Abstract
Histocompatibility testing for stem cell and solid organ transplantation has become increasingly complex as newly discovered HLA alleles are described. HLA typing assignments reported by laboratories are used by physicians and donor registries for matching donors and recipients. To communicate effectively, a common language for histocompatibility terms should be established. In early 2010, representatives from Clinical, Registry, and Histocompatibility organizations joined together as the Harmonization of Histocompatibility Typing Terms Working Group to define a consensual language for laboratories, physicians, and registries to communicate histocompatibility typing information. The Working Group defined terms for HLA typing resolution, HLA matching, and a format for reporting HLA assignments. In addition, definitions of verification typing and extended typing were addressed. The original draft of the Definitions of Histocompatibility Typing Terms was disseminated to colleagues from each organization to gain feedback and create a collaborative document. Commentary gathered during this 90-day review period were discussed and implemented for preparation of this report. Histocompatibility testing continues to evolve; thus, the definitions agreed on today probably will require refinement and perhaps additional terminology in the future.
Introduction
The definitions herein are intended as general concepts. There will be exceptions to these general definitions. These definitions do not imply any specific requirements for HLA typing but are only meant to define useful terms.
Definitions of typing resolution
HLA laboratories should have a written agreement with each entity requesting HLA typing for transplantation regarding the specifications for the resolution of typing. The following terms might be used in the agreement. These definitions do not imply any specific requirements for typing; that decision is made by the HLA typing laboratory in compliance with testing standards and requirements of the test requesting entity.
Allelic resolution
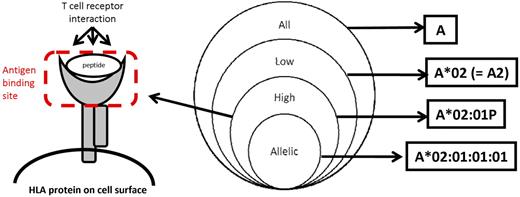
The DNA-based typing result is consistent with a single allele as defined in a given version of the WHO HLA Nomenclature Report as described on the reference Web site (http://www.hla.alleles.org).1 An allele is defined as a unique nucleotide sequence for a gene as defined by the use of all of the digits in a current allele name. Examples include A*01:01:01:01 and A*02:07 (designations based on the IMGT/HLA database Version 3.1.0, July 20102 ; Figures 1 and 2; Table 1).
HLA typing resolution. The Venn diagram illustrates increasing levels of HLA typing resolution. The figure on the left shows the antigen binding site of an HLA class I molecule. High-resolution HLA typing defines the specific DNA sequence of the antigen binding site. Allelic resolution defines a single allele as defined by a unique DNA sequence for the HLA gene; in certain instances, the allele name may include synonymous DNA substitutions within the coding region, differences in the noncoding region, and changes in expression. An example of allelic resolution is A*01:01:01:01 for which synonymous DNA substitutions and differences in the noncoding region have been defined. A*02:07 is also an example of allelic resolution; this allele has not been found to have synonymous DNA substitutions or differences in the noncoding region to date.
HLA typing resolution. The Venn diagram illustrates increasing levels of HLA typing resolution. The figure on the left shows the antigen binding site of an HLA class I molecule. High-resolution HLA typing defines the specific DNA sequence of the antigen binding site. Allelic resolution defines a single allele as defined by a unique DNA sequence for the HLA gene; in certain instances, the allele name may include synonymous DNA substitutions within the coding region, differences in the noncoding region, and changes in expression. An example of allelic resolution is A*01:01:01:01 for which synonymous DNA substitutions and differences in the noncoding region have been defined. A*02:07 is also an example of allelic resolution; this allele has not been found to have synonymous DNA substitutions or differences in the noncoding region to date.
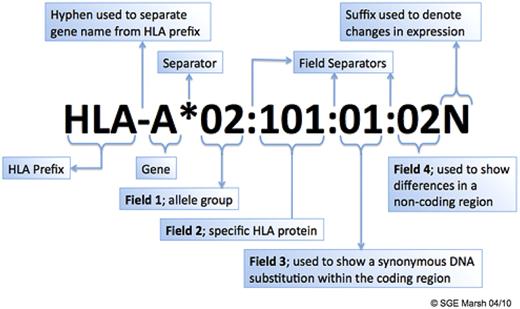
Convention for HLA allele naming. The figure illustrates the meaning of each component of an HLA allele name. Fields 3 and 4 may not be used in a name if no synonymous DNA substitutions or differences in a noncoding region are found for a particular allele. For example, based on the IMGT/HLA database Version 3.1.0, July 2010,2 A*02:07 has not been found to have synonymous DNA substitutions or differences in the noncoding region; thus, fields 3 and 4 have not been assigned for this allele. Courtesy of Prof Steven Marsh, Anthony Nolan Research Institute, London, United Kingdom (www.hla.alleles.org).
Convention for HLA allele naming. The figure illustrates the meaning of each component of an HLA allele name. Fields 3 and 4 may not be used in a name if no synonymous DNA substitutions or differences in a noncoding region are found for a particular allele. For example, based on the IMGT/HLA database Version 3.1.0, July 2010,2 A*02:07 has not been found to have synonymous DNA substitutions or differences in the noncoding region; thus, fields 3 and 4 have not been assigned for this allele. Courtesy of Prof Steven Marsh, Anthony Nolan Research Institute, London, United Kingdom (www.hla.alleles.org).
Nomenclature of HLA alleles
| Nomenclature . | Indication . |
|---|---|
| HLA | The HLA region and prefix for an HLA gene |
| HLA-DRB1 | A particular HLA locus, ie, DRB1 |
| HLA-DRB1*13 | A group of alleles that encode the DR13 antigen or sequence homology to other DRB1*13 alleles |
| HLA-DRB1*13:01 | A specific HLA allele |
| HLA-DRB1*13:01:02 | An allele that differs by a synonymous mutation from DRB1*13:01:01 |
| HLA-DRB1*13:01:01:02 | An allele that contains a mutation outside the coding region from DRB1*13:01:01:01 |
| HLA-A*24:09N | A “null” allele, an allele that is not expressed |
| HLA-A*30:14L | An allele encoding a protein with significantly reduced or “Low” cell surface expression |
| HLA-A*24:02:01:02L | An allele encoding a protein with significantly reduced or “Low” cell surface expression, where the mutation is found outside the coding region |
| HLA-B*44:02:01:02S | An allele encoding a protein that is expressed as a “Secreted” molecule only |
| HLA-A*32:11Q | An allele that has a mutation that has previously been shown to have a significant effect on cell surface expression, but where this has not been confirmed and its expression remains “Questionable” |
| Nomenclature . | Indication . |
|---|---|
| HLA | The HLA region and prefix for an HLA gene |
| HLA-DRB1 | A particular HLA locus, ie, DRB1 |
| HLA-DRB1*13 | A group of alleles that encode the DR13 antigen or sequence homology to other DRB1*13 alleles |
| HLA-DRB1*13:01 | A specific HLA allele |
| HLA-DRB1*13:01:02 | An allele that differs by a synonymous mutation from DRB1*13:01:01 |
| HLA-DRB1*13:01:01:02 | An allele that contains a mutation outside the coding region from DRB1*13:01:01:01 |
| HLA-A*24:09N | A “null” allele, an allele that is not expressed |
| HLA-A*30:14L | An allele encoding a protein with significantly reduced or “Low” cell surface expression |
| HLA-A*24:02:01:02L | An allele encoding a protein with significantly reduced or “Low” cell surface expression, where the mutation is found outside the coding region |
| HLA-B*44:02:01:02S | An allele encoding a protein that is expressed as a “Secreted” molecule only |
| HLA-A*32:11Q | An allele that has a mutation that has previously been shown to have a significant effect on cell surface expression, but where this has not been confirmed and its expression remains “Questionable” |
The table was adapted from one kindly provided by Prof Steven Marsh, Anthony Nolan Research Institute, London, United Kingdom (www.hla.alleles.org).
High resolution
A high-resolution typing result is defined as a set of alleles that encode the same protein sequence for the region of the HLA molecule called the antigen binding site and that exclude alleles that are not expressed as cell-surface proteins. The antigen binding site includes domain 1 and domain 2 of the class I α polypeptides, and domain 1 of the class II α and domain 1 of the class II β polypeptide chains.
Low resolution
A DNA-based typing result at the level of the digits composing the first field in the DNA-based nomenclature. Examples include: A*01; A*02. If the resolution corresponds to a serologic equivalent, this typing result should also be called low resolution.
Other levels of resolution
If high resolution cannot be obtained or if the laboratory's agreement with the entity requesting the testing limits the typing efforts to a subset of alleles, the laboratory may report its results at a level of resolution that falls between high resolution and low resolution. Examples are to consider only those alleles expected to be found in the local population or that are designated as common and well defined.3 A third example is typing that assigns a G group designation (eg, A*02:01:01G).
Replacement of the term “confirmatory typing”
Activities encompassed within the term “confirmatory typing” have become unclear as typing practices and matching criteria have changed over time. It is recommended that the following 2 terms be used in the place of the words “confirmatory typing” for better clarity. “Verification typing” and “extended typing” as defined in the next 2 sections describe 2 distinct activities that may be performed separately or concurrently.
Verification typing
HLA typing performed on an independent sample (or, for a cord blood unit, from an attached segment or from the unit itself) with the purpose of verifying concordance of that typing assignment with the initial HLA typing assignment. Concordance does not require identical levels of resolution for the 2 sets of typing but requires the 2 assignments to be consistent with one another.
Extended typing
HLA typing performed to add additional information to an existing HLA assignment. This additional HLA typing: (1) may include assignments at additional HLA loci (eg, to type HLA-C for an HLA-A, -B, -DRB1 typed volunteer donor) and/or (2) may include increased resolution at any previously typed HLA locus (eg, to type an individual with HLA-B serologic assignments to identify the HLA-B alleles). For some cases, extended typing will fulfill requirements for verification typing at a particular locus. In these cases, a combined term “verification/extended typing” might be used.
Format for reporting HLA assignments
HLA typing assignments must be clearly understood by the end user. HLA laboratories should have a written agreement with each transplantation entity requesting HLA typing regarding the specifications for the typing. The agreement can include the loci to be tested, the level of resolution of the typing, and the format in which typing results will be reported. The typing assignments must conform with World Health Organization nomenclature for factors of the HLA system (http://www.hla.alleles.org)1 and comply with other applicable international conventions (eg, multiple allele code definitions as designated by the National Marrow Donor Program as described by Bochtler et al4 ). It might also include the transplant center's matching requirements if the end user requests that the laboratory evaluate the extent of matching.
Unresolved alternative assignments
It is strongly recommended that the report to the end user include all uncertainty in the typing assignment relevant to the level of resolution as stated in the written agreement. This means that genotypes and/or alleles that have not been excluded should be listed in the report. If it is not possible to provide a list of all unresolved alternatives on the report, the laboratory should indicate that alternative assignments exist and provide a rationale for the HLA assignment that is selected for inclusion in the report. The impact of any uncertainty in HLA assignments for either the potential donor or patient on matching should be addressed in the report.
Database used in interpretation of typing results
The version of the IMGT/HLA database used to interpret the HLA results should be available to the end user on request.
Reporting a string of alleles
Slashes should be used to separate a string of alternative alleles (eg, A*02:01/02:02/02:07/02:20 to mean A*02:01 or A*02:02 or A*02:07 or A*02:20). Based on resolution requirements, allele names might be truncated from the right; for example, A*02:01 is understood to include all silent substitutions, differences outside the coding region, and expression codes that begin with the digits 02:01. Left truncation leads to confusion of the allele family and allele number and thus should not be used.
Matching
When providing HLA assignments for a potential donor and patient for allogeneic transplantation, the laboratory should include a description of the matching status of the pair as defined by local, national, or international standards.
Directionality of match
The laboratory may wish to refer to directionality (or vector) of the mismatch in cases where the patient or a potential donor is homozygous at a locus or has a nonexpressed allele. If the patient is homozygous and 1 of the HLA assignments is identical to an assignment of the heterozygous donor (eg, patient A*01:01:01:01, potential donor A*01:01:01:01, A*23:01), the mismatch may be referred to as a mismatch in the host-versus-graft vector direction. If the potential donor is homozygous and 1 of the HLA assignments is identical to an assignment of the heterozygous patient, the mismatch may be referred to as a mismatch in the graft-versus-host vector direction.
Matching within a family
HLA haplotypes identical by descent.
This phrase may be used when (1) parental HLA assignments are available, (2) all 4 haplotypes are unequivocally defined in the family, (3) the HLA assignments of the parents are clearly distinguishable from one another, and (4) the assignments include HLA class I and class II loci to the extent that potential recombinations have been ruled out. The patient and potential donor who share both haplotypes may be described as HLA identical by descent.
HLA identical for all loci tested.
This phrase may be used to refer to matching of related donors who appear to share the HLA loci tested with the patient based on segregation within the family. This phrase would refer to matching in which not all HLA loci are tested (eg, HLA-A, -B, -C, DRB1 typed but not DQB1 or DPB1), so the possibility of recombination is not excluded.
Families where segregation to confirm identity by descent is not possible.
When it is not possible to unequivocally define haplotypes, the phrases used to describe matching should be those used for an unrelated donor as described in the next section.
Matching of patient to unrelated donor or matching within a family where identity by descent cannot be ascertained
Matched for {insert} at {insert} resolution.
A phrase like this might be used to refer to the number of loci tested (ie, 2 assignments at 3 loci yielding 6 assignments to include HLA-A, -B, -DRB1 or 4 loci yielding 8 assignments with the addition of HLA-C or 10 assignments with the addition of HLA-DQB1 or 12 assignments with DPB1), the potential identity of the assignments (eg, 8/8, 7/8, or 9/10), and the level of resolution used to determine the potential identity (eg, high resolution or low resolution). The number of loci to be included in this grading of match should be agreed locally with the service users.
For example, an umbilical cord blood unit may be described as “matched for 6/6 at low resolution for HLA-A and HLA-B and at allelic level resolution for HLA-DRB1” with the patient. For example, an adult volunteer donor may be described as “matched for 9/10 at high resolution” with the patient.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article has been published jointly by invitation and consent in both Blood and Human Immunology. All rights reserved. No part of this document may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage and retrieval system, without written permission by The American Society of Hematology and The American Society for Histocompatibility and Immunogenetics.
Acknowledgments
These definitions have been reviewed by representatives from the following organizations: American Association of Blood Banks, American Society for Histocompatibility and Immunogenetics, College of American Pathologists, European Federation for Immunogenetics, Foundation for the Accreditation of Cellular Therapy, National Marrow Donor Program, World Marrow Donor Association, and American Society for Blood and Marrow Transplantation. The extent to which each organization is able to incorporate the definitions herein can be found on the Web site of each organization.
Information on the current status of this document can be found at http://www.hla.alleles.org.
Wellcome Trust
Authorship
Contribution: All authors were members of the Harmonization of Histocompatibility Typing Terms Working Group and as such participated in discussions, writing, and editing the definitions of histocompatibility typing terms.
Conflict-of-interest disclosure: C.T. is employed by Laboratory Corporation of America Holdings, a commercial company that performs HLA typing, and is Co-Chair of the American Society for Histocompatibility and Immunogenetics Quality and Standards Committee. H.N. is a Consultant for National Marrow Donor Program. The remaining authors declare no competing financial interests.
Correspondence: Dawn R. Wagenknecht, HLA-Vascular Biology Laboratory, Franciscan St Francis Health, 1600 Albany St, Beech Grove, IN 46107; e-mail: dawn.wagenknecht@franciscanalliance.org.