Abstract
Effector memory T cells (TEM) do not cause graft-versus-host disease (GVHD), though why this is has not been elucidated. To compare the fates of alloreactive naive (TN) or memory (TM) T cells, we developed a model of GVHD in which donor T cells express a transgene-encoded TCR specific for an antigenic peptide that is ubiquitously expressed in the recipient. Small numbers of naive TCR transgenic (Tg) T cells induced a robust syndrome of GVHD in transplanted recipients. We then used an established method to convert TCR Tg cells to TM and tested these for GVHD induction. This allowed us to control for the potentially different frequencies of alloreactive T cells among TN and TM, and to track fates of alloreactive T cells after transplantation. TEM caused minimal, transient GVHD whereas central memory T cells (TCM) caused potent GVHD. Surprisingly, TEM were not inert: they, engrafted, homed to target tissues, and proliferated extensively, but they produced less IFN-γ and their expansion in target tissues was limited at later time points, and local proliferation was reduced. Thus, cell-intrinsic properties independent of repertoire explain the impairment of TEM, which can initiate but cannot sustain expansion and tissue damage.
Introduction
In allogeneic stem cell transplantation (alloSCT), a potentially curative therapy for malignant and nonmalignant disorders of hematopoiesis, donor T cells can attack recipient tissues, resulting in GVHD.1 Removal of allograft T cells prevents GVHD, but abrogates T cell–mediated immune reconstitution and graft-vs-leukemia (GVL). The goal of alloSCT research is to limit GVHD without sacrificing the positive effects of donor T cells. In a CD4-mediated mouse model of GVHD, we showed that naive phenotype T cells (TN) cause GVHD, while effector memory phenotype cells (TEM) do not.2 Importantly, despite their inability to cause GVHD, TEM engrafted, responded to antigen and mediated GVL.3 Several labs have reported similar findings for both CD4 and CD8 T cells using either major- or minor-histocompatibility antigen (miHAg) disparate systems.4-9 Thus, the failure of TEM to cause GVHD is a generalizable phenomenon. However, the mechanisms that limit the ability of TEM to cause GVHD are unknown. A better understanding of these mechanisms could lead to new targets for preventing and treating GVHD induced by TN. Further, understanding how and why TEM, TN, and TCM differ is important for developing such cells as therapeutics for promoting GVL and immune reconstitution with a lower risk for GVHD.
We considered 3 main hypotheses to explain why TEM fail to cause GVHD. First, there could be differences in migration and priming, because TEM do not express CD62L and CCR7, and they are therefore relatively excluded from lymph nodes,10,11 which have been proposed as key sites for alloreactive T-cell activation. Second, relative to TN, TEM may have a TCR repertoire with fewer alloreactive specificities.12 Third, TEM may be inherently limited in their ability to induce GVHD because of reduced clonal expansion or less pathogenic effector functions. Studies from Beilhack and our group indicated that trafficking alone is not responsible for the inability of TEM to cause GVHD.13,14 However, the roles of TCR repertoire and inherent limitations in TEM are currently unresolved.9
It has been difficult to distinguish the effects of repertoire and intrinsic functional properties of TEM in GVHD models. In polyclonal models of GVHD, the specificities of the disease-causing T cells are unknown and/or cannot be measured, precluding a precise estimation of the frequency of such cells. Tracking alloreactive and pathogenic T cells by expression of activation markers is also not feasible, as essentially all donor T cells after transplant express them.15
A complementary approach to address the issue of repertoire, which is not limited by these technical barriers, would be to compare the GVHD-inducing ability of TN and TM with identical specificities, using TCR Tg model systems. Indeed, there have been several reports of GVHD models in which disease is mediated by TCR Tg T cells of a single specificity. These include the CD8-mediated, major MHC-mismatched, 2C Tg model (Ld-specific)16-18 ; the CD4-mediated, MHC-mismatched 3BBM74 (I-Abm12-specific)19 and D10 (I-Ab-specific) models20 ; and the CD4-mediated miHAg-mismatched TEα model.20-22 These models have improved our understanding of GVHD but they each have limitations. Disease was mild in the MHC-mismatched models and required very large numbers of T cells to induce. In the TEα model, the alloantigen is highly expressed and restricted to the host MHCII+ hematopoietic compartment. This model causes only moderate disease in a sublethal irradiation model even with high doses of T cells (4 × 106), and the recipients develop only some of the features of GVHD.20-22 Thus, despite the great utility of TCR Tg models in other scenarios of T cell–mediated immunopathology, such as diabetes or EAE, use of TCR Tg models of GVHD has been relatively limited, to date.
Recognizing the potential utility of this approach to address the question of repertoire versus cell-intrinsic differences in the GVHD-inducing potential of TN vs TM, we sought to generate a TCR Tg model: (1) that reproduces the clinical and histopathologic GVHD induced by polyclonal cells; (2) that is induced by the transfer of a smaller numbers of cells, better modeling the number of alloreactive T cells transferred in polyclonal systems; and (3) in which hematopoietic and nonhematopoietic cells express the target antigen, also paralleling the typical clinical GVHD situation. We chose to transfer CD4+ TS1 TCR Tg T cells,23 which recognize the S1 epitope of influenza hemagglutinin (HA), into irradiated recipients in which HA is expressed at low levels in all cells by virtue of the HA104 Tg (HA104 mice24 ). We found that the transfer of small numbers of TS1 TN into irradiated HA104 Tg recipients precipitated the major clinical and histopathologic features of GVHD.
An additional advantage of this model is that the TS1 Tg mouse has been used extensively to study T cell memory.25-34 Protocols for the generation of the required numbers of TM are available based on in vitro priming followed by in vivo resting.25,26,30,33,34 Similar systems have been used to generate TM by others in other TCR Tg systems.35-37 This method is advantageous, because TM generated by infection or immunization with killed virus in vivo are very few and their frequencies decay with time (Hataye et al38 and K.W.J., W.D.S., and M.J.S., unpublished observations, June 2009). This limitation of cell number precluded the use of this approach in our studies,which required purification and transfer to many simultaneous recipients. Importantly, TM produced via the method we used have authentic memory cell properties, gene expression and behavior.25-37,39 Such cells function similarly to memory T cells generated by influenza infection in vivo, including providing protective immunity.29,30,33,34
We compared the GVHD-inducing ability of TS1 TN and TEM and found an inherent defect in the ability of TEM to cause GVHD. We were able to trace this defect to differential accumulation of TN and TEM in the colon, but, surprisingly, not in spleen, as well as to a decrease in cytokine production at both sites. We also used the system to isolate TCM that were definitively Ag-experienced. This allowed us to address whether TCM can cause GVHD, a point that is currently controversial,5,40 showing clearly that they do cause GVHD. Taken together, these experiments help explain the intriguing finding that TEM are incompetent at causing disease. They also have important implications for the use of TM in the alloSCT setting in patients.
Methods
Mice
Mice were from the following sources: BALB/c (National Cancer Institute or The Jackson Laboratory), BALB/c RAG2−/− (Taconic), BALB/c TS1 and B10.D2 TS1 (Adam Adler, University of Connecticut), and BALB/c Thy1.1 (The Jackson Laboratory). BALB/c TS1 RAG2−/−, B6.C TS1 RAG1−/− and BALB/c TS1 Thy1.1 RAG2−/− were generated at Yale (see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Hosts were HA104 heterozygotes24 except in Figure 1A and B in which homozygotes were used.
Bone marrow transplantation
Seven to 12-week-old hosts received 750 cGy and BALB/c HA-negative RAG−/− BM alone or in combination with sorted TS1 TN, TCM, or TEM. Recipients were weighed 2-3 times per week. Mice that lost 30% of their body weight were killed and scored as dead; their last recorded weight remained in the dataset.
Histopathology
Flow cytometry
Antibodies were from commercial sources or lab-prepared as described in supplemental Methods. Surface and intracellular cytokine staining and dead cell exclusion were as described3 and as in supplemental Methods. For BrdU analysis, mice were injected with 3 mg of BrdU (Sigma-Aldrich) 30 minutes before sacrifice. BrdU staining and apoptosis assays were performed as in supplemental Methods. Colon and hepatic cells were isolated as described in supplemental Methods.
Memory cell generation
TS1 CD4 T cells were enriched to > 75% purity from splenocytes using a CD4+ T cell–enrichment kit (StemCell Technologies). TS1 cells (106 cells/mL) were cultured with HA S1 peptide 110-119 (SFERFEIFPK; 10ug/mL; Keck Facility, Yale University) and T cell–depleted, irradiated splenocytes (3 × 106 cells/mL) for 3 days.25,26 BALB/c or B6.C TS1 cells (5 × 106) were transferred intravenously into each RAG−/− BALB/c or B6.C recipient, respectively.
Cell sorting
TS1 were sort-purified by excluding cells expressing F4/80, CD11b, CD8α, Gr-1, CD25, and Ter119. TN (CD62L+ CD44dim) were sorted from unmanipulated TS1 mice and TCM (CD62L+ CD44+) and TEM (CD62L− CD44+) from “memory” mice. Sorted cells were 40%-50% CD4+TS1+. Purities of TN, TEM, and TCM among TS1 were > 99%.
Statistics
The significance of comparisons of weight change and the percentage of BrdU+ TS1 in colon were calculated by Student t test. The significance of comparisons of histopathology scores, numbers of TS1 cells, and percentages of TS1 that were BrdU+ (in spleen), cytokine+, or apoptotic were calculated by the Mann-Whitney U test.
Results
A novel TCR-transgenic model of GVHD
To resolve the contribution of TCR repertoire to the different abilities of TEM and TN to induce GVHD, we transplanted TS1 CD4 Tg into BALB/c HA104 Tg mice, which express HA as a model miHAg. TS1 mice were RAG2-deficient, ensuring that all TS1 cells express a single TCR.
HA104 mice were irradiated and reconstituted with RAG−/− BALB/c BM and 1000, 3000, or 9000 sort-purified BALB/c TS1 TN (CD44dimCD62L+). As negative controls, we transferred BM without TS1 cells into irradiated HA104 recipients and 3000 TS1 TN into irradiated BALB/c recipients lacking the HA104 Tg. Transplant of TS1 cells into HA104 recipients induced death and weight loss. Death was dose-dependent, with 100% mortality in mice that received 9000 TS1 cells, 40% in mice that received 3000 TS1 cells, and 10% in mice that received 1000 TS1 cells (Figure 1A). There was no significant difference in weight loss between recipients of 3000 and 1000 TS1 cells (Figure 1B). Importantly, there was no death or long-term weight loss in irradiated HA104 mice that received BM cells alone or in HA-negative BALB/c mice that received 3000 or 9000 TS1 cells (Figure 1 and not shown), verifying that disease is dependent on both donor TS1 cells and expression of HA by recipients.
A TCR Tg model of GVHD. (A-B) HA104 mice received 750cGy and 8 × 106 BALB/c RAG−/− BM cells alone or in combination with 9000, 3000, or 1000 sorted TS1 TN. As an additional control, wt mice received BM cells in combination with 3000 TN. Shown are survival (A) and weight loss (B). P values are relative to BM only controls. Data are representative of 2 independent experiments. (C-D) HA104 mice received 750cGy and 8 × 106 RAG−/− BM cells alone or in combination with 3000 sorted TS1 TN. On day 21, mice were killed and tissues were taken for histopathology analysis. Representative skin, colon, and liver pathology are shown in panel C. Pathology scores are shown in panel D. Pathology data were combined from 2 independent experiments.
A TCR Tg model of GVHD. (A-B) HA104 mice received 750cGy and 8 × 106 BALB/c RAG−/− BM cells alone or in combination with 9000, 3000, or 1000 sorted TS1 TN. As an additional control, wt mice received BM cells in combination with 3000 TN. Shown are survival (A) and weight loss (B). P values are relative to BM only controls. Data are representative of 2 independent experiments. (C-D) HA104 mice received 750cGy and 8 × 106 RAG−/− BM cells alone or in combination with 3000 sorted TS1 TN. On day 21, mice were killed and tissues were taken for histopathology analysis. Representative skin, colon, and liver pathology are shown in panel C. Pathology scores are shown in panel D. Pathology data were combined from 2 independent experiments.
To investigate whether TS1 cells induce pathology typical of GVHD, HA104 mice were transplanted with 3000 TS1 TN and histopathology was evaluated on day 21 (Figure 1C-D). TS1 recipients developed skin and colon disease and mild, but statistically significant liver disease. Skin pathology included dermal fibrosis, fat loss, inflammation, epidermal interface changes and mild follicular drop-out. In the colon there were inflammatory infiltrates, apoptotic bodies, neutrophilic cryptitis and crypt loss. Affected liver had central perivenulitis and bile duct injury. These pathologic findings are typical of GVHD induced by polyclonal cells. In samples taken at later time points, pathologic changes typical of GVHD were also observed below. GVHD-type pathologic changes in the colon were always observed, while there was some variability in the intensity of skin disease (K.W.J., W.D.S, and M.J.S., unpublished observations, May 2010), as is sometimes the case in miHAg mismatched systems.41 Notably, TS1 Tg T cells on the B6.C RAG−/− background (H-2d) also caused GVHD in BALB/c HA104 mice, with a similar clinical and pathologic syndrome (data not shown and supplemental Figure 1).
Generating Memory TS1 Cells
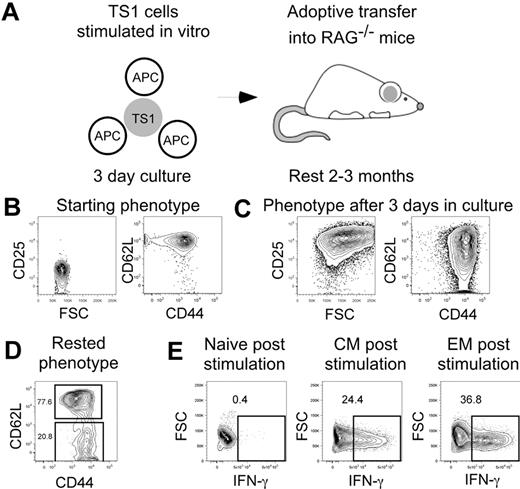
Having established that TS1 cells induce GVHD in HA104 mice, we next generated TS1 TM so we could compare TS1 TN, TEM, and TCM in the HA104 GVHD model (Figure 2A). TS1 cells were stimulated in vitro for 3 days with HA peptide and APCs and then rested in syngeneic RAG−/− recipients for 2-3 months to allow memory differentiation, as demonstrated in prior work.25-34 By day 3 of culture, TS1 cells increased in FSC and uniformly up-regulated CD25. CD44 was also up-regulated and a fraction of cells lost expression of CD62L (Figure 2B,C). After residence in RAG−/− mice for 2-3 months, ∼ 80% of TS1 cells were CD62L+CD44+ and therefore of a TCM phenotype, while ∼ 20% of TS1 cells were CD62L−CD44+ and therefore of a TEM phenotype (Figure 2D). All memory cells were CD25− and FoxP3−, as were TS1 TN (not shown).
Generation of TS1 memory cells. TS1 TN were stimulated in vitro with HA peptide and irradiated T cell-depleted splenocytes for 3 days. Cells were then transferred into RAG−/− mice and allowed to rest for 2-3 months (design, A). Shown are representative flow cytometry showing the phenotypes before culture (B), after 3 days in culture (C) and after resting in RAG−/− mice (D). Data are representative of > 10 experiments. IFN-γ production in TN, TCM and TEM post stimulation with PMA and ionomycin is shown in panel E (data are from 1 experiment).
Generation of TS1 memory cells. TS1 TN were stimulated in vitro with HA peptide and irradiated T cell-depleted splenocytes for 3 days. Cells were then transferred into RAG−/− mice and allowed to rest for 2-3 months (design, A). Shown are representative flow cytometry showing the phenotypes before culture (B), after 3 days in culture (C) and after resting in RAG−/− mice (D). Data are representative of > 10 experiments. IFN-γ production in TN, TCM and TEM post stimulation with PMA and ionomycin is shown in panel E (data are from 1 experiment).
To confirm that our TS1 TM behaved as previously reported, sort-purified TS1 TCM and TEM recovered from RAG−/− recipients or fresh TN were stimulated with PMA and ionomycin for 4 hours and assayed for production of IFN-γ by intracellular cytokine staining (Figure 2E). As predicted, and consistent with their memory phenotypes, TS1 TEM and TCM, but not TN, produced IFN-γ.
TS1 TEM cause minimal GVHD while TS1 TCM and TN induce similar disease.
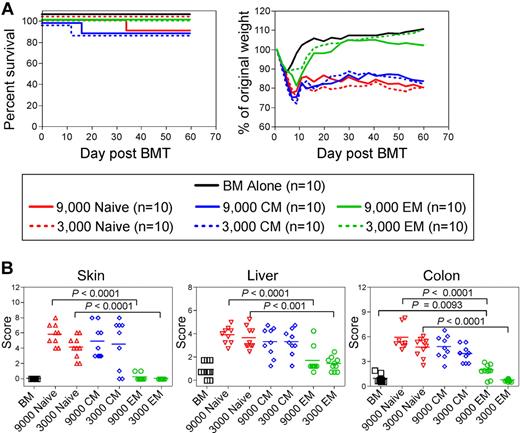
We compared the ability of TS1 TN, TEM, and TCM to induce GVHD by transferring graded doses of sort-purified TS1 TN, TCM, or TEM, with BALB/c BM cells, into irradiated HA104 recipients. Although all TS1 recipients initially lost more weight than did BM-only controls, weight loss in the TN and TCM recipients was far more severe and prolonged as compared with that in TEM recipients (Figure 3A). Weight change in the 3000 TEM group did not differ at most time points from that in the BM alone control. However, TEM were not inert, as 9000 TEM induced statistically significant weight loss, albeit mild, relative to BM alone controls. Weight changes in the TCM and TN recipient groups were similar at all time points. Although mortality was limited in this experiment, all deaths occurred in the TN and TCM groups. In other experiments, TN and TCM were more lethal, with death in as many as 50% of mice receiving 3000 TN and 20% of mice receiving 3000 TCM. In an analysis of survival in 5 independent experiments in which TS1 TN, TEM and TCM were infused, no TEM recipients died (combined survival data, supplemental Figure 2). Overall, TS1 TCM were less lethal than were TS1 TN though this difference was not reflected in weight loss and histopathologic changes (Figures 3 and 4).
TS1 TN and TCM cause severe GVHD whereas TS1 TEM cause only mild GVHD. HA104 mice received 750cGy and 8 × 106 RAG−/− BM cells alone or in combination with 9000 or 3000 TS1 TN, TCM or TEM. Mice were killed at day 60 and tissues were taken for histopathologic analysis. Survival and weight loss are shown in panel A. P < .0001 comparing weight loss in recipients of TN or TCM vs recipients of BM alone on days 7 to 60. P < .0001 comparing weight loss in TEM vs TN recipients on days 13-60. P < .0001 on days 9-23 and P < .05 on days 46-60 comparing weight loss in recipients of 9000 TEM vs recipients of BM alone. Weight data in recipients of 3000 TS1 cells are representative of > 3 independent experiments; weight loss in recipients of 9000 TS1 TEM is from one experiment. Mice were killed at the conclusion of the experiment and histopathology was scored in panel B. Pathology is from one experiment with n = 9-10 per group. See text for details.
TS1 TN and TCM cause severe GVHD whereas TS1 TEM cause only mild GVHD. HA104 mice received 750cGy and 8 × 106 RAG−/− BM cells alone or in combination with 9000 or 3000 TS1 TN, TCM or TEM. Mice were killed at day 60 and tissues were taken for histopathologic analysis. Survival and weight loss are shown in panel A. P < .0001 comparing weight loss in recipients of TN or TCM vs recipients of BM alone on days 7 to 60. P < .0001 comparing weight loss in TEM vs TN recipients on days 13-60. P < .0001 on days 9-23 and P < .05 on days 46-60 comparing weight loss in recipients of 9000 TEM vs recipients of BM alone. Weight data in recipients of 3000 TS1 cells are representative of > 3 independent experiments; weight loss in recipients of 9000 TS1 TEM is from one experiment. Mice were killed at the conclusion of the experiment and histopathology was scored in panel B. Pathology is from one experiment with n = 9-10 per group. See text for details.
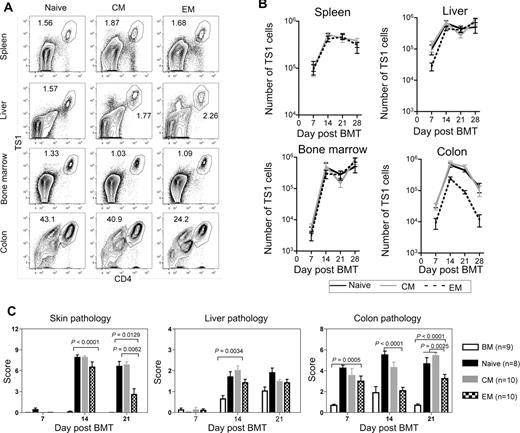
Fewer colon TS1 cells accumulate in recipients of TEM than in recipients of TN or TCM. HA104 mice received 750cGy and 8 × 106 RAG−/− BM cells in combination with 3000 TS1 TN, TCM, or TEM. On days 7, 14, 21, and 28, mice were killed and spleen, liver, BM, and colon cells were isolated. In addition, skin, colon, and liver samples were taken for analysis of histopathology. Representative FACS plots of CD4 and TS1 staining in the spleen, liver, BM and colon of TN, TCM, and TEM recipients 14 days after transplantation are shown in (A). Total numbers of TS1 in spleen, liver, BM and colons of TN, TCM and TEM recipients are shown in (B). In the colon, P values comparing the number of TS1 cells in TN versus TEM recipients were as follows: day 7, P = .0021; day 14, P = .0015; day 21, P < .0001; day 28, P = .0286. Skin, liver and colon pathology scores are shown in (C). For day 21 colon scores, P = .0025 comparing scores in recipients of TEM to the combined scores from TN and TCM recipients. Results shown are combined from 2 independent experiments. In the first experiment, a minimum of 4 mice per group were killed on days 7,14, 21, and 28. In the second experiment a minimum of 4 mice per group were killed on days 7, 14, and 21. Day 21 pathology scores for TN recipients are also shown in Figure 1D.
Fewer colon TS1 cells accumulate in recipients of TEM than in recipients of TN or TCM. HA104 mice received 750cGy and 8 × 106 RAG−/− BM cells in combination with 3000 TS1 TN, TCM, or TEM. On days 7, 14, 21, and 28, mice were killed and spleen, liver, BM, and colon cells were isolated. In addition, skin, colon, and liver samples were taken for analysis of histopathology. Representative FACS plots of CD4 and TS1 staining in the spleen, liver, BM and colon of TN, TCM, and TEM recipients 14 days after transplantation are shown in (A). Total numbers of TS1 in spleen, liver, BM and colons of TN, TCM and TEM recipients are shown in (B). In the colon, P values comparing the number of TS1 cells in TN versus TEM recipients were as follows: day 7, P = .0021; day 14, P = .0015; day 21, P < .0001; day 28, P = .0286. Skin, liver and colon pathology scores are shown in (C). For day 21 colon scores, P = .0025 comparing scores in recipients of TEM to the combined scores from TN and TCM recipients. Results shown are combined from 2 independent experiments. In the first experiment, a minimum of 4 mice per group were killed on days 7,14, 21, and 28. In the second experiment a minimum of 4 mice per group were killed on days 7, 14, and 21. Day 21 pathology scores for TN recipients are also shown in Figure 1D.
Multiple surviving mice from one experiment were killed at day 60 and pathology was scored (Figure 3B). Although potentially biased by death in the TN and TCM recipients, which would presumably eliminate mice with the most severe pathology, there were significant differences between the recipients of TEM and TCM or TN. Recipients of TS1 TN and TCM developed typical histopathologic GVHD of the skin, liver and colon whereas TEM induced no skin or liver GVHD. 9000, but not 3000, TEM induced only mild pathology in the colon. Disease in TN and TCM recipients did not significantly differ. In 2 other experiments, colon disease was always significantly milder in TEM recipients compared with TN recipients; however, TN-induced skin disease was variable (mild in one experiment and absent in another, not shown). Liver disease was also mild and not significantly different in these 2 other experiments (not shown). In both of these experiments, TS1 TN induced death in 35%-50% of mice, which most likely eliminated the mice with the most severe pathology. In parallel experiments in the B6.C TS1→HA104 system, TN and TCM induced severe clinical GVHD whereas again TS1 TEM induced only mild weight loss, significantly less than induced by either TN or TCM (supplemental Figure 1).
In sum these experiments demonstrate that when TEM and TN have an identical TCR, TEM are inherently limited in their ability to cause GVHD, whereas TCM behave similarly to TN. Thus, repertoire alone cannot completely account for the different abilities of TEM and TN to induce GVHD.
Expansion of TS1 TN, TEM, and TCM in HA104 Tg recipients
To investigate why TS1 TEM induce less GVHD than do TS1 TN or TCM, we tested the hypothesis that the lack of GVHD relates to differences in expansion of TEM vs TN or TCM progeny posttransfer. HA104 mice were irradiated and reconstituted with BALB/c RAG−/− BM with no T cells or with 3000 TS1 TN, TCM, or TEM. At weekly intervals posttransplant, TS1 cells in spleen, liver, BM and colon were enumerated by flow cytometry (Figure 4A-B). Strikingly, in spleen and BM similar numbers of TS1 cells were recovered in TEM, TN, and TCM recipients at all time points. In liver, aside from a small lag in TEM recipients at day 7, TS1 numbers were also similar in all recipient groups. Thus, alloreactive TS1 TEM engraft, survive, and robustly expand in vivo. However, there were substantially fewer TS1 cells in the colons of TEM recipients than in TN or TCM recipients at all time points, and by day 28 the difference was approximately 20-fold (Figure 4B). TEM thus have a selective defect in accumulation in the colon, which likely contributes to their reduced ability to cause clinical GVHD.
TS1 TEM induce early GVHD that is not sustained
Given that TEM expanded at early time points, we compared histopathology in TEM, TCM and TN recipients at days 7, 14, and 21 after transplant (Figure 4C; representative pathology, supplemental Figure 3). There was no skin GVHD in any group on day 7. Skin scores were similar in all TS1 recipient groups on day 14, but by day 21 TEM recipients had lower scores than did TN or TCM recipients. This was not driven by fat loss (a measure of weight loss) as P values were significant even when fat scores were not included (not shown). Liver GVHD was only detectable on day 14, and TEM induced mild but significant disease that was similar to that induced by TN or TCM. In the colon, TN and TCM recipients had disease at all time points while in TEM recipients significant pathology was only seen on days 7 and 21. However, scores were lower in TEM recipients than in recipients of TN or TCM at day 14 and as compared with scores in TN and TCM recipients combined at day 21. Therefore, although TEM recipients had little GVHD at late time points, there was histologic disease at early times, albeit of a relatively milder nature, suggesting that TEM can initiate, but are limited in their ability to sustain, a pathologic GVHD response.
TS1 TN outcompete TS1 TEM when cotransferred
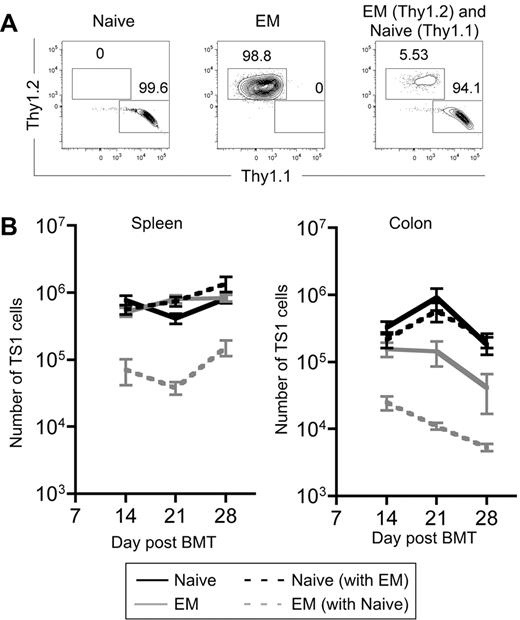
The ability of TEM progeny to accumulate to a significant level in spleen, BM and liver was unexpected, given the lack of clinical GVHD. An informative way to compare the fitness of 2 populations is in a competition assay wherein latent defects not apparent when single populations are tested can be revealed. This is especially relevant for testing the impact of TEM in GVHD, as in the clinic mixed populations of T cells are infused. We therefore cotransferred 3000 Thy1.1 TS1 TN and 3000 Thy1.2 TS1 TEM into irradiated HA104 recipients. The Thy1 difference allowed the fates of the 2 types of cells to be separately tracked. As controls, 3000 TS1 Thy1.1 TN or Thy1.2 TEM were transferred individually. Mice were killed on days 14, 21, and 28 and spleen and colon cells were analyzed.
As in prior experiments, when transferred individually, progeny of TS1 TEM and TN expanded similarly in spleen while TEM expanded less in colon. However, the presence of TS1 TN markedly suppressed the expansion of TS1 TEM in both spleen and colon at all time points (Figure 5A-B). Thus, the presence of TN inhibits the expansion of TEM and suggests that in vivo, even if there are alloreactive TEM, their function and presumably pathogenicity will be suppressed by alloreactive TN. Clinical GVHD, as measured by weight loss, was similar in TN and TN+TEM recipients (supplemental Figure 4), indicating that TEM do not suppress TN-induced GVHD, consistent with the failure of TEM to suppress the accumulation of TN progeny in spleen and colon.
Progeny of TS1 TEM are outcompeted by progeny of TS1 TN. HA104 mice received 750cGy and 8 × 106 RAG−/− BM cells in combination with 3000 Thy1.1 TS1 TN, 3000 Thy1.2 TS1 TEM, or a mix of both. On days 14, 21, and 28 spleen and colon cells were isolated. Representative flow cytometry plots showing Thy1.1 and Thy1.2 expression on splenic CD4+TS1+ cells on day 14 are shown in (A). The numbers of TS1 cells in the spleen and colon are shown in (B). P < .05 comparing numbers of TEM that are transferred alone to numbers of TEM that are cotransferred with TN in the spleen and colon for all time points analyzed except for the colon on day 28, for which P = .138. Days 14 and 21 are combined from 2 independent experiments; day 28 is from 1 experiment. There were 8 mice per group for days 14 and 21 and 3 mice per group for day 28.
Progeny of TS1 TEM are outcompeted by progeny of TS1 TN. HA104 mice received 750cGy and 8 × 106 RAG−/− BM cells in combination with 3000 Thy1.1 TS1 TN, 3000 Thy1.2 TS1 TEM, or a mix of both. On days 14, 21, and 28 spleen and colon cells were isolated. Representative flow cytometry plots showing Thy1.1 and Thy1.2 expression on splenic CD4+TS1+ cells on day 14 are shown in (A). The numbers of TS1 cells in the spleen and colon are shown in (B). P < .05 comparing numbers of TEM that are transferred alone to numbers of TEM that are cotransferred with TN in the spleen and colon for all time points analyzed except for the colon on day 28, for which P = .138. Days 14 and 21 are combined from 2 independent experiments; day 28 is from 1 experiment. There were 8 mice per group for days 14 and 21 and 3 mice per group for day 28.
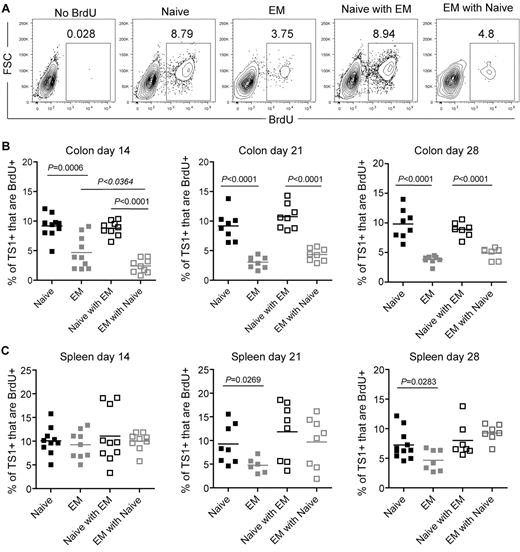
To further investigate why there were fewer TS1 cells in colons of recipients of TEM than TN, we used BrdU pulse-labeling to assess the frequency of dividing TS1 cells in both the individual and competitive situations. On days 14, 21, and 28 cohorts were given BrdU 30 minutes before sacrifice, which labels only cells currently in S phase. This short pulse excludes the possibility of cells being labeled outside the colon and subsequently migrating into tissues. At all time points a smaller fraction of TEM than TN TS1 progeny recovered from colon were dividing, and this percentage was further suppressed by competing TN at day 14, but not on days 21 or 28 (Figure 6A-B). In spleen, BrdU incorporation by TS1 cells was similar in all groups at day 14 (Figure 6C). On days 21 and 28, however, a smaller fraction of TEM than TN progeny incorporated BrdU, though the difference was less marked than that in the colon. In contrast, there were no significant differences among any of the groups in TS1 cell apoptosis, nor did the competitive presence of TN increase cell death among TEM (supplemental Figure 5). Thus, the reduced accumulation of TEM in the colon is explained at least in part by a reduced ability to divide in situ. The further suppression of TEM by TN (Figure 5A-B) primarily occurs by mechanisms other than diminishing proliferation of TEM.
Fewer colon TS1 proliferate in TEM than in TN recipients. Mice were transplanted as in Figure 5. On days 14, 21, and 28, mice received BrdU 30 minutes before sacrifice. (A) Representative BrdU staining on CD4+TS1+ cells recovered from colon on day 14. The no BrdU control depicts a recipient of TS1 TN and TEM that was not injected with BrdU. Percentages of colon (B) and splenic (C) CD4+TS1+ cells that are BrdU+. For each time point, data were combined from 2 independent experiments with similar results.
Fewer colon TS1 proliferate in TEM than in TN recipients. Mice were transplanted as in Figure 5. On days 14, 21, and 28, mice received BrdU 30 minutes before sacrifice. (A) Representative BrdU staining on CD4+TS1+ cells recovered from colon on day 14. The no BrdU control depicts a recipient of TS1 TN and TEM that was not injected with BrdU. Percentages of colon (B) and splenic (C) CD4+TS1+ cells that are BrdU+. For each time point, data were combined from 2 independent experiments with similar results.
Because the integrin α4β7 promotes recruitment of T cells into bowel, we investigated whether TS1 cells in TN, TEM, and TCM recipients differed in its expression. In the spleen at day +28 the MFI of α4β7 staining was highest in TS1 TN recipients as compared with TS1 TEM recipients (supplemental Figure 6). α4β7 expression of TCM was between that of TS1 TN and TEM. In the colon, however, α4β7 was low in all TS1 recipients, consistent with prior reports that α4β7 is down-regulated in bowel-infiltrating T cells.42 Overall, it is likely that differences in numbers of colonic TS1 cells can be attributed to both differences in proliferation as well as the small differences seen in α4β7 expression.
Activation, phenotype, and effector functions of TS1 progeny
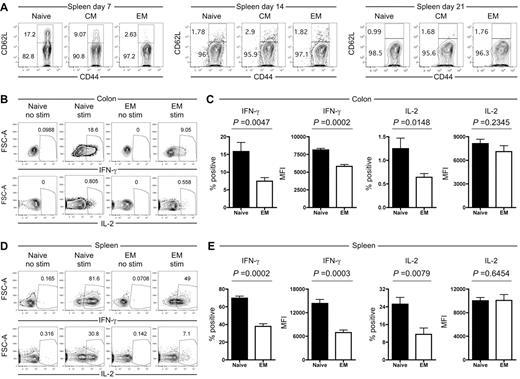
In addition to quantitative differences, we investigated whether progeny of TS1 TN, TCM and TEM differ qualitatively in ways that may contribute to their reduced ability to induce GVHD. In the spleen, the majority of TS1 cells in all recipients were CD62L−CD44+ and therefore of an effector/TEM phenotype (Figure 7A). However, at day 7 a substantial fraction of TS1 cells in TN and TCM recipients maintained CD62L expression, though by day 14 all TS1 cells had down-regulated CD62L. As expected, there was little if any reexpression of CD62L by TEM progeny. In spleen and colon, CD69 expression was elevated on all TS1 T cells regardless of origin (supplemental Figure 7). In contrast, CD25 was uniformly elevated only in colon-resident TS1 progeny of both TN and TEM recipients (supplemental Figure 7), suggesting a difference in environment affecting the quality of T cell activation. Consistent with the activated phenotype,43 splenic and colon TS1 cells in both TN and TEM recipients also up-regulated GITR (supplemental Figure 7). In sum these data, together with the BrdU data (Figure 6), reflect sustained in situ activation of TS1 T cells locally in the colon as well as spleen.
Progeny of TS1 TEM produce less IFN-γ than progeny of TS1 TN. HA104 mice received 750cGy and 8 × 106 RAG−/− BM cells in combination with 3000 TS1 TN, TCM or TEM. (A) Expression of CD62L and CD44 for CD4+ TS1+ cells in the spleen on days 7, 14 and 21. (B-E) HA104 recipients were transplanted with 3000 TS1 TN or TEM as described in panel A. On day 21, spleen and colon cells were isolated, stimulated with PMA and ionomycin and stained for IFN-γ and IL-2. Representative FACS plots with and without stimulation are shown for the colon (B) and for the spleen (D). The percentages of TS1 cells that are IFN-γ+ or IL-2+ and the MFIs of the IFN-γ+ or IL-2+ cells are shown for the colon (C) and the spleen (E). Data are combined from 2 independent experiments (total of 8 mice per group).
Progeny of TS1 TEM produce less IFN-γ than progeny of TS1 TN. HA104 mice received 750cGy and 8 × 106 RAG−/− BM cells in combination with 3000 TS1 TN, TCM or TEM. (A) Expression of CD62L and CD44 for CD4+ TS1+ cells in the spleen on days 7, 14 and 21. (B-E) HA104 recipients were transplanted with 3000 TS1 TN or TEM as described in panel A. On day 21, spleen and colon cells were isolated, stimulated with PMA and ionomycin and stained for IFN-γ and IL-2. Representative FACS plots with and without stimulation are shown for the colon (B) and for the spleen (D). The percentages of TS1 cells that are IFN-γ+ or IL-2+ and the MFIs of the IFN-γ+ or IL-2+ cells are shown for the colon (C) and the spleen (E). Data are combined from 2 independent experiments (total of 8 mice per group).
To evaluate effector functions of TS1 progeny in TN and TEM recipients, we stimulated splenocytes and cells extracted from colon with PMA and ionomycin and stained for intracellular IFN-γ, IL-2, and IL-17. At day 21 post transplantation, in colon, ∼ 16% of TS1 TN progeny produced IFN-γ as compared with ∼ 7.5% of TS1 TEM progeny (Figure 7B-C). The IFN-γ MFI was also higher among IFN-γ+ TS1 TN progeny. The frequencies of IL-2+ (Figure 7B,C) and IL-17+ cells (supplemental Figure 8) in the colon were < 1.5% in all groups, suggesting that they were less important for pathogenesis relative to IFN-γ+ cells. Nonetheless, there were differences consistent with less effector function deriving from TEM in that the frequency of IL-2+ cells as well as the MFI of IL-17 staining were both lower in TS1 TEM progeny. In the spleen, we saw a parallel picture, although the frequencies of cytokine-producing cells in both TN and TEM recipients were higher than in the colon (Figure 7D-E). Seventy percent and 25% of TN progeny produced IFN-γ and IL-2 respectively, in contrast to 38% and 11.5% of TEM progeny, respectively. Again, similar to colon, the MFI of IFN-γ but not IL-2 was higher among IFN-γ producing TS1 TN progeny. Overall, analysis of cytokine secretion clearly showed enhanced effector function generated by TN progeny compared with TEM progeny, with the most striking effects seen with IFN-γ but subtle effects observed with both IL-2 and IL-17.
We also considered whether there were differences in expression of the inhibitory receptor PD-1. PD-1 was uniformly up-regulated on TS1 in both spleen and colon in TS1 TN, TEM, and TCM recipients as early as day 7 after transplantation (not shown). Strikingly, at day 28, the MFI of PD-1 was significantly higher in TS1 TEM progeny than in TN progeny (supplemental Figure 7), suggesting that TEM progeny may be more susceptible to inhibition by PD ligands, which in turn could contribute to both their reduced numbers and diminished IFN-γ and IL-2 production.
Discussion
The main goal of the present studies was to determine whether differences in TCR repertoire account for the relative inability of CD4+ TEM to induce GVHD. We approached this by devising a system in which highly purified TM and TN with identical repertoires could be compared. We developed a TCR Tg model of GVHD and used it to compare the abilities of TEM, TCM, and TN to induce GVHD. We found that TEM differ from TN in their ability to induce GVHD, even when expressing the same TCR, and thus there are repertoire-independent explanations for the reduced ability of TEM to cause GVHD. In contrast, TCM behaved similarly to TN.
The role of repertoire differences between TN and TEM in GVHD has been controversial. Dutt et al found that prior vaccination of donors with host splenocytes increased the potency of CD4+ TEM to induce GVHD.9 However, disease induced by T cells from primed donors was different in character from that induced by TN. Such differences could have been because of shifts in the repertoire and recognition of different allogeneic epitopes, intrinsic differences between TN and TEM, or both. These possibilities could not be distinguished in that polyclonal system because alloreactive and GVHD-inducing T cells could not be tracked, as was possible in the TCR Tg model. Zhang et al reported that donor CD4+CD62L− cells isolated from mice with GVHD induced GVHD in secondary recipients whereas fresh TEM did not.44 However, these studies do not bear on the role of repertoire among TM, because CD4 cells extracted from hosts undergoing GVHD would have been activated effectors rather than resting TM, as they would have been continuously exposed to alloantigen.
Our studies used TN and TM with a fixed TCR, allowing a focus on the repertoire-independent differences. By comparing equal numbers of cells with an identical specificity that only differ in their histories of antigen experience, we could directly test the inherent differences. In this well-controlled setting, we could, reach the clear conclusion that TEM have substantial inherent and repertoire-independent defects compared with TN. Clinically and histopathologically, TS1 TEM caused only mild and transient early disease, which was much less severe than that caused by TS1 TN, even when almost 10-fold more TEM were transferred. In this context, it is important to point out that our studies do not contradict a role for repertoire—it could also play a role if different between TN and TEM. Rather, our data complement studies investigating repertoire, such as those by Dutt et al9 and our own recent studies,45 by demonstrating that TEM and TN do differ fundamentally independent of repertoire. Thus, both polyclonal and TCR-restricted models of GVHD can make unique contributions to understanding the roles of repertoire and intrinsic defects in the reduced potential of TEM to cause GVHD.
Our TCR Tg model of GVHD differs from prior TCR Tg models in important ways. We chose to target a peptide antigen ubiquitously expressed at a low level in the host, as this recapitulates a typical miHAg targeted in a polyclonal system. Hence, there is presentation of the peptide by both recipient and donor antigen presenting cells (APCs). This is clinically realistic–given that donor APCs promote CD4-mediated GVHD by presenting hostderived peptides46-48 —and allows the testing of Tg TN, TEM, and TCM under conditions in which donor T cells can continue to encounter Ag-bearing APCs as GVHD evolves. In contrast, prior TCR Tg systems have mostly used TCR Tgs that are reactive against the host MHC and as a consequence T cell stimulation diminishes as host APCs are cleared. Perhaps for this reason, other systems required the transfer of very large numbers of donor T cells, at least 10 to 1000 fold greater than in our system.16-21,49-51 Further, our Tg T cells were on a RAG−/− background, assuring that TN and TM repertoires were not skewed by endogenous TCR rearrangements that could be selected for as the immune process progresses. Importantly, our model mirrors key features of GVHD in polyclonal systems. TS1 recipients lost weight and developed hunched posture. At T cell doses of only 3000 to 9000 per recipient, GVHD was lethal in a substantial fraction of mice. Skin, liver and bowel had histopathologic changes characteristic of typical GVHD caused by polyclonal T cells.
Whether TCM can cause GVHD has also been an area of controversy. It was previously reported that a mix of polyclonal CD4 and CD8 TCM did not induce GVHD,5 whereas we found that purified polyclonal CD8+ TCM did induce GVHD,40 though milder than that induced by TN. Here we found that TCM and TN both induced similar weight loss and histopathologic disease, though TCM were modestly but significantly less lethal. That TS1 TCM were less lethal than TS1 TN indicates that TCM also have repertoire-independent differences from TN. Separation of TCM and TN is based on the degree of CD44 expression. In a polyclonal system we could achieve this convincingly with CD8 cells. However, we were unable to cleanly separate CD4 TCM from TN based on CD44 expression, which in our hands overlapped because of the broad and continuous distribution of expression levels (B.E.A, W.D.S., and M.J.S., unpublished observations, October 2010). This difficulty was overcome in the TS1 system because we know that CD44+CD62L+ TCM were stimulated during the in vivo culture period, and became homogeneously CD25+, indicating that they were definitively Ag-experienced. Despite the clear induction of GVHD by Ag-induced TCM, it is worth noting that our results do not exclude that a subset of “natural” TCM, perhaps generated via Ag-independent means, may yet be impaired in GVHD-inducing capacity.
An advantage of our model is the ability to quantitatively track the TS1 progeny posttransplant. One of the most surprising findings from such studies was that TEM expand in the spleen and elsewhere, despite their relative inability to cause GVHD. This seems to contradict findings in polyclonal systems; however, we believe our finding is not necessarily inconsistent with observations in polyclonal GVHD models. Negrin and colleagues demonstrated reduced expansion of TEM relative to TN in the FVB→BALB/c model.6 Our group similarly recovered fewer progeny of CD4+ TEM relative to TN in the B6bm12→B6 system.3 A likely explanation to reconcile these results with those in the TS1 system is that in the polyclonal models alloreactive cells were more frequent among TN than TEM. If so, this would argue for a contribution of repertoire in addition to repertoire-independent effects in the polyclonal situation. Also of relevance, we found that TEM expansion was markedly inhibited by the presence of Ag-specific TN, potentially indicating that TEM can be affected by surrounding T cells, which could have impacted results in polyclonal settings.
Another difference between the TS1 and polyclonal models is that polyclonal TEM likely included cells that arose via homeostatic expansion rather than Ag-specific activation.52 In the TCR Tg system, all TEM are Ag-experienced; such T cells might represent a particular subset among the polyclonal TEM phenotype cells with more potent ability to expand on encountering alloantigen in a GVHD setting. Nonetheless, though TCR Tg T cells do indeed expand, albeit to a limited extent in colon, they do not cause sustained clinical disease, in agreement with polyclonal models.
An important question is why TEM are less capable of causing GVHD. Among the most interesting observations was that despite robust expansion in spleen, far fewer progeny of TEM accumulated in colon than did progeny of TN or TCM, commensurate with reduced pathology induced there by TEM. One possible explanation for this would be that fewer TEM progeny entered the colon. This is possible, as expression of α4β7, required for optimal trafficking into bowel,53 was greater in a subset of progeny of TS1 TN versus TEM (supplemental Figure 6), although it did not differ in cells already resident in colon. However, migration differences are unlikely to fully explain different numbers of TN and TEM progeny in the colon, as the frequency of TS1 cells in S phase was substantially greater in TN recipients (Figure 6), indicating that TEM are relatively less proliferative in the colon. That so many TS1 are dividing in situ is in itself a significant finding and suggests initial priming in secondary lymphoid tissues is but the first step in alloreactive T-cell activation, and that further stimulation and division occurs in target tissues, even weeks posttransplant. Continued uniform expression by colonic TS1 cells of activation markers such as CD69, CD25 and GITR also supports this conclusion.
In addition to cell number, at time points when cell numbers and disease scores were diverging between recipients of TN and TEM, TEM were less able to generate cytokines, chiefly IFN-γ. Both cell frequency and the intensity of intracellular staining were lower in progeny of TEM than TN. Though why TEM progeny are less proliferative and functional remains to be determined, an important observation is that they up-regulate PD-1 expression to a greater extent. PD-1 is well-documented, particularly in CD8 T cells54 but also in CD4 T cells,55 to mediate exhaustion and reduced functional capabilities. It also limits the capacity of naive polyclonal T cells to cause GVHD in MHC-mismatched GVHD models.56 Thus, while more work is required to understand qualitative differences between TN and TEM progeny and how this relates to pathogenesis, our findings are important mechanistic clues, particularly in conjunction with the observed differences in cell numbers.
TS1 TEM showed an even more pronounced impairment in cell accumulation when in competition with TS1 TN. In the spleen, competition with TN reduced the total number of TS1 TEM whereas in isolation TN and TEM expanded to almost equal extents. This observation could indicate additional and different impairments in TEM that only operate in the context of more “fit” competitors. It is possible that TN actively inhibit division or promote the death of TEM. In any case, it is interesting that TN were more successful than TEM in competing for limiting factors or niches that promote division and survival.
Overall, our studies have provided a key insight into why TEM fail to cause GVHD. While TEM may have a different repertoire than TN, by controlling for this we found that TEM have cell-intrinsic defects that include reduced ability to proliferate in target tissues as well as an inability to cause sustained tissue damage. Critically, we found that TEM can indeed engraft, be activated and significantly expand in secondary lymphoid tissue; this in turn could explain why TEM can carry out some beneficial effector functions, such as GVL and recall responses to immunogens.2,3 The surprising capacity of TEM to expand bodes well for their use to provide GVL and immunity without clinically significant GVHD. Future work will focus on the nature of the cell intrinsic defects that restrict both proliferation as well as presumably effector function. The system we describe in this report should be useful for such studies, which should shed light on the basic biology of TEM as well as provide concepts that could guide the prevention and therapy of GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yale Animal Resources Center technicians for excellent animal care.
This work was supported by National Institutes of Health grants R01-HL066279 (M.J.S. and W.D.S.), P01-AI064343 (W.D.S. and A.J.D.), R01-AI24541 (A.J.C.), and T32-HL007974 (B.E.A.). W.D.S. is a recipient of a Clinical Scholar award from the Leukemia & Lymphoma Society.
National Institutes of Health
Authorship
Contribution: K.W.J. designed and performed experiments, analyzed data, and wrote the paper; B.E.A. designed and performed experiments and analyzed data; C.Z. performed experiments; histopathology slides were scored by J.M.M. (skin) and A.J.D. (colon and liver); A.J.C. created the HA104 mice; D.L.F. developed the methods to create TS1 memory cells, provided technical advice and edited the manuscript; and W.D.S. and M.J.S. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark J. Shlomchik, Yale University School of Medicine, 333 Cedar St, PO Box 208035, New Haven, CT 06520-8035; e-mail: mark.shlomchik@yale.edu.
References
Author notes
W.D.S. and M.J.S. contributed equally to this article.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal