstrated significant reduction: 26.9% in the 30 U/kg/infusion subgroup and 38% in the 60 U/kg/infusion subgroup (P < .0001). At month 6, the percentage decreases were 22.2% and 29.9%, respectively (P < .0001). (B) Liver volume reduction at month 9 was 10.5% in the 30 U/kg/infusion subgroup (P = .004) 1 and 11.1% in the 60 U/kg/infusion subgroup (P < .0001). In patients with hepatomegaly (defined as liver volume > 1.5 times normal where normal volume was calculated as 2.5% body weight) reduction in liver volume was 13.9% at month 9 for both dose groups combined. The error bars represent SE.
There was early achievement of the primary end point of reduction in spleen volume by all patients. Statistically significant spleen reduction was achieved at month 9: 26.9% (95% confidence interval [CI]: −31.9, −21.8) in the 30 U/kg/infusion group and 38.0% (95% CI: −43.4, −32.8) in the 60 U/kg/infusion group (both P < .0001) which was also statistically significant at 6 months in both dosing groups: 22.2% (95% CI: −24.8, −19.6) in the 30 U/kg/infusion group and 29.9% (95% CI: −36.7, −23.2) in the 60 U/kg/infusion group (both P < .0001). There was a statistically significant difference (95% CI: 1.38, 15.67) between groups at month 9 (P = .021).
Statistically significant reductions in liver volume were noted in both groups at month 9: 10.5% (95% CI: −17.0, −4.0) in the 30 U/kg/infusion group (P = .004) and 11.1% (95% CI: −15.0, −7.4) in the 60 U/kg/infusion group (P < .0001). Here too, there were statistically significant changes at 6 months: 7.56% (95% CI: −12.0, −3.3) in the 30 U/kg/infusion group and 7.51% (95% CI: −12.0, −3.2) in the 60 U/kg/infusion group (both P = .002). There was no statistically significant difference between groups. Patients who entered the study with hepatomegaly (11 patients in the 30 U/kg/infusion group and 7 patients in the 60 U/kg/infusion group), defined as > 1.5 times expected liver volume (calculated as 2.5% of body weight),5,6 demonstrated a greater decrease in liver volume at month 9 (14%).
Figure 3 shows changes in hemoglobin concentration, platelet counts, and the biomarker, chitotriosidase levels. Mean increases in hemoglobin concentration from baseline were statistically significant at 6 months: 1.5 g/dL in the 30 U/kg/infusion group (95% CI 0.2-2.8; P = .0002) and 1.7g/dL in the 60 U/kg/infusion group (95% CI: 0.6-2.8; P = .0002) which continued at month 9: 1.6 g/dL in the 30 U/kg/infusion group (95% CI: 0.3-3.5; P = .001) and 2.2 g/dL in the 60 U/kg/infusion group (95% CI: 0.6-3.8; P < .0001). There was no statistically significant difference between groups at 6 or 9 months. Changes in hemoglobin concentration at 6 and 9 months in patients anemic at baseline (2 patients in the 30 U/kg/infusion group and 8 patients in the 60 U/kg/infusion group) were 2.3 g/dL and 3.2 g/dL, respectively (95% CI: 1.6-3.0 and 95% CI: 2.2-4.2, respectively).
Change from baseline in hematologic parameters and biomarkers. (A) Change from baseline in hemoglobin levels. Mean hemoglobin values at baseline were at the lower limit of the normal range and improved to within normal limits at month 9. Significant increases in mean hemoglobin levels were observed between baseline and month 9 for both dose groups (see “Results”) and for anemic patients (see “Results”). (B) Change from baseline in platelet counts: a significant increase in platelet counts from baseline was observed (see “Results”) in the 60 U/kg dose group at month 9 (mean increase = 41 494/mm3) and in the 30 U/kg dose group (mean increase = 11 427/mm3). (C) Gaucher disease severity as evidenced by a decrease in chitotriosidase levels at month 9. Disease severity and response to treatment was monitored by measurement of chitotriosidase levels before first-dose administration and at visits 7, 14, and 20. All patients achieved decreases in chitotriosidase levels by month 9. The mean decrease in chitotriosidase levels was ∼ 50% from baseline by month 9 (P < .0001 at 30 U/kg/infusion and P = .0016 at 60 U/kg/infusion). The error bars represent SE.
Change from baseline in hematologic parameters and biomarkers. (A) Change from baseline in hemoglobin levels. Mean hemoglobin values at baseline were at the lower limit of the normal range and improved to within normal limits at month 9. Significant increases in mean hemoglobin levels were observed between baseline and month 9 for both dose groups (see “Results”) and for anemic patients (see “Results”). (B) Change from baseline in platelet counts: a significant increase in platelet counts from baseline was observed (see “Results”) in the 60 U/kg dose group at month 9 (mean increase = 41 494/mm3) and in the 30 U/kg dose group (mean increase = 11 427/mm3). (C) Gaucher disease severity as evidenced by a decrease in chitotriosidase levels at month 9. Disease severity and response to treatment was monitored by measurement of chitotriosidase levels before first-dose administration and at visits 7, 14, and 20. All patients achieved decreases in chitotriosidase levels by month 9. The mean decrease in chitotriosidase levels was ∼ 50% from baseline by month 9 (P < .0001 at 30 U/kg/infusion and P = .0016 at 60 U/kg/infusion). The error bars represent SE.
Improvement in platelet counts was seen in both dose groups at 6 and 9 months but only results from the 60 U/kg/infusion group achieved the prespecified α level of statistical significance at month 9 (mean increase of 41 494/mm3; 95% CI: 17 658-65330; P = .003). There was a statistically significant difference in platelet response between groups at month 9 (P = .042).
Although the trial was not a priori powered for dose-response and comparisons across dose groups, there were overt differences between the 2 dose-arms in the magnitude of reduction in spleen volume and increases of platelet counts. Summary of mean differences between groups (posthoc analysis) showed a statistically significant difference for mean changes between groups in spleen volume (P = .0212) and platelet counts (P = .0313) but not in mean changes in liver volume (P = .3042), hemoglobin levels (P = .6861), or chitotriosidase levels (P = .9375). All patients achieved ∼ 50% decrease in chitotriosidase levels by month 9.
There was no deterioration in bone density in any patient. Preliminary results of QCSI scans18 showed increases in fat fraction at month 9 in all 8 patients evaluated (data not shown).
Pharmacokinetic analysis
Mean Cmax, AUC0−t, and AUC0−∞ were higher for patients in the 60 U/kg/infusion group than for those in the 30 U/kg/infusion group, although there was an overlap in the respective ranges. Pharmacokinetic graphs showing a dose-dependency were comparable with those described in healthy volunteers.13
Discussion
This pivotal study, with approximately the same number of patients as in the other seminal ERT trials in Gaucher disease,1-3,19 demonstrates safety of plant cell–expressed taliglucerase alfa by virtue of no serious adverse events, low rate of Ab formation, no neutralizing Abs, and a low incidence of hypersensitivity reactions, which are similar to those of imiglucerase.1-4 Nonetheless, the small sample size is a limitation of the study. Safety is also comparable with that of velaglucerase alfa (VPRIV; Shire HGT), manufactured in a human fibroblast cell line19,20 that was in clinical trials at approximately the same time as taliglucerase alfa, and like taliglucerase alfa, was allowed by regulatory agencies (FDA, European Medicines Agency [EMA]), and the Israeli Ministry of Health) in Early Access Programs as a stop-gap measure for patients requiring ERT during the global imiglucerase shortage.10,11 Velaglucerase alfa was approved in many countries in 2010. ERTs in general are safer than the commercially available substrate reduction agent, miglustat (Zavesca, Actelion Pharmaceuticals Ltd), which was also used during the global shortage despite the caveat of its FDA/EMA restricted indications to patients who are unwilling/unable to receive IV ERT.21
With regard to efficacy and improvement in disease-specific, clinically relevant features, the results presented herein are comparable with and within the same time frame as those achieved by other ERTs.1-3,19 Moreover, given that the patients in the trials of the 1990s1-3 were more severely affected at baseline, and knowing that the more severe the organomegaly at baseline the greater the response,22 the current data are all the more remarkable.
One of the unique features of this clinical trial is the selection of reduction in spleen volume as the single primary end point. While the previously used hematologic parameters are indeed responsive to specific therapy and blood samples can be conveniently transported to a central laboratory for uniformity in reporting, both hemoglobin and platelet counts are quite commonly affected by (non-Gaucher disease) associated diseases that may or may not be evident at screening. In addition, anemia is seen in only one-third of untreated patients at baseline23 and in some cases this is because of iron deficiency. In contrast, almost all untreated and symptomatic patients with Gaucher disease present with splenomegaly24 which has almost always shown reduction in volume after disease-specific therapy.1-3,19,21-23 Thus, change in splenic size was agreed as a primary endpoint that would more specifically track responsiveness to Gaucher-specific therapy. This approach was vindicated in finding early achievement of the primary end point for reduction in spleen volume at both doses, with another interesting finding of a differential between splenic volume reduction and platelet response, but no comparable differential in liver and hemoglobin parameters.
The discrepancy in platelet response, robust in the high-dose group and less robust in the low-dose group, may or may not be a random effect, that is, it may be a true dose-dependent response to taliglucerase alfa or may be because of inclusion to the low-dose group (in the random assignment) of some patients whose platelets respond poorly to ERT. The hypothetical possibility of a difference because of dose-response cannot be assessed without larger cohorts of patients exposed to taliglucerase alfa at the 2 doses. However, this differential response to dose is inline with the less robust response of platelets at lower doses with imiglucerase.22,23,25 Yet, this may be the less likely scenario because both dose groups responded equally well in terms of spleen reduction. It should be noted that even excellent responders may not achieve normalization of all 4 major clinical features,5,6 which is particularly true of those with severe thrombocytopenia, where only a doubling of platelet counts can be expected within the first few years of ERT.5,6 Thus, particularly with regard to response by platelets, the concept of “poor” responders is a credible explanation for our idiosyncratic findings.
After the recent publication associating poor platelet response to ERT with the presence of intrasplenic lesions,26 all MRI scans were re-examined posthoc, but there was no such correlation in either dose group.
The issue of the minimally effective dose remains unresolved despite use of a 30 U/kg/infusion group because even lower doses have been reported to be effective.22,23,25 However, the results of the current trial provide for the first time a statistically meaningful dose-response analysis based on a prospective, double-blind study (as versus retrospective data23,25 ). Furthermore, long-term analysis will be possible as these patients enter the extension protocol while continuing at the same dose. After completion of 24 months it might be possible to evaluate long-term efficacy of the 30 U/kg/infusion regimen of taliglucerase alfa. In addition, data from the increasing numbers of patients receiving taliglucerase alfa variously under a switch-over trial (PB-06-002: NCT 00712348), the Expanded Access Protocol (PB-06-004: NCT 00962260), and a pediatric protocol (PB-06-005: NCT 00258778) as well as compassionate-use programs, may allow further assessment of safety and efficacy including dosage effects beyond what has been derived from the current study.
In summary, the goal of producing a safe and effective enzyme for Gaucher disease has been achieved in a plant cell system that is unique and has potential ramifications for further development. The safety and efficacy profiles of taliglucerase alfa as described herein in adult patients with Gaucher disease justify its use clinically. The use of this efficient production system should reduce the cost of therapy, which heretofore has been among the most expensive in the world. Finally and importantly, this study underscores proof of principle for the use of a nonmammalian plant cell–derived protein expression system as a safe and effective platform for production of complex glycosylated recombinant human proteins, where the plant system can be viewed as a biologic firewall to possible contamination by viruses that may be virulent to humans. This seminal demonstration may lead to production of additional therapeutic proteins using this platform.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the professionalism and dedication of the nursing and ancillary staff at each of the various trial sites.
This work was supported by Protalix Biotherapeutics.
Authorship
Contribution: A.Z. was the lead principal investigator of the trial with the largest patient enrollment, was part of the team to develop the trial protocol, served in an advisory capacity with regulatory agencies, had access to all data from all sites, was involved in data analysis, and was involved in writing the original manuscript and all subsequent revisions; E.B.-A. participated in study design, regulatory activities, and data management, and reviewed all versions of the manuscript; R.C. had access to all data, and reviewed all versions of the manuscript; M. Petakov, F.B.-F., E.T.M., S.E.S.-M., D. Amato, G.D., F.G., R.H., H.R., P.G., A.M., M. Phillips, and G.A. were involved in patient monitoring at the various sites of the clinical trial and reviewed all versions of the manuscript; G.P. was the lead study monitor, participated in the study design, regulatory activities, and data management, and reviewed all versions of the manuscript; D.E. was the site coordinator at the largest enrollment center, had access to all data from all sites, was involved in data analysis, and was involved in writing the original manuscript and all subsequent revisions; M.S. and S.H. participated in regulatory activities and data management, and reviewed all versions of the manuscript; and D. Aviezer participated in study design, regulatory activities, and data management, was involved in writing the original manuscript, and reviewed all versions of the manuscript.
Conflict-of-interest disclosure: Protalix Biotherapeutics is the commercial sponsor of this study. Protalix developed taliglucerase alfa and, together with some of the co-authors, initiated the study design and protocol. The company provided all of the investigational drug, as well as study-specific equipment, administrative support, and grants for travel by the principal investigators to attend meetings for the study and writing the manuscript. The company was involved in approval of the manuscript as drafted by the authors and was aware of the authors' decision to submit the final manuscript to Blood. Protalix Biotherapeutics received a limited grant from the Chief Scientist Office of the Israeli Ministry of Industry to support this development program. A.Z. receives consultancy fees from, and has share options in, Protalix Biotherapeutics and sits on their scientific advisory board. E.B.-A., R.C., M.S., S.H., and D. Aviezer are employees of Protalix Biotherapeutics. M. Petakov, F.B.-F., E.T.M., S.E.S.-M., D. Amato, G.D., F.G., R.H., H.R., P.G., A.M., M. Phillips, and D.E. received grants and/or support for travel to meetings for the study, and provision of medicine, equipment, and administrative support was given to each trial site. E.B.-A. is a vice president of Protalix Biotherapeutics. R.C. is the senior medical director of Protalix. G.P. is an employee of Target Health Inc which provides regulatory consultancies for Protalix Biotherapeutics. M.S. and S.H. are employees of Protalix Biotherapeutics. D. Aviezer is the president and chief executive officer of Protalix Biotherapeutics. G.A. declares no competing financial interests.
Correspondence: Ari Zimran, MD, Gaucher Clinic, Shaare Zedek Medical Center, One Bezek Road, PO Box 3235, Jerusalem 91031, Israel; e-mail: azimran@gmail.com.