Abstract
Advances in autologous hematopoietic cell transplantation (HCT) strategies have resulted in a growing number of long-term survivors. However, these survivors are at increased risk of developing cardiovascular complications due to pre-HCT therapeutic exposures and conditioning and post-HCT comorbidities. We examined the incidence and predictors of congestive heart failure (CHF) in 1244 patients undergoing autologous HCT for a hematologic malignancy between 1988 and 2002. The cumulative incidence of CHF was 4.8% at 5 years and increased to 9.1% at 15 years after transplantation; the CI for female lymphoma survivors was 14.5% at 15 years. The cohort was at a 4.5-fold increased risk of CHF (standardized incidence ratio = 4.5), compared with the general population. The risk of CHF increased substantially for patients receiving ≥ 250 mg/m2 of cumulative anthracycline exposure (odds ratio [OR]: 9.9, P < .01), creating a new and lower threshold for cardiac surveillance after HCT. The presence of hypertension among recipients of high-dose anthracycline (≥ 250 mg/m2) resulted in a 35-fold risk (OR: 35.3, P < .01) of CHF; the risk was nearly 27-fold (OR: 26.8, P < .01) for high-dose anthracycline recipients with diabetes, providing evidence that hypertension and diabetes may be critical modifiers of anthracycline-related myocardial injury after HCT and creating targeted populations for aggressive intervention.
Introduction
Autologous hematopoietic cell transplantation (HCT) has been increasingly used as a curative option for many hematologic malignancies since the mid-1980s.1 Advances in HCT strategies have contributed to incremental changes in survival of 10% per decade, resulting in a growing number of long-term survivors.1-3 However, these survivors are at risk for developing treatment-related complications that significantly affect the quantity and quality of survival.4-6 A recent study found that whereas allogeneic HCT survivors have the highest burden of morbidity after HCT, the risk for severe or life-threatening conditions in autologous HCT recipients remains substantial, with the cumulative incidence exceeding 30% at 10 years after HCT.7
A serious complication after autologous HCT is the development of congestive heart failure (CHF), which can often occur years after the completion of therapy.4,8-11 Long-term HCT survivors have a nearly 3-fold risk of cardiovascular complications compared with age-matched controls,7 and the risk of death due to cardiac dysfunction is greater than 4-fold for female autologous HCT recipients.2 Exposure to cardiotoxic therapies such as anthracycline chemotherapy and/or chest radiation has long been identified as an important mediator of CHF in cancer patients.12 However, less is known regarding the incidence and predictors of CHF after autologous HCT. Potentially cardiotoxic exposures unique to HCT include conditioning with high-dose (HD) chemotherapy (especially cyclophosphamide) and total body irradiation (TBI).9 In addition, HCT survivors are at increased risk of developing cardiovascular risk factors such as essential hypertension and diabetes mellitus, due in part to conditioning-related exposures such as TBI.5,8 The modifying influence of these cardiovascular risk factors on the risk of CHF after cardiotoxic therapy has also not been fully investigated.
We used both a retrospective cohort study design and a nested case-control approach to describe the magnitude of risk of CHF after autologous HCT, and evaluated the role of patient demographics, pre-HCT therapeutic exposures, transplantation conditioning regimens, and post-HCT cardiovascular risk factors in the development of CHF after autologous HCT.
Methods
Cohort analysis
A total of 1327 consecutive patients underwent autologous HCT for a hematologic malignancy at City of Hope (COH) between 1988 and 2002. Medical records maintained at COH were the primary source of data for the current study and were used to abstract the following information: demographics, disease status at HCT, conditioning-related exposures, and post-HCT cardiac dysfunction. The COH long-term follow-up program follows patients who have undergone HCT. The following protocol is used to ensure complete follow-up after HCT. If the date of last medical visit at COH is not recent or if there are any gaps in the patient's history within the window of interest, a standard protocol is used to identify and contact physicians who are treating patients outside COH to obtain relevant details regarding patient health. If the physician is not available or is unable to provide recent information, the patient is contacted to obtain this information. The human subjects committee at COH approved the protocol. Informed consent was provided according to the Declaration of Helsinki. Patients with documented cardiac dysfunction before HCT (n = 43, 3.2%) or who actively refused participation in the long-term follow-up program (n = 40, 3.0%) were excluded from the current study; 1244 patients were included in the retrospective cohort analysis.
The case definition of CHF was according to the American Heart Association (AHA)/American College of Cardiology (ACC) publication, Guidelines for the Diagnosis and Management of Chronic Heart Failure in the Adult13 ; this required clinician documentation of patient symptoms (dyspnea and fatigue) and signs (edema and rales) consistent with CHF. Diagnostic echocardiogram reports were used to document the extent of cardiac compromise. Patients who developed transient cardiac dysfunction because of a potentially reversible acute complication such as sepsis and who subsequently had no evidence of cardiac dysfunction during follow-up were excluded.
The cumulative incidence of CHF after HCT was calculated taking into consideration the competing risk of death.14 The time to risk was computed from the date of HCT to the date of onset of CHF, the date of last contact, or the date of death, whichever came first. The log-rank test was used to compare the various subpopulations. Univariate analyses were performed to compare demographics, underlying diagnosis, disease status at HCT, and conditioning-related exposures between patients who developed CHF and those who did not using either χ2 and Fisher exact tests for dichotomous or t tests for continuous variables. Cox proportional hazards regression analysis was used to calculate relative risk (RR) estimates and their 95% confidence intervals (95% CI), adjusted for covariates. Variables in the regression model included sex, ethnicity/race, underlying diagnosis, age at HCT (< 35, 35-44, 45-54, and ≥ 55 years), and subsequent HCT after initial autologous HCT. Patients receiving transplantations in first or second remission after acute myeloid, lymphoblastic leukemia, Hodgkin lymphoma, or non-Hodgkin lymphoma were considered to be at standard risk for relapse; the remainder were considered to be at high risk. Exposure to high-dose chemotherapeutic agents and TBI for conditioning were recorded as dichotomous variables.
The 2005 National Hospital Discharge Survey data were used to generate age- and sex-specific rates of CHF in the U.S. general population (International Classification of Diseases, 9th revision, clinical modification code 428.0, 428.2-428.4).15 These age- and sex-specific rates were used to calculate expected number of cases in our cohort. The standardized incidence ratio (SIR) was calculated by obtaining the ratio of the observed and expected number of cases. The 95% CIs were estimated using a method described by Haenszel.16 Absolute excess risk (AER) was defined as the mean excess number of CHF cases per 10 000 survivors per year over and above those that would be observed in an age- and sex-matched general population. AER was calculated by subtracting the number of expected events from those observed in this cohort, dividing this by person-years of follow-up for the autologous HCT cohort, and multiplying the result by 10 000. Data were analyzed using SPSS Version 18.0 (IBM) and SAS Version 9.2 software. All statistical tests were 2-sided and P < .05 was considered statistically significant.
Case-control analysis
A total of 88 patients (7.1% of the entire cohort) developed CHF. For each patient, up to 3 controls were selected from within the cohort according to the following matching criteria: age at HCT (± 5 years), year of HCT (± 2 years), and length of follow-up (control follow-up was equivalent to or exceeded that of the index case). For all cases and controls, medical records were used to abstract pre- and post-HCT treatment-related information, as well as details regarding specific post-HCT cardiovascular risk factors. On occasion, medical record information was obtained from more than one hospital if portions of treatment were provided outside COH. The following data were collected.
Therapeutic exposures.
Therapeutic exposures included chemotherapy (cumulative dose per square meter body surface area) and radiation therapy (total dose, field, and dose per fraction). The anthracycline cardiotoxicity risk score17,18 was calculated by multiplying the cumulative dose of each anthracycline treatment by a factor that reflects the cardiotoxic potential of each drug and then summing the individual results (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Specific alkylating agents examined in this analysis included cyclophosphamide, ifosfamide, procarbazine, melphalan, and cisplatin. Chest radiation included radiation to the following fields: mantle, mediastinal, or lung.
Cardiovascular risk factors.
Chronic health conditions that could potentially increase the risk of CHF, including diabetes, hypertension, thyroid disease, and dyslipidemia, were captured if they were diagnosed by their treating physician (either a primary care physician or a hematologist/oncologist) and/or if a patient was receiving medications for their management. To be included as a pre-HCT cardiovascular risk factor, the condition had to be present during the initial pre-HCT evaluation. To be considered as a post-HCT cardiovascular risk factor, the health condition could have been diagnosed after HCT or may have been a pre-HCT condition that persisted after HCT. All post-HCT health conditions had to have developed before the onset of CHF in cases or for a comparable period of follow-up in controls.
Cases and controls were compared with respect to pre-HCT therapeutic exposures and pre- and post-HCT comorbidities using conditional logistic regression for dichotomous outcomes, and linear regression adjusting for matching set for continuous outcomes. HD anthracycline was defined as cumulative lifetime exposure of ≥ 250 mg/m2. To better understand the interaction between individual post-HCT cardiovascular risk factors (hypertension, diabetes, thyroid disease, and dyslipidemia) and HD anthracycline, we created separate conditional logistic regression models that allowed us to evaluate the added risk of CHF in the following categories: no cardiovascular risk factors and no HD anthracycline (referent group), cardiovascular risk factors and no HD anthracycline, no cardiovascular risk factors and HD anthracycline, and cardiovascular risk factors and HD anthracycline. Each of the models was adjusted for underlying diagnosis, sex, pre-HCT exposure to chest radiation, and individual pre-HCT cardiovascular risk factors.
Of the 88 cases included in this study, 22 were part of a case-control study published previously.19 However, the previous publication did not use a cohort study design, and was limited to subset of 1+–year survivors of both allogeneic and autologous HCT who developed CHF ≥ 1 year after HCT. In addition, because of limitations in sample size, we were unable to explore the interaction between individual cardiovascular risk factors and pre-HCT exposure to HD anthracycline.
Results
Clinical presentation of CHF
Median time to presentation for the 88 cases with CHF was 2.3 years from HCT (range, 1 month to 14.7 years), and median age at presentation was 52.4 years (range, 17.5-71.0 years). A detailed cardiac evaluation was available for 80 cases, confirmed by an echocardiogram in 77 cases; for the remaining 8 cases, diagnosis of CHF relied on primary care physician report.
Dyspnea on exertion was the most common presenting symptom (81.8%), followed by fatigue (72.7%), weight gain (62.5%), and orthopnea (43.2%). Seventy-three patients (83.0%) reported at least 3 symptoms associated with CHF. Mean left ventricular ejection fraction (LVEF) was 37% (range, 13%-53%), and all evaluable cases (n = 77) had a greater than 15% reduction of LVEF from their pre-HCT measurements.
Cohort study
As of December 31, 2008, the median follow-up for the entire cohort was 5.3 years (range, 0.1-20.5 years); for the 536 (43.1%) patients alive at last contact, it was 9.0 years (range, 1.6-20.5 years). Overall, the cohort provided 7195 person-years of follow-up, with 83% of the cohort followed through December 2008 (if alive) or up to the date of CHF diagnosis or death.
The clinical characteristics of the cohort are summarized in Table 1. The major indications for HCT included non-Hodgkin lymphoma (48.1%), Hodgkin lymphoma (22.8%), acute leukemia (14.6%), and multiple myeloma (14.5%). The most frequently used conditioning agents were cyclophosphamide (85.9%), etoposide (82.9%), TBI (59.2%), carmustine (22.4%), melphalan (20.7%), and busulfan (11.0%). The majority of patients (56.9%) received a preparative regimen that included cyclophosphamide, etoposide, and TBI, and an additional 24.9% received cyclophosphamide and etoposide but without TBI. Ninety (7.6%) patients underwent a subsequent allogeneic HCT at a median of 3.2 years (range, 0.6-13.6 years) after their autologous HCT.
As seen in Table 1, patients with CHF were significantly more likely to be female (59.1% vs 41.0%, P < .01) and older at HCT (46.5 vs 43.3 years, P = .03) compared with those without CHF. Race/ethnicity, conditioning exposure, and disease status at HCT were comparable between those who did and did not develop CHF.
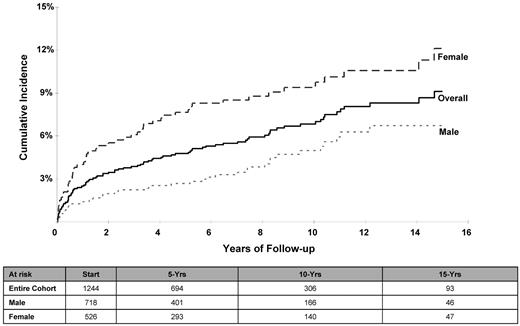
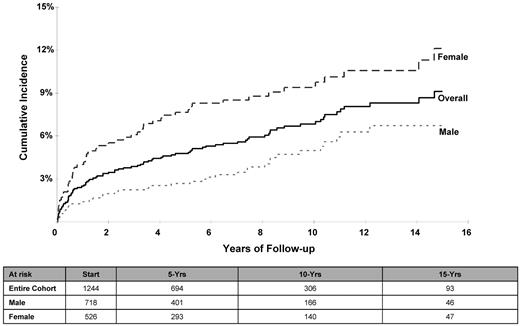
The cumulative incidence (± SE) of CHF at 5, 10, and 15 years after HCT was 4.8% ± 0.6%, 6.8% ± 0.8%, and 9.1% ± 1.0%, respectively (Table 2 and Figure 1). The cumulative incidence was higher for females compared with males: 12.1% ± 1.8% versus 6.7% ± 1.1% at 15 years (P < .01); the risk was especially high for female lymphoma patients (14.5% ± 2.3%) at 15 years compared with male lymphoma patients (6.1% ± 1.3%) or nonlymphoma patients of either sex (male: 8.8% ± 2.1%; female: 6.0% ± 2.0%; P < .01). Multivariate Cox regression analysis revealed an increased risk of CHF for females (RR = 2.4; P < .01) and for patients undergoing HCT for lymphoma (referent group: nonlymphoma; lymphoma RR = 1.5; P = .05); the risk of CHF increased with age at HCT (referent group: < 35 years at HCT; 35-44 years: RR = 1.9, P = .05; 45-54 years: RR = 2.5, P < .01; and ≥ 55 years: RR = 4.1, P < .01).
Cumulative incidence of CHF after HCT for the entire cohort and separated by sex.
Cumulative incidence of CHF after HCT for the entire cohort and separated by sex.
Patients who had received autologous HCT were at a 4.5-fold increased risk of developing CHF compared with an age- and sex-matched general population (SIR = 4.46, 95% CI: 3.58-5.47, Table 3). The AER for CHF was 95.5 per 10 000 person-years of follow-up or 0.96% per year. The greatest excess risk was observed for patients who underwent HCT before the age of 35 years (SIR = 17.5, 95% CI: 9.37-29.36).
Case-control study
The 88 patients with CHF (cases) were matched with 218 autologous HCT recipients who did not develop CHF (controls); 77 cases (88%) were matched with 2 or more controls. Table 4 summarizes the therapeutic exposures experienced before HCT, as well as the prevalence of pre- and post-HCT cardiovascular risk factors for cases and controls. Cumulative pre-HCT anthracycline exposure was significantly higher among cases compared with controls (309.4 vs 237.0 mg/m2, P < .01). Cases were significantly more likely to have hypertension before HCT (10.5% vs 8.7%, P < .01) and after HCT (28.4% vs 8.3%, P < .01) and were more likely to have diabetes after HCT (13.6% vs 4.6%, P < .01) compared with controls. In addition, cases were significantly more likely to have multiple cardiovascular risk factors after HCT (17.0% vs 6.4%, P < .01) compared with controls. The mean pre-HCT LVEF in cases was comparable to that of the controls (61.2% vs 62.8%, P = .08).
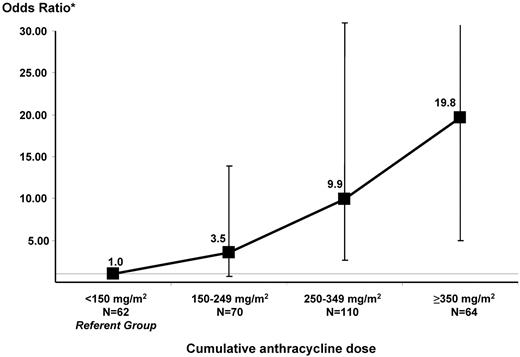
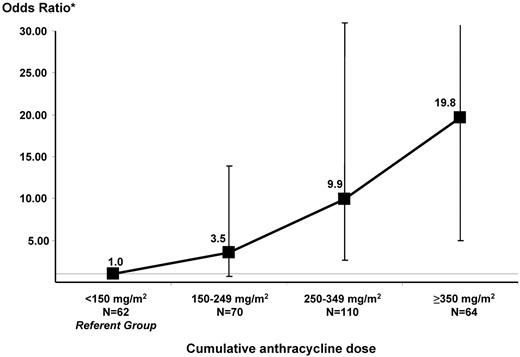
Figure 2 depicts the increasing risk of CHF with increasing cumulative anthracycline exposure after adjusting for sex, underlying diagnosis, and exposure to chest radiation. The referent group consisted of HCT survivors exposed to < 150 mg/m2 anthracyclines. There was a significant increase in risk beginning at dose category 250-349: odds ratio (OR): 9.9, P < .01; the OR was 19.8 (P < .01) for patients treated with ≥ 350 mg/m2 anthracyclines.
Magnitude of risk of CHF by increments of cumulative anthracycline dose. Matching criteria for cases and controls included: age at HCT (±5 years), year of HCT (±2 years), and duration of follow-up. The model was also adjusted for sex, underlying diagnosis (lymphoma vs nonlymphoma), and pre-HCT exposure to chest radiation.
Magnitude of risk of CHF by increments of cumulative anthracycline dose. Matching criteria for cases and controls included: age at HCT (±5 years), year of HCT (±2 years), and duration of follow-up. The model was also adjusted for sex, underlying diagnosis (lymphoma vs nonlymphoma), and pre-HCT exposure to chest radiation.
As described in the “Methods” section, we created separate logistic regression models to better understand the interaction between individual post-HCT cardiovascular risk factors (hypertension, diabetes, thyroid disease, and dyslipidemia) and HD anthracycline. As shown in Table 5, hypertension among patients exposed to HD anthracycline was associated with a 35-fold (OR = 35.3, P < .01) increase in risk of developing CHF, and diabetes conferred a nearly 27-fold (OR = 26.8, P < .01) risk. Six patients (2% of total) who were treated with HD anthracyclines had both hypertension and diabetes after HCT; these patients were included in both the hypertension and diabetes regression models. Post-HCT dyslipidemia (OR = 5.4, P = .01) and hypothyroidism (OR = 3.3, P = .7) were associated with a more modest risk among patients exposed to HD anthracyclines.
Outcome after CHF
Of the 88 patients with CHF, 60 have died (overall survival of 40% at 5 years after CHF diagnosis). The most common cause of death was relapse/progression of primary disease (46.7% of all deaths); heart failure accounted for 18.3% of the deaths.
Discussion
Patients undergoing HCT are at increased risk of developing cardiovascular complications because of pre-HCT therapeutic exposures (eg, chemotherapy and radiation) and conditioning and post-HCT comorbidities. Overall outcomes after these complications are generally poor, with a 5-year survival rate of < 50%.19,20 Previous studies have been limited by small sample sizes, relatively short follow-up after HCT, lack of pre-HCT therapeutic exposure data in assessment of CHF risk, and lack of information regarding cardiovascular risk factors. These factors limited the ability of these studies to describe the magnitude of risk with precision, to identify the role of pre-HCT therapeutic exposures, and to assess the contribution of post-HCT comorbidities in at-risk survivors. In addition, none of the previous studies escribed the cumulative incidence of CHF after autologous HCT. The current study overcomes these limitations by comprehensively evaluating the risk of CHF in a large cohort of autologous HCT recipients with 7195 person-years of follow-up.
Among the 1244 patients who underwent autologous HCT for hematologic malignancies at COH between 1988 and 2002, the estimated cumulative incidence of CHF was 4.8% at 5 years, and increased to 9.1% at 15 years after transplantation. Female lymphoma survivors were at an especially high risk, with the 15-year cumulative incidence approaching 15%. There is a paucity of data regarding the incidence of CHF in survivors of hematologic malignancy treated with conventional therapy or HCT. Aleman et al21 reported a 1.5% incidence of CHF or cardiomyopathy at 15 years in Hodgkin lymphoma survivors treated with conventional therapy using a combination of radiation ± low-dose (< 280 mg/m2) anthracycline. A subsequent study22 of patients with advanced-stage Hodgkin lymphoma treated with higher-dose anthracycline (cumulative dose 400 mg/m2) without radiation reported an 8% incidence of CHF or myocardial infarction at 11.5 years from diagnosis. For the current study, the pre-transplantation cumulative anthracycline dose approached 300 mg/m2 in patients with lymphoma and 18% had also received chest radiation, potentially explaining in part the high incidence of CHF.
Overall, the risk of CHF for HCT survivors was increased 4.5-fold compared with the age- and sex-matched general population, and the AER was 95.5 per 10 000 person-years of follow-up or 0.96% per year. Within our cohort, we found a significantly increased risk of CHF with increasing age at HCT. At the same time, compared with an age- and sex-matched general population, we showed a higher risk among the younger cohort. This association between older age at treatment and cardiovascular risk has been reported in non-HCT cancer populations,21,23 and is likely related to the increasing background risk of cardiovascular events and cardiovascular risk factors with increasing age. Conversely, the higher risk among younger HCT patients compared with the general population is due in part to the very low rates of CHF among the young individuals in the general population.
In the nononcology population, the overall incidence of heart failure is similar in men and women.24 However, it is clear that women with heart failure differ from men with respect to etiology, diagnosis, prognosis, and treatment.24,25 For example, the attributable risk of hypertension is significantly greater in women compared with men, indicating that there may be sex differences in cardiac response to the increase in afterload.25,26 In the current study, female survivors were more than twice as likely to develop CHF after HCT compared with males, despite adjustment for age at exposure and underlying diagnosis. This magnitude of risk persisted after adjustment for cumulative anthracycline dose, body mass index and cardiovascular risk factors including hypertension in the case-control model (data not shown). Our findings are consistent with previous reports in other cancer survivor populations that have demonstrated a higher risk of anthracycline-related CHF in women.4,21,27 The underlying mechanism for the higher risk of anthracycline-related CHF in female cancer survivors is not clear. Differences in body composition between men and women could alter the disposition and thus the metabolism of anthracyclines. With the exception of idarubicin (used in a very small proportion of patients), most anthracyclines do not reach a high concentration in adipose tissue.27,28 For women with a higher percentage of body fat for the same body surface area, equivalent doses of anthracyclines could lead to greater concentrations in nonadipose tissues such as the heart and therefore lead to more cardiotoxicity than in their male counterparts.
Anthracyclines (doxorubicin, daunomycin, idarubicin, and mitoxantrone) are widely used in the treatment of cancer. Therapy-related CHF, a well-recognized sequela of treatment with anthracyclines, can occur immediately after drug administration or years after the completion of therapy.12,29,30 The cardiotoxicity associated with anthracyclines is dose dependent and is the result of direct myocardial injury from reactive oxygen species or cardiotoxic drug metabolites.30 Our findings are in agreement with others that have demonstrated increased risk of cardiotoxicity with increasing dose of anthracycline.12,29,30 Compared with survivors treated with < 150 mg/m2 anthracycline, individuals treated with relatively modest doses of 250 to 349 mg/m2 were at a nearly 10-fold risk of developing CHF, identifying a threshold for surveillance after HCT. Conversely, we did not find an association between HD chemotherapy or TBI used for conditioning and risk of CHF. The majority of our patients received conditioning that included cyclophosphamide, etoposide, and TBI. The role of alternative conditioning regimens such as carmustine, etoposide, cytarabine, and melphalan in the development of CHF remains to be determined.
Few studies to date have evaluated the association between the risk of post-HCT CHF and pre-HCT therapeutic exposures (eg, anthracyclines and chest radiation) and cardiovascular risk factors. Hypertension is the most common risk factor for CHF in the general population, conferring a 2-fold risk for occurrence of CHF, and carries the highest population-attributable risk for CHF.24,26 In animal models, there is evidence that hypertension may accelerate left ventricular myocardial remodeling known to occur after anthracycline exposure.31 Conversely, the pathophysiology of heart failure in patients with diabetes is more complex and can be caused by unrecognized (silent) myocardial infarction or metabolic derangement due to hyperglycemia.32 In the current study, nearly one-third of all cases were diagnosed with hypertension before the onset of CHF. The presence of hypertension among recipients of HD anthracycline was associated with a 35-fold increased risk of CHF, whereas the risk was nearly 27-fold for HD-anthracycline recipients who developed diabetes, providing further evidence that hypertension and diabetes may be critical modifiers of anthracycline-related left ventricular myocardial injury and creating targeted populations for aggressive intervention.
The current study represents the first large-scale attempt to describe the magnitude of risk of CHF and the modifying effect of cardiovascular risk factors using validated clinical end points. However, a retrospective review of medical records is limited by the amount of information available in the records. Recognizing the logistical challenges of collecting pre-HCT treatment and post-HCT cardiovascular risk factor information on all 1244 study participants, we conducted a nested case-control study to comprehensively evaluate the modifying role of these risk factors on the prevalence of CHF after HCT. Our analysis did not include other cardiovascular risk factors such as the duration and intensity of tobacco exposure or details regarding physical activity. However, the prevalence of any smoking was analyzed and found to be comparable between cases and controls. Previous studies using self-reported information from a similar population have demonstrated that a large majority of HCT survivors quit smoking after HCT, with less than 15% reporting current use of tobacco.33
This study was limited to cases with clinical evidence of heart failure (ACC/AHA stage C or D), and we were able to obtain relevant cardiac imaging studies for nearly all of the cases to demonstrate compromised cardiac function. Furthermore, a diagnostic history and physical examination performed by a cardiologist or a primary physician was required as part of the clinical documentation of CHF. Our cases did not include those individuals who may have compromised cardiac function but were asymptomatic. However, the focus of this study was on understanding the impact of therapeutic exposures and cardiovascular risk factors on the development of clinical heart failure, and therefore asymptomatic cardiac dysfunction was beyond the scope of this report.
In summary, we found that the risk of CHF after autologous HCT continues to increase with time from HCT, and that female lymphoma survivors are at an especially high risk. Pre-HCT exposure to HD anthracycline (≥ 250 mg/m2) was an independent predictor of CHF, and conventional cardiovascular risk factors such as hypertension and diabetes were critical modifiers of this risk. These data form the basis for developing novel paradigms for prevention that include targeted screening in autologous HCT survivors at high risk for CHF (eg, female sex and cumulative lifetime anthracycline exposure ≥ 250 mg/m2), behavior modification after HCT (eg, adoption of healthy lifestyle and aggressive management of cardiovascular risk factors), and pharmacologic intervention (ACE inhibitors or β-blockers) for asymptomatic survivors with early evidence of cardiac dysfunction after HCT.
The online version of this article contains a data supplement.
Presented in part at the Annual Meeting of the American Society of Clinical Oncology, June 4, 2011, Chicago, IL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by the National Institutes of Health (2 K12 CA001727-14 to S.H.A., R01 CA078938 to S.B., and P01 CA30206 to S.J.F.) and by a Leukemia & Lymphoma Society Scholar Award for Clinical Research 2191-02 (to S.B.).
National Institutes of Health
Authorship
Contribution: S.H.A. designed the research, collected, assembled, analyzed, and interpreted the data, and wrote the manuscript; C.-L.S. and F.L.W. analyzed and interpreted the data; T.S., G.M., and L.F. collected and assembled the data; K.V. and S.J.F. provided study patients and collected and assembled the data; and S.B. designed the research, analyzed and interpreted the data, and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Saro Armenian, DO, MPH, City of Hope, 1500 East Duarte Rd, Duarte, CA 91010-3000; e-mail: sarmenian@coh.org.