Abstract
We generated MHC-independent chimeric antigen receptors (CARs) directed to the GD2 antigen expressed by neuroblastoma tumor cells and treated patients with this disease. Two distinguishable forms of this CAR were expressed in EBV-specific cytotoxic T lymphocytes (EBV-CTLs) and activated T cells (ATCs). We have previously shown that EBV-CTLs expressing GD2-CARs (CAR-CTLs) circulated at higher levels than GD2-CAR ATCs (CAR-ATCs) early after infusion, but by 6 weeks, both subsets became low or undetectable. We now report the long-term clinical and immunologic consequences of infusions in 19 patients with high-risk neuroblastoma: 8 in remission at infusion and 11 with active disease. Three of 11 patients with active disease achieved complete remission, and persistence of either CAR-ATCs or CAR-CTLs beyond 6 weeks was associated with superior clinical outcome. We observed persistence for up to 192 weeks for CAR-ATCs and 96 weeks for CAR-CTLs, and duration of persistence was highly concordant with the percentage of CD4+ cells and central memory cells (CD45RO+CD62L+) in the infused product. In conclusion, GD2-CAR T cells can induce complete tumor responses in patients with active neuroblastoma; these CAR T cells may have extended, low-level persistence in patients, and such persistence was associated with longer survival. This study is registered at www.clinialtrials.gov as #NCT00085930.
Introduction
Adoptively transferred T cells can recognize tumor-associated antigens presented in association with MHC molecules on the cell surface. However, many cancer cells and solid tumors have defects in antigen processing and presentation,1,2 including down-regulation of and/or failure to express MHC molecules.3,4 Introducing tumor-specific chimeric antigen receptors (CARs) into adoptively transferred T cells allows them to recognize tumor-associated antigens in an MHC-independent manner while retaining their cytotoxic activity. Until recently, however, CAR-T cells had shown little evidence of antitumor activity against solid tumors and had only brief persistence in vivo.5-7 It has been believed that engagement of the chimeric receptor by tumor antigens failed to provide the requisite costimulatory signals necessary for optimal expansion, function, and persistence because tumor cells, unlike professional antigen-presenting cells, lack costimulatory ligands and may express inhibitory ligands. Therefore, efforts have been made to incorporate the signaling endodomains of costimulatory molecules, such as CD28, OX40, and 41BB into CARs.8-12
We and others have described an alternative approach in which CARs are expressed on cytotoxic T lymphocytes (CTLs) specific for endogenous viral antigens.13-15 In principle, these CAR-CTLs should receive superior costimulation in vivo, provided they engage, on professional antigen-presenting cells, the antigen for which their native receptors are specific: unlike tumor cells, professional antigen-presenting cells express a multiplicity of costimulatory ligands. We have previously reported in 11 pediatric patients with neuroblastoma the short-term fate and antitumor activity of T cells genetically modified with a first-generation CAR designed to recognize GD2.16 The trial was designed as a phase 1, dose-escalation, safety study in which we could directly compare the in vivo proliferation and short-term persistence of autologous activated T cells (ATCs) and autologous Epstein Barr-virus specific T cells (EBV-CTLs), each modified with a distinguishable GD2-specific CAR (GD2-CAR). We found that CAR expressing EBV-CTLs (CAR-CTLs) survived in the circulation at an initially higher level over the 6-week study period than GD2-CAR expressing activated T cells (CAR-ATCs), a difference we attributed to superior costimulation for CAR-CTLs provided when the cells engaged EBV antigens on professional antigen-presenting cells through their native receptors. Notwithstanding this initial difference, by the end of the 6-week period, both CAR-CTLs and CAR-ATCs had either become undetectable or were present at extremely low levels in peripheral blood samples.16
We now report our analysis of the long-term fate of these low-level persisting CAR-CTLs and CAR-ATCs, in a total of 19 subjects with high-risk neuroblastoma, including the original 11 patients who have been followed for up to 4 years. We have tracked the low-level persistence of CAR-CTLs or CAR-ATCs beyond the initial 6-week study period previously reported, identified the characteristics of the infused lines that determine whether such persistence occurs, and assessed the association of long-term persistence with disease outcome. Our results show that CAR-CTLs and CAR-ATCs may persist at low levels for as long as 4 years, with no evident difference in the long-term (> 6 weeks) detection levels of either cell type. The duration of persistence of either CAR-CTLs or CAR-ATCs is highly concordant with the proportion of helper (CD4+) and central memory (CD45RO+CD62L+) cells within the infused product. Three of 11 subjects with active disease had a complete response to CAR–T-cell infusion, and persistence of either CAR-CTLs or CAR-ATCs in the circulation for 6 weeks or more was associated with superior time to progression in subjects with active disease.
Methods
Study eligibility
This protocol was performed after review and approval by the Baylor College of Medicine Institutional Review Board, the Recombinant DNA Advisory Committee, and the United States Food and Drug Administration. This study is registered at www.clinialtrials.gov as #NCT00085930. Subjects with a history of high-risk disease, relapsed/refractory disease, and/or patients who were unable to receive or complete standard up-front therapy for neuroblastoma were eligible to participate. All patients were EBV-seropositive, had recovered from all previous therapy, and had no evidence of severe intercurrent infection. Informed consent and appropriate assent for participation were obtained from each subject or subject's guardian before both procurement of patient blood for CAR-T-cell generation and T-cell infusion in accordance with the Declaration of Helsinki.
The initial results from the first 11 patients treated on this study have been previously published.16 The current report describes the longer-term clinical and CAR–T-cell analyses from these patients and from 8 additional subjects treated: 1 at dose level 3 and 7 at dose level 1. Briefly, the study was designed as a phase 1 clinical trial to evaluate safety and the maximum tolerated dose of CAR–T cells in patients with neuroblastoma. Patients were treated on all 3 dose levels (dose level [DL] 1, 2 × 107 cells/m2; DL 2, 5 × 107 cells/m2; and DL 3, 1 × 108 cells/m2), and no dose-limiting toxicity was identified.
Generation and analysis of infused cell lines
Generation of autologous lymphoblastoid cell lines, EBV-CTLs, and ATCs was performed per current Good Manufacturing Practice guidelines as previously published.16,17 Retroviral transduction to generate CAR-CTLs and CAR-ATCs occurred according to our standard operating procedures.16 All cell products were tested for sterility, immunophenotype, identity, and cytotoxic specificity for EBV and GD2 before release.
Frozen aliquots of CAR-CTLs and CAR-ATCs were used to assess the memory cell population within each infused product. Cells were stained with the monoclonal antibodies to CD45RO, CD45RA, CD3, CD4, CD8, CD62L, and CCR7 (BD Biosciences). A FACSCalibur instrument and CellQuest Version 5.2.1 software (both from BD Biosciences) were used for all flow cytometric analyses. Naive cells were defined as CD45RA+CD3+; CD4+ and CD8+ effector memory and central memory cells were defined as CD45RO+CCR7−CD62L− and CD45RO+CD62L+, respectively.
Follow-up testing
PBMC samples were obtained at predefined time points to determine persistence of the infused cellular products and to look for evidence of replication competent retroviruses. Extracted DNA from PBMCs was sent to the Viral Production Laboratory at Indiana University for replication competent retrovirus testing as per our standard operating procedures. Real-time quantitative PCR was used to assess the presence and persistence of CAR–T cells as previously published.16 More than 85% of the quantitative PCR assays were performed in triplicate (limited only by clinical sample availability), and all samples (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), were run with a negative control represented by nontransduced PBMCs. Detection levels are reported as the percentage of positive cells based on a standard curve generated from a single-cell clone from a tumor cell line transduced with each transgene construct. The sensitivity of the assay is comparable with the detection of 1 transduced cell diluted in 1000 to 6000 nontransduced cells and therefore corresponds to 0.0001% to 0.002% of the tumor cell line.
Clinical assessment
Disease burden before T-cell infusion was assessed by CT or MRI of the primary site of disease, meta-iodobenzylguanidine, and/or bone scan to evaluate for metastatic sites, and bone marrow aspirate and biopsy to evaluate disease in those patients with known or suspected bone marrow positivity. Preinfusion images were repeated 6 weeks later to monitor response. Further imaging was obtained per standard of care directed by the patient's primary oncologist. Response was determined using the International Recommendations for Neuroblastoma Response Criteria.18 Best response was determined by continued comparison of images with pretreatment studies.
Statistical analysis
GD2 T-cell lines that were never detected in the peripheral blood of patients were not included in the statistical analysis specific to CAR–T-cell persistence (5 ATC lines: 4 from DL 1 and 1 from DL2; 5 CTL lines: all from DL1). Descriptive statistics (mean and SDs, or median and ranges) were used to summarize the data. As the data on CAR- ATC and CAR-CTL are correlated within subjects, the relationships between the duration of detection (log-transformed) and the number of CD4+ cells, as well as the number of central memory cells, respectively, were analyzed by linear regression models using the generalized estimating equations method. Overall survival (OS) was estimated by the Kaplan-Meyer method. Time to progression (TTP) was analyzed by the cumulative incidence function using Gray method.19 TTP was defined as the time from first injection to disease progression per the International Recommendations for Neuroblastoma Response Criteria for patients with active disease at the time of GD2 T-cell infusion, where death was the competing risk. A P value < .05 was considered statistically significant.
Results
Patient characteristics
In total, 19 patients have been treated with autologous CAR-CTLs and CAR-ATCs (Table 1). Ten of the 19 patients were female, and the median age at the time of infusion was 7 years (range, 3-20 years). Clinically, 8 patients had no evidence of active disease, 5 of whom had a history of relapsed disease and 3 who were infused after completing therapy for high-risk disease; 11 patients had active disease; 4 of these had limited disease (3 with solitary bone lesions and 1 with bone marrow disease); the remaining 7 had active, bulky disease at the time of infusion.
Safety data
Autologous CAR-CTLs and CAR-ATCs were generated and infused into each patient. Three patients received 2 separate infusions of GD2-specific T cells; therefore, a total of 44 products have been given over the course of this study. All infusions were negative for replication competent retroviruses. No severe or dose-limiting toxicities have been identified. Three patients had grade 1 to 3 localized pain as determined by the National Cancer Institute Common Toxicity Criteria Version 2.0, approximately 2 weeks after cell infusion: 2 at a site of biopsy-proven tumor necrosis16 and 1 in her lower leg at a site with no evidence of active disease.
Analysis of GD2-specific T-cell lines
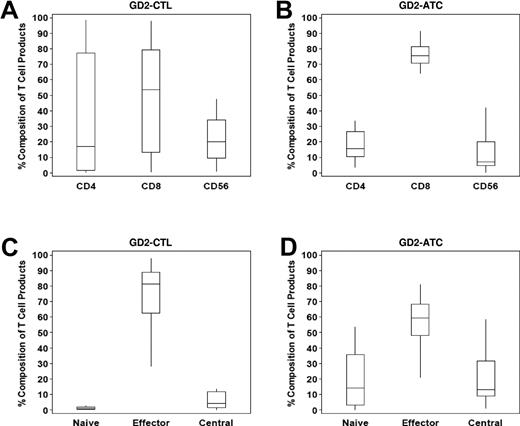
Both GD2-specific ATC lines and EBV-CTL lines were polyclonal, with median CD4::CD8 percentages of 15.8::75.6 and 16.9::53.4 for ATC and CTL lines, respectively. A small number of CD3−CD56+ NK cells were also identified within each product (Figure 1A-B). More than 80% of the CTL population were effector memory cells (CD45RO+CCR7−CD62L−), whereas ATCs were composed of 59.4% effector memory, 12.9% central memory (CD45RO+CD62L+), and 13.9% naive (CD45RA+CD3+) cells (Figure 1C-D). Chromium release assays for the additional lines infused confirmed that CAR-ATCs and CAR-CTLs both killed GD2+ target cells, whereas only CAR-CTLs killed EBV+ targets16 (data not shown).
Composition of GD2–T-cell products. The cellular composition of GD2-CTL (A) and GD2-ATC (B) lines was analyzed using flow cytometry. Both products were polyclonal with a predominance of CD8+ T cells, followed by a smaller percentage of CD4+ cells and NK cells within each product line. Furthermore, as expected, GD2-CTL lines were terminally differentiated and composed of mostly effector memory cells (C), whereas GD2-ATCs (D) had a higher percentage of naive and central memory cells within each T-cell line.
Composition of GD2–T-cell products. The cellular composition of GD2-CTL (A) and GD2-ATC (B) lines was analyzed using flow cytometry. Both products were polyclonal with a predominance of CD8+ T cells, followed by a smaller percentage of CD4+ cells and NK cells within each product line. Furthermore, as expected, GD2-CTL lines were terminally differentiated and composed of mostly effector memory cells (C), whereas GD2-ATCs (D) had a higher percentage of naive and central memory cells within each T-cell line.
Persistence data
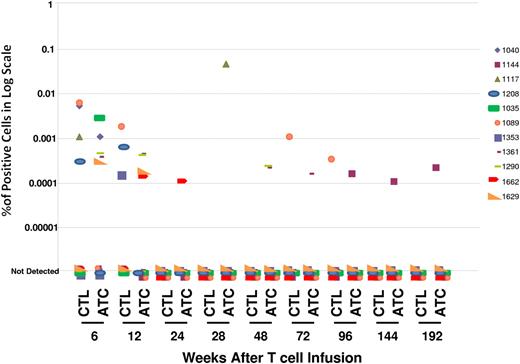
As previously reported,16 by 6 weeks after infusion, PCR signals derived from either CAR-ATC and CAR-CTL were both low or absent. Overall, PCR signals derived either from the CAR expressed by ATCs or by CTLs at or after 6 weeks was detectable in the circulation of 11 of 19 (58%) patients treated (Figure 2). Of the 11 patients, CAR-CTLs alone were present in 3, CAR-ATCs alone in 6, and both CAR-CTLs and CAR-ATCs in 2. Figure 2 also shows that, in all subjects, transgenic signal could nonetheless persist at low levels for 96 (CAR-CTLs) to 192 weeks (CAR-ATCs).
First-generation GD2-CAR T cells can be detected in the peripheral blood of patients for a prolonged period of time. Real-time quantitative PCR was used to assess the presence and persistence of CAR–T cells. GD2-ATCs or GD2-CTLs could be detected, at or after 6 weeks after infusion, in the circulation of 11 of 19 (58%) patients. Although the levels of detection were low, transgenic signals could be identified at 96 weeks for CAR-CTLs and 192 weeks for CAR-ATCs after infusion. The estimate of frequency of CAR– T cells was obtained using standard curves of DNA from tumor cell lines transduced with the retroviral vectors encoding each CAR. Integrant analysis showed between 6 and 8 proviral integration sites per cell. Sensitivity assays in which transduced T cells were diluted with nontransduced T cells showed unequivocal detection ability when 1 transduced cell was diluted in 1000 to 6000 nontransduced cells, corresponding to 0.0001% to 0.002% of the tumor cell lines used for the standard curve.
First-generation GD2-CAR T cells can be detected in the peripheral blood of patients for a prolonged period of time. Real-time quantitative PCR was used to assess the presence and persistence of CAR–T cells. GD2-ATCs or GD2-CTLs could be detected, at or after 6 weeks after infusion, in the circulation of 11 of 19 (58%) patients. Although the levels of detection were low, transgenic signals could be identified at 96 weeks for CAR-CTLs and 192 weeks for CAR-ATCs after infusion. The estimate of frequency of CAR– T cells was obtained using standard curves of DNA from tumor cell lines transduced with the retroviral vectors encoding each CAR. Integrant analysis showed between 6 and 8 proviral integration sites per cell. Sensitivity assays in which transduced T cells were diluted with nontransduced T cells showed unequivocal detection ability when 1 transduced cell was diluted in 1000 to 6000 nontransduced cells, corresponding to 0.0001% to 0.002% of the tumor cell lines used for the standard curve.
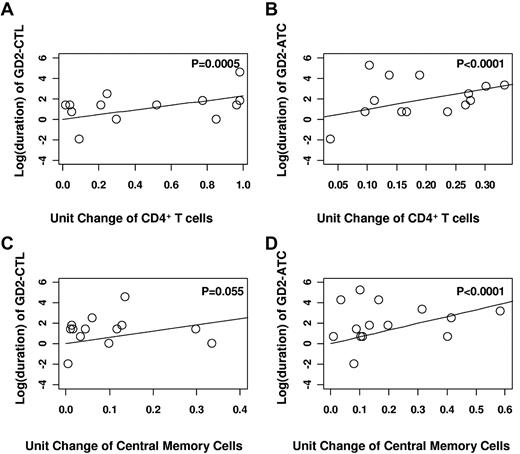
Phenotypic analysis of T-cell lines before infusion allowed us to assess which characteristics were associated with subsequent longer-term in vivo persistence. Regression analysis showed a highly statistically significant association between prolonged detection and the number of CD4+ cells and central memory cells (Figure 3). Each 1-unit increase in the number of CD4+ cells within the infused product was associated with a 2.28 increase in the log (duration) of CAR-CTL and a 9.83 increase in the log (duration) of CAR-ATC (P = .0005 and P < .0001, respectively; Figure 3A-B). In addition, each 1-unit increase in the percentage of central memory cells was associated with a 6.1 increase in the log (duration) of CAR-CTL and a 6.63 increase in the log (duration) of CAR-ATC (P = .055 for CAR-CTLs and P < .0001 for CAR-ATCs) in the T-cell product (Figure 3C-D).
Prolonged detection of GD2–T cells within the peripheral blood of patients was highly concordant with the percentage of helper (CD4+ cells) and central memory cells (CD45RO+CD62L+) in the infused product. Using regression analysis, it was determined that each 1-unit increase in the number of CD4+ cells within the infused product was associated with a 2.28 increase in the log (duration) of CAR-CTL (A) and a 9.83 increase in the log (duration) of CAR-ATC (B). Further, each 1-unit increase in the percentage of central memory cells was associated with a 6.1 increase in the log (duration) of CAR-CTL (C) and a 6.63 increase in the log (duration) of CAR-ATC (D) in the T-cell product.
Prolonged detection of GD2–T cells within the peripheral blood of patients was highly concordant with the percentage of helper (CD4+ cells) and central memory cells (CD45RO+CD62L+) in the infused product. Using regression analysis, it was determined that each 1-unit increase in the number of CD4+ cells within the infused product was associated with a 2.28 increase in the log (duration) of CAR-CTL (A) and a 9.83 increase in the log (duration) of CAR-ATC (B). Further, each 1-unit increase in the percentage of central memory cells was associated with a 6.1 increase in the log (duration) of CAR-CTL (C) and a 6.63 increase in the log (duration) of CAR-ATC (D) in the T-cell product.
Clinical response
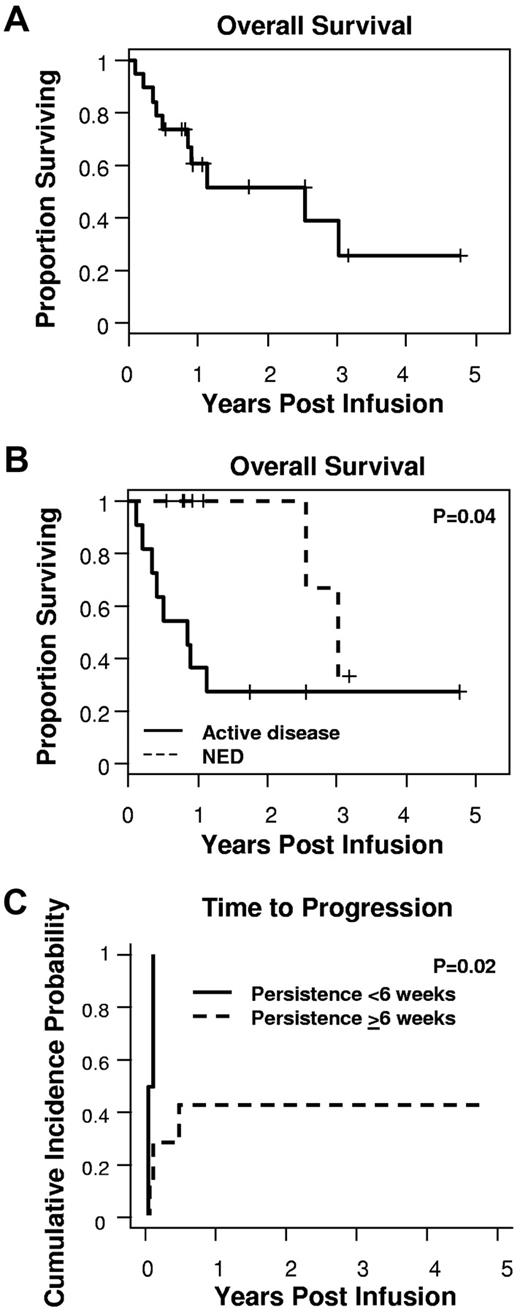
With a median follow-up of 329 days (range, 39-1740 days), the median OS was 931 days (Figure 4A). As expected, patients with no evidence of active disease at the time of CAR–T-cell infusion had a longer OS than those with active disease (P = .04, log-rank test; Figure 4B). Strikingly, however, the persistence of even low levels of either CAR-ATCs or CAR-CTLs at or beyond 6 weeks was associated with a significantly longer TTP (P = .02; Figure 4C) in patients with active disease. This difference was not attributable to a shorter overall time of follow-up for the “persistent” versus “nonpersistent” groups (median follow-up for the “persistent” group, 329 days [range, 39-1740 days] vs 219 days [range, 76-413 days] for the “nonpersistent” group), representing instead a true difference in progression-free survival. Of the 11 patients with active disease at the time of GD2 T-cell infusion, 3 patients achieved complete response. One subject had bone marrow disease that cleared within 6 weeks, but subsequently relapsed16 ; 2, however, have achieved sustained complete response for > 60 months and > 21 months after infusion. In all 3 of these subjects, CAR-ATCs or CAR-CTLs persisted > 6 weeks.
Detection of CAR–T cells for 6 weeks or more was associated with prolongation of TTP. (A) With a median follow-up of 329 days, the median OS was 931 days. (B) Patients with active disease at the time of infusion had a shorter OS compared with those with no evidence of disease. (C) In addition, for those with active disease at the time of infusion, the lack of CAR-ATCs or CAR-CTLs at or beyond 6 weeks was associated with a significantly shorter TTP.
Detection of CAR–T cells for 6 weeks or more was associated with prolongation of TTP. (A) With a median follow-up of 329 days, the median OS was 931 days. (B) Patients with active disease at the time of infusion had a shorter OS compared with those with no evidence of disease. (C) In addition, for those with active disease at the time of infusion, the lack of CAR-ATCs or CAR-CTLs at or beyond 6 weeks was associated with a significantly shorter TTP.
Discussion
Genetic modification of T cells with CARs specific for tumor-associated antigens is an attractive option for cancer immunotherapy because it allows MHC-independent antigen recognition that nonetheless retains the normal T-cell cytotoxic effector mechanisms. We have previously studied the effects of T cells expressing a CAR specific for the tumor-associated antigen GD2 in children with neuroblastoma. We compared the short-term (< 6 weeks) levels and persistence of CAR-CTLs and CAR-ATCs each expressing a GD2-CAR that was distinguishable by a separate specific sequence tag. As previously reported,16 CAR-CTLs initially circulated at higher levels than CAR-ATCs, but by 6 weeks both T-cell types were low or undetectable. In the current study, we report prolonged follow-up of a larger study cohort and show that both CAR-CTLs and CAR-ATCs can persist long-term, that persistence correlates with the phenotype of the cells infused, and that the continued presence of even low levels of CAR–T cells is associated with superior TTP and sustained complete response, even in patients with active relapsed or resistant disease at the time of infusion.
GD2-specific monoclonal antibodies have been effective agents for the control of neuroblastoma, although some patients do not respond at all and others have disease progression during and/or after treatment.20-30 We reasoned that targeting GD2 with a CAR on effector T cells might have several advantages over current soluble GD2 antibodies. First, the multivalent display of CAR molecules on a T-cell surface could produce a targeting agent with higher overall avidity than an antibody in soluble bivalent form, leading to increased binding of malignant cells whose antigen expression was too low to elicit soluble antibody binding.31,32 Second, the additional cytotoxic effector mechanisms used by T cells might kill tumor cells more readily than the mechanisms recruited by antibody alone. Finally, the potential of CAR–T cells to persist in the circulation and potentially at the sites of tumor, for longer than antibody might lead to superior long-term control of resurgent malignancy.33 These potential advantages, however, carry reciprocal concerns, such as the risk of off-target adverse effects because of low affinity but high avidity cross-reactivity with potential damage to normal tissues expressing low levels of GD2 antigen, and the possible persistence of these unwanted effects for a prolonged period of time. Fortunately, the current study shows that neither of these potential concerns is indeed realized. Thus, after infusion of > 40 products in 19 patients followed for up to 5 years, the only treatment-related adverse events we observed were low-grade fever and mild to moderate local pain at the site of tumor necrosis in 2 subjects and unexplained local pain in 1 subject. In particular, none of the recipients developed the neurologic pains or dysfunction that have been associated with GD2-monoclonal antibody infusion and attributed to activation of the complement cascade by the Fc component of the monoclonal antibody, a component lacking in our CAR.34
Our previous study showed that, for the first several weeks after infusion, CAR-CTLs have an initial numeric advantage in the peripheral blood compared with CAR-ATCs. By 6 weeks after infusion, however, this difference was no longer discernible. It is now evident from our longer-term follow-up that there is not an inevitable continuing decay in CAR signal from either cell population. Instead, low levels of each cell type may persist for up to 96 weeks for CAR-CTLs and 192 weeks for CAR-ATCs. There is an extremely strong correlation between the proportion of CD4+ helper cells and CD45RO+CD62L+ central memory cells present in infused CAR-CTLs and CAR-ATCs and the subsequent duration of signal detection in patient peripheral blood samples.
The correlation noted above is consistent with preclinical studies in rodents and monkeys, which show that the long-term survival of adoptively transferred T cells requires both a helper population and an effector memory subset,35 and that selection of these populations improves T-cell survival and persistence in vivo. Similarly, adoptive transfer of tumor infiltrating lymphocytes to human subjects has revealed that persistence is greater in those patients who receive tumor infiltrating lymphocytes containing CD4+ cells compared with CD8+ T-cell clones.36 Our current data show that the association of persistence with the presence of CD4+ and CD45RO+CD62L+ T-cell subsets can be extended to human subjects receiving genetically retargeted activated T cells and EBV-CTLs.
Importantly, our data also suggest that long-term persistence, even at low levels of CAR–T cells, is associated with an improved outcome and longer TTP. This apparent benefit on tumor recurrence/progression is perhaps surprising, given the low level of effector cells present in the peripheral blood. It is possible, however, that this circulating compartment does not accurately reflect the presence of effector cells at sites of metastasis or in draining lymph nodes, or the dynamic state of effector T-cell production and their tumor trafficking. The alternative explanation is that the apparent association between CAR–T-cell persistence and longer TTP does not reflect a genuinely sustained therapeutic benefit from the CAR–T cells but is instead a consequence of a superior “immune environment” in those persons whose malignancy is intrinsically less aggressive. Given our demonstration that the cell subset composition of the infused cell line strongly influences subsequent CAR–T-cell persistence, it should be possible to prospectively distinguish between a causal and a consequential effect of CAR–T-cell persistence by enriching CAR–T-cell lines for CD4+ and/or CD62L+ populations.37,38 Because we observed an association between CAR–T-cell persistence and the phenotype of the infused line, such enrichment should lead to a higher percentage of patients whose CAR–T cells can be detected for at least 6 weeks and who would therefore be anticipated to have a prolonged TTP, if the link is indeed causal and not merely associative.
In conclusion, our long-term follow-up of 19 patients who received GD2-specifc CAR-modified T lymphocytes shows that the long-term low-level presence of CAR-expressing T cells is associated with clinical benefits, including complete remission in 3 patients, which has persisted long term in 2. Because the infusion of CAR–T cells also appears to have long-term safety, it is appropriate to implement cell selection approaches that will enhance in vivo CAR–T-cell survival and determine whether this modification can further improve their antitumor efficacy and the clinical outcome.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yu-Feng Lin for her multiyear dedication to the patients and conduct of this study as well as all of the primary oncologists, parents, and, most importantly, the patients for their participation in this study.
This work was supported by the National Institutes of Health (grant PO1 CA94237) and the General Clinical Research Centers at Baylor College of Medicine (RR00188). C.U.L. was supported by grants T32HL092332 and 5K12CA090433 from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: C.U.L., B.S., G.D., H.L., M.-F.W., C.M.R., H.E.H., and M.K.B. wrote the manuscript; C.U.L., B.S., G.D., E.Y., E.L., O.D., A.P.G., C.M.R., and H.E.H. generated autologous cell products, evaluated release criteria, and performed follow-up testing; M.P., G.D., A.P.G., C.R., Z.M., C.M.R., and M.K.B. developed the clinical grade GD2 CARs and manufactured the CTL and ATC products; and C.U.L., G.D.M., H.V.R., H.E.H., and M.K.B. evaluated patients, administered products, and assessed clinical response.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chrystal U. Louis, 1102 Bates St, Suite 1770.00, Houston, TX 77030; e-mail: culouis@txch.org.