Abstract
We studied the effects of TNF-α and Fas-induced death signaling in hematopoietic stem and progenitor cells (HSPCs) by examining their contributions to the development of bone marrow failure syndromes in Tak1-knockout mice (Tak1−/−). We found that complete inactivation of TNF-α signaling by deleting both of its receptors, 1 and 2 (Tnfr1−/−r2−/−), can prevent the death of 30% to 40% of Tak1−/− HSPCs and partially repress the bone marrow failure phenotype of Tak1−/− mice. Fas deletion can prevent the death of 5% to 10% of Tak1−/− HSPCs but fails to further improve the survival of Tak1−/−Tnfr1−/−r2−/− HSPCs, suggesting that Fas might induce death within a subset of TNF-α-sensitive HSPCs. This TNF-α/Fas-induced cell death is a type of receptor-interacting protein-1 (RIP-1)–dependent programmed necrosis called necroptosis, which can be prevented by necrostatin-1, a specific RIP-1 inhibitor. In addition, we found that the remaining Tak1−/− HSPCs died of apoptosis mediated by the caspase-8–dependent extrinsic apoptotic pathway. This apoptosis can be converted into necroptosis by the inhibition of caspase-8 and prevented by inhibiting both caspase-8 and RIP-1 activities. We concluded that HSPCs are heterogeneous populations in response to death signaling stimulation. Tak1 mediates a critical survival signal, which protects against both TNF-α/Fas-RIP-1–dependent necroptosis and TNF-α/Fas-independent apoptosis in HSPCs.
Introduction
In hematopoietic stem and progenitor cells (HSPCs), the survival and death machinery is tightly controlled by a complex interplay between intrinsic signals and stimuli from the surrounding bone marrow (BM) microenvironment, inducing a dynamically balanced network of pro-survival and anti–survival influences. Disruption of this balance can result in hematopoietic disorders, such as myeloproliferative disorders and bone marrow failure (BMF).1-5
Prosurvival signaling in HSPCs has been very well studied. Genetic studies clearly demonstrate the role of the survival machinery in regulating numbers and functions of HSPCs.1-4 Transgenic overexpression of Bcl-2, an important pro-survival gene, increases HSPC numbers and improves hematopoietic reconstitutive capacity in mice. Mice with deletions of pro-survival genes, such as Bcl-xl, Mcl-1, or survivin, develop severe BMF because of the apoptotic loss of hematopoietic cells, including HSPCs.1-4 Importantly, the expression of these pro-survival genes is stimulated by hematopoietic cytokines in HSPCs. Hematopoietic cytokines, such as SCF, IL-3, thrombopoietin, IL-6, and IL-11 stimulate the survival of HSPCs as shown by both in vitro culture and genetic studies. Via Jak/Stat, Raf/Mapk, and PI3K/Akt, multiple signaling pathways can be activated by hematopoietic cytokines in HSPCs, resulting in self-renewal, proliferation, differentiation, and survival. Among these, the PI3K/Akt pathway has been proposed to be the mediator of the major survival signal.6 On activation, PI3K inactivates proapoptotic signals, such as Bad and FoxO3 via a serine/threonine phosphorylation cascade,7,8 or stimulates the activation of pro-survival signals, such as Nf-κB,9 which in turn up-regulate the expression of pro-survival genes.
Anti–survival signaling in HSPCs is still not well understood. Previous studies suggested that proinflammatory cytokines, including TNF-α,10 IL-1β,11 IFN-α/γ, as well as FasL (Fas ligand), exercise negative regulation over hematopoiesis and thus were implicated in a number of BMF syndromes.12-15 However, recent studies suggested that these proinflammatory cytokines stimulate dual signals for HSPC functions.16-19 A negative signal provokes programmed cell death and/or differentiation by inducing the production of reactive oxygen species (ROS) and by activating cell-extrinsic apoptotic signaling, mediated by caspase-8.20,21 A positive signal, however, promotes proliferation and survival by inducing the activation of TGF-β–activated kinase-1(Tak1)–mediated signaling.22-25 Therefore, under normal homeostatic conditions, because of high levels of Tak1 activity, HSPCs are relatively resistant to proinflammatory cytokine-induced cell death.16,26 The role of apoptotic cell death induced by these factors in BMF syndromes remains somewhat ambiguous. In addition, recent studies have demonstrated that some of these proinflammatory factors also induce a receptor-interacting protein-1 (RIP-1)–dependent programmed cell death called necroptosis in other cell types. It was suggested that RIP-1 and caspase-8 exist within the same protein complex, called a ripoptosome, induced by inflammatory factors that functionally antagonize each other. Caspase-8 represses necroptosis by degrading RIP-1 and stimulates type I or type II apoptosis by directly activating caspase-3 or by promoting mitochondrial-dependent caspase-9 activation. Caspase-8–induced apoptosis can be converted into RIP-1-dependent necroptosis when caspase-8 activity is repressed.27-32 The role of RIP-1–dependent necroptosis in hematopoietic cells has not been studied.
Tak1 is a member of the Map3k family of enzymes and is a key mediator of inflammatory cytokine-induced survival signals.33 On activation, Tak1 phosphorylates downstream signaling molecules, including Ikkβ, Mkk3/6, and Mkk4/7; these in turn activate Nf-κB, p38, and c-Jun N-terminal kinase (Jnk) signaling cascades, respectively.33 As a consequence, Tak1 signaling is involved in regulating cell proliferation and survival by inducing the production of several cytokines and the expression of many survival genes (including Bcl-xl, Mcl-1, and cIAP). Gene knockout studies done in animals suggested that Tak1 mediates a major survival signal in almost all types of cells studied, including skin keratinocytes,34 lymphocytes,35 intestinal enterocytes,36 and mouse embryonic fibroblasts.37 This Tak1-mediated survival signaling plays an essential role in protecting these cells from apoptosis induced by inflammatory cytokines and other unfavorable insults. Tak1 deletion leads to excessive activation of these cells in response to inflammatory cytokine stimulation, resulting in deregulation of the inflammatory reaction and apoptotic injury in corresponding tissues. We have found that Tak1 is also essential for the survival of hepatocytes and hematopoietic cells, including HSPCs.38 Interferon-inducible Tak1-knockout mice (MxCre+Tak1fx/fx, Tak1−/− hereafter) developed severe BMF and liver failure because of the massive death of their mutant HSPCs and hepatocytes after induction of Tak1 deletion.38
To study the molecular mechanisms underlying BMF syndromes in our inducible Tak1−/− mice and also to investigate the factors responsible for inducing the death of Tak1−/− HSPCs, we generated Tak1/Tnfr1, Tak1/Tnfr2, Tak1/Tnfr1/r2, Tak1/Fas, and Tak1/Tnfr1/r2/Fas compound-mutant mice to examine whether inactivation of TNF-α signaling, Fas signaling, or the simultaneous inactivation of both of these was able to prevent the BM damage seen in Tak1−/− mice, and if so, to what degree. In addition, using in vitro assays, we also examined other potential causes of cell death in Tak1−/− HSPCs. We found that death signaling induced by TNF-α and Fas, via activation of RIP-1, accounted for only 30% to 40% of HSPC loss in Tak1−/− mice (based on CFU data). Other unknown factor(s), acting through the caspase-8–dependent extrinsic apoptotic pathway, induce the death of the remaining 60% to 70% of Tak1−/− HSPCs.
Methods
Mice
All experiments using mice were performed according to the guidelines of Loyola University Medical Center and were approved by the Loyola University Institutional Animal Care and Use Committee. Mx1cre+Tak1fx/fx mice37,38 were crossed with Fas−/− mice (B6.MRL-Faslpr/J from The Jackson Laboratory) and Tnfr1−/−r2−/− mice (B6.129S-Tnfrsf1atm1Imx Tnfrsf1btm1Imx/J from The Jackson Laboratory), respectively, to generate heterozygous compound-mutant mice. These heterozygous compound-mutant mice were further crossed to produce Mx1cre+Tak1fx/fxFas−/−, Mx1cre+Tak1fx/fxTnfr1−/−, Mx1cre+Tak1fx/fxTnfr2−/−, Mx1cre+Tak1fx/fxTnfr1−/−r2−/− mice. Mx1cre+Tak1fx/fxFas−/− and Tak1fx/fxTnfr1−/−r2−/− mice were further crossed to generate Mx1cre+Tak1fx/fxTnfr1−/−r2−/−Fas−/− mice. Genotypes of all mice were determined by PCR assay using primers listed in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). At 6 to 8 postnatal weeks, all mice, including WT controls (Cre−) and heterozygous compound-mutant mice, were injected with 5 μg/g weight polyriboinosinic acid/polyribocytidylic acid (poly I:C; GE Healthcare Life Sciences) every other day for a total of 3 injections to induce deletion of the Tak1 gene.
Mouse hematopoietic phenotype analysis
Mice were killed 7 to 8 days after the first poly I:C injection. Peripheral blood (PB), spleens, and BM were collected. PB were analyzed for WBC, platelet, and RBC counts by Hemavet 950FS (Drew Scientific). After RBC lysis, mononucleated cells (MNCs) in PB, spleens, and BM samples were counted and stained with surface markers for flow cytometric analysis as described previously.38
Histologic analysis
Femurs from different genotypes of mice were fixed in zinc formaldehyde at room temperature for 3 days. Femurs were then decalcified by 10% EDTA buffer for 2 weeks; tissues were then transferred into 70% ethanol until embedding. Embedding and cutting of sections were performed according to standard protocols from the Pathology Department, Loyola University Medical Center. Slides were then stained with H&E.
Reagents
Lipopolysaccharide (LPS), as well as ROS scavengers N-acetyl-L-cysteine and butylated hydroxyanisole, were purchased from Sigma-Aldrich. RIP-1 inhibitor necrostatin-1 (Nec-1) was purchased from Santa Cruz Biotechnology. IL-3, IL-6, and SCF were purchased from eBioscience. TNF-α, IL-1β, IFN-α, FasL, TRAIL, and IFN-γ were purchased from BD Biosciences.
HSPC isolation, retroviral infection, and in vitro culturing
High-titer retrovirus was produced by cotransfecting Phoenix cells with a retroviral vector containing the indicated genes together with packaging vectors using Calphos Mammalian Transfection Kit (Clontech). Retroviral supernatants were harvested 24 and 48 hours after transfection. Retroviral plasmids used in this study include: MSCV-Cre-GFP and MSCV-Cre-YFP vectors; MSCV-Bcl-xl-GFP, MSCV-cIAP1-GFP, and MSCV-SOD-GFP vectors, which were generated by subcloning Bcl-xl, cIAP, and SOD genes from pMIG-Bcl-xl, pcdna3.1-hciap1, and pF151-pcDNA3.1(+)SOD1WT plasmids (Addgene) into the MSCV-GFP retroviral vector; MSCV-Tak1-GFP vector, which was generated by subcloning the Tak1 gene from pCMV-HA-Tak1 plasmid39 into the MSCV-GFP vector; and MSCV-Δ-caspase-8-GFP, MSCV-Δ-caspase-9-GFP, and MSCV-Δ-FADD-GFP vectors (kindly provided by Dr Strasser of the Walter and Eliza Hall Institute of Medical Research).40
The c-kit+ HSPCs from indicated genotypes of mice were enriched by EasySep Mouse CD117 Positive Selection Kit (StemCell Technologies). HSPCs were cultured in 6-well plates in HSPC culture medium consisting of RPMI 1640 supplemented with 10% FBS, 100 ng/mL mSCF, 50 ng/mL mIL-6, and 10 ng/mL mIL-3 and were infected with high-titer retrovirus. Infected cells were either incubated in HSPC medium for analysis of changes in the percentage of GFP+ cells or were purified by FACS for CFU and apoptosis analysis as described in Figures 3, 5 and 6.
CFU assay
BM MNCs directly isolated from the indicated genotypes of mice or purified infected HSPCs were seeded into MethoCult GF M3434 medium (StemCell Technologies) and incubated at 37°C, 100% humidity, and 5% CO2 for 10 days. Numbers of colonies were counted according to the manufacturer's instructions.
Annexin V and 7-AAD staining to analyze for apoptosis
Infected c-kit+ HSPCs were purified by FACS and incubated in HSPC medium for 24 hours. Cells were then stained with allophycocyanin-conjugated annexin V followed by 7-amino-actinomycin D (7-AAD) staining in binding buffer following the manufacturer's instructions (BD Biosciences). Death of infected cells was examined for the percentages of annexin V+ and annexin V+/7AAD+ cells by flow cytometry.
Statistical analysis
All data were analyzed using the 2-tailed Student t test with GraphPad Prism Version 5.04 software to identify significant differences between groups. Values are expressed as means ± SEM. Differences were regarded as significant at P < .05.
Results
The BMF phenotype of Tak1−/− mice is partially suppressed by the inactivation of TNF-α signaling
TNF-α acts by binding to and activating its specific receptors. Two TNF-α–specific receptors, Tnfr1 (p55) and Tnfr2 (p75), have been identified.41 To investigate whether the BMF phenotype of Tak1−/− mice can be prevented by TNF-α signal inactivation, we generated Mx1Cre+Tak1fx/fxTnfr1−/−, Mx1Cre+Tak1fx/fxTnfr2−/−, and Mx1Cre+Tak1fx/fxTnfr1−/−r2−/− compound-mutant mice (Tak1−/−Tnfr1−/−, Tak1−/−Tnfr2−/−, and Tak1−/−Tnfr1−/−r2−/− hereafter, respectively). Genotypes of the mice were verified by PCR assay, as described in supplemental Figure 1. We previously found that mice with heterozygous Tak1 deletion did not display phenotypic hematopoietic changes.38 Therefore, we focused on Tak1 homozygous knockouts in our studies. Six to 8 weeks after birth, all mice, including Cre-negative Tak1fx/fx wild-type controls, were injected with poly I:C to induce Tak1 gene deletion. High efficiency Tak1 gene deletion was confirmed in hematopoietic cells from Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− mice by PCR assay (supplemental Figure 1). We found that, as was the case with Tak1−/− mice, Tak1−/−Tnfr1−/− mice, Tak1−/−Tnfr2−/− mice, and most Tak1−/−Tnfr1−/−r2−/− mice died on days 8 to 10 after injection. Only 2 of 10 Tak1−/−Tnfr1−/−r2−/− mice were able to survive up to 15 and 20 days, respectively, and these showed hunched backs and significantly reduced body weights. Substantial hematopoietic cell infiltration was observed in the livers of these 2 surviving mice when examined on day 15 and day 20, respectively, suggesting excessive inflammatory reactions. However, the death of hepatocytes was obviously reduced compared with Tak1−/− mice (supplemental Figure 2). In addition, we found that BM from these 2 mice was hypocellular, as shown by a reduction in the number of total MNCs (1.2 × 107and 0.96 × 107 respectively, approximately one-third of WT mice). We speculated that the Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− mice might die of super-inflammatory reactions induced by overproduced inflammatory cytokines, such as IFN-γ (Figure 1G).
BMF phenotype in Tak1−/− mice is partially prevented by inactivation of TNF-α signaling. Femurs, tibias, spleens, and PB of mutant mice with indicated genotypes were collected on day 8 after induction of Tak1 gene deletion. (A) H&E-stained BM sections from indicated genotypes of mice (20 × 0.7 air). Bar represents 100 μm in length. (B-C) Numbers of MNCs from 2 hind legs (B) and spleen (C) of each mouse were counted. (D-E) WBC counts and plt (platelet) numbers in PB of mice were analyzed by CBC. (F) The percentage of reticulocytes was examined in PB by methylene blue staining. (G) Concentration of IFN-γ in PB of mice was measured by ELISA. Data are the average of 4 mice from each genotype. **Significant difference compared with WT control mice (P < .01). $$Significant increase compared with Tak1−/− mice (P < .01).
BMF phenotype in Tak1−/− mice is partially prevented by inactivation of TNF-α signaling. Femurs, tibias, spleens, and PB of mutant mice with indicated genotypes were collected on day 8 after induction of Tak1 gene deletion. (A) H&E-stained BM sections from indicated genotypes of mice (20 × 0.7 air). Bar represents 100 μm in length. (B-C) Numbers of MNCs from 2 hind legs (B) and spleen (C) of each mouse were counted. (D-E) WBC counts and plt (platelet) numbers in PB of mice were analyzed by CBC. (F) The percentage of reticulocytes was examined in PB by methylene blue staining. (G) Concentration of IFN-γ in PB of mice was measured by ELISA. Data are the average of 4 mice from each genotype. **Significant difference compared with WT control mice (P < .01). $$Significant increase compared with Tak1−/− mice (P < .01).
To compare changes in hematopoiesis, mice were killed on day 8 after the first poly I:C injection. Histologic sections showed a significant disruption in BM structure and a hypocellular phenotype for Tak1−/−Tnfr2−/− mice, which was comparable to that of Tak1−/− mice. This Tak1−/− BM defect was substantially prevented in Tak1−/−Tnfr1−/− mice and was improved still further in Tak1−/−Tnfr1−/−r2−/− mice (Figure 1A). Consistent with this finding, we also found that the number of MNCs in BM and spleens of Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− mice was significantly increased compared to Tak1−/− and Tak1−/−Tnfr2−/− mice (Figure 1B-C). As a consequence, WBC counts and reticulocyte percentages were also significantly increased in Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− mice compared with Tak1−/− and Tak1−/−Tnfr2−/− mice (Figure 1D,F). Interestingly, the number of platelets in Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− mice was comparable to what was seen in Tak1−/− and Tak1−/−Tnfr2−/− mice, suggesting that the defects in Tak1 deletion-related thrombopoiesis were not prevented by the inactivation of TNF-α signaling (Figure 1E). It needs to be noted here that, although the BM structure and cellularity of Tak1−/−Tnfr1−/−r2−/− mice were much improved compared with Tak1−/−Tnfr1−/− mice, as shown by histologic section studies (Figure 1A), still, because of the relatively smaller body size of Tak1−/−Tnfr1−/−r2−/− mice, the absolute numbers of MNCs in BM and spleens of these mice are still comparable with those in Tak1−/−Tnfr1−/− mice. These data suggested that TNF-α–induced signaling contributes significantly to the BMF phenotype of Tak1−/− mice. Both Tnfr1 and Tnfr2 are involved in mediating TNF-α–induced repressive signaling in hematopoietic cells. Consistent with previous studies, we found that Tnfr1 is the major mediator of TNF-α–induced signaling in hematopoietic cells.10,41-43
The depletion of HSPCs in Tak1−/− mice is partially prevented by the inactivation of TNF-α signaling
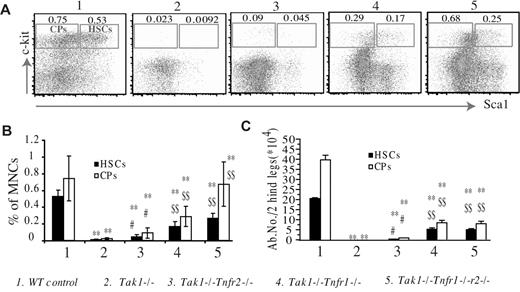
To investigate whether the depletion of Tak1−/− HSPCs could also be prevented by inactivating TNF-α signaling, we analyzed the percentages and absolute numbers of phenotypic HSCs (lineage−c-kit+Sca1+) and committed hematopoietic progenitors (CPs, lineage−c-kit+Sca1−) in the BM of mutant mice on day 8 after the induction of Tak1 deletion. We found that the percentages of HSCs and CPs were significantly increased in Tak1−/−Tnfr2−/− mice compared with Tak1−/− mice. Such percentages were further increased in Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− mice, although they had still not returned to normal levels. However, no significant difference was observed between Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− mice. Consistently, the absolute numbers of HSCs and CPs were significantly increased in Tak1−/−Tnfr2−/− mice compared with those in Tak1−/− mice. These numbers were significantly further increased in Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− mice; no significant difference was observed between the latter 2 types of mice. The absolute numbers of HSCs and CPs in Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− mice were only 25% to 35% of those observed in WT mice (Figure 2).
The depletion of Tak1−/− HSPCs is partially prevented by inactivation of TNF-α signaling. BM of mutant mice was collected on day 8 after induction of Tak1 gene deletion. (A-B) Percentages of phenotypic HSCs (Lin−Sca+c-Kit+) and CPs (Lin−Sca−c-Kit+) in total BM MNCs were analyzed by flow cytometry. BM MNCs were stained with FITC-lineage cocktail, PE-Sca1, and allophycocyanin-c-kit antibodies. HSCs and CPs were analyzed by gating first on the lin−/lo population (lineagenegative/low); percentages of HSCs and CPs in total BM MNCs were then examined. (C) Absolute numbers of HSCs and CPs were counted. Data are the average of 4 mice from each genotype. **Significant reduction compared with WT control mice (P < .01). #Significant increase compared with Tak1−/− mice (P < .05). $$Significant increase compared with Tak1−/Tnfr2−/− mice (P < .01).
The depletion of Tak1−/− HSPCs is partially prevented by inactivation of TNF-α signaling. BM of mutant mice was collected on day 8 after induction of Tak1 gene deletion. (A-B) Percentages of phenotypic HSCs (Lin−Sca+c-Kit+) and CPs (Lin−Sca−c-Kit+) in total BM MNCs were analyzed by flow cytometry. BM MNCs were stained with FITC-lineage cocktail, PE-Sca1, and allophycocyanin-c-kit antibodies. HSCs and CPs were analyzed by gating first on the lin−/lo population (lineagenegative/low); percentages of HSCs and CPs in total BM MNCs were then examined. (C) Absolute numbers of HSCs and CPs were counted. Data are the average of 4 mice from each genotype. **Significant reduction compared with WT control mice (P < .01). #Significant increase compared with Tak1−/− mice (P < .05). $$Significant increase compared with Tak1−/Tnfr2−/− mice (P < .01).
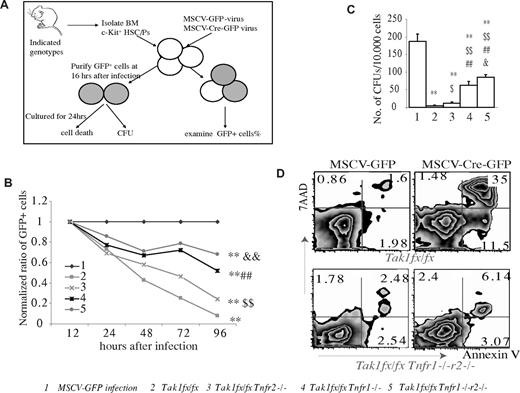
Out of concern that increased inflammatory cytokines, such as IFN-γ, might affect hematopoietic phenotype, to study the role of Tnfr1 and Tnfr2-mediated signaling in the survival of Tak1−/− HSPCs, we isolated c-kit+ HSPCs (including HSCs and CPs) from Tak1fx/fxTnfr1−/−, Tak1fx/fxTnfr2−/−, and Tak1fx/fxTnfr1−/−r2−/− mice and infected them in vitro with MSCV-Cre-GFP retrovirus to induce Tak1 deletion. c-kit+ HSPCs from Tak1fx/fx mice were infected with MSCV-Cre-GFP and MSCV-GFP virus (empty vector only) separately and were studied in parallel as positive and negative controls, respectively. Infected cells were cultured in HSPC culture medium with daily medium changes (Figure 3A). The percentages of GFP+ cells (representing infected cells) were examined at the indicated time points. These percentages from each sample were first normalized to the percentages of GFP+ cells at 12 hours in the same sample to obtain a ratio of increased or decreased GFP+ cells in each sample at the indicated time points. These ratios from each sample were further normalized to the ratio from the MSCV-GFP–infected control group samples at the corresponding time points. This then set the ratio of GFP+ cells at 1 for the 12 hour time point for all experimental samples, and the ratio of GFP+ cells at 1 at all time points for MSCV-GFP control samples. The capacity of Tnfr1 and r2 deletions to prevent the depletion of Tak1−/− HSPCs was evaluated by examining the dynamic changes in GFP+ cell ratios in infected cells (Figure 3B). We found that this dynamic analysis of infected cells ratio assay was very sensitive, reliable, and reproducible in studying gene transduction-related cell loss (such as Cre transduction-related depletion of Tak1−/− HSPCs in this study). As expected, the ratio of GFP+ cells in Cre-infected Tak1fx/fx HSPCs was reduced rapidly during culturing, whereas this reduction in GFP+ cell ratio was significantly retarded by deletion of Tnfr2, Tnfr1, or both of these simultaneously. The ability of Tnfr1 deletion to prevent depletion of Tak1−/− HSPCs was significantly less than that observed for the deletion of both Tnfr1 and r2 but was significantly greater than for the deletion of Tnfr2 only. However, this ratio in the Tak1fx/fxTnfr1−/−r2−/− group was still significantly reduced compared to the MSCV-GFP control group. These data were consistent with in vivo data, supporting the conclusion that inactivation of TNF-α signaling can only partially prevent the death of Tak1−/− HSPCs. Both Tnfr1 and r2 contribute to the TNF-α–induced depletion of Tak1−/− HSPCs (Figure 3B). In agreement with previous studies,43,44 we found Tnfr1 to be the major mediator of TNF-α signaling in HSPCs. This conclusion was further confirmed by CFU assays (Figure 3C).
Both Tnfr1 and Tnfr2 are involved in mediating the TNF-α–induced death of Tak1−/− HSPCs. (A) Schematic diagram of the experimental design. c-kit+ HSPCs were isolated from BM of mice with indicated genotypes. These HSPCs were infected with MSCV-Cre-GFP virus to induce Tak1 deletion in vitro. Infected cells were cultured in HSPC medium with medium changes every day. MSCV-GFP–infected Tak1fx/fx HSPCs were studied in parallel as controls (group 1 in B-C). (B) The percentages of infected cells (GFP+) from each sample were examined by flow cytometry at indicated time points and normalized to the percentage of MSCV-GFP–infected control group cells at corresponding time points. (C-D) Infected cells were purified by FACS and seeded for CFU assay. Exactly 30 000 GFP+ cells from each genotype were sorted and seeded into 3 plates. Numbers of colonies for each plate were counted on day 10 after cell seeding. Data shown in the figure are representative of 3 independent experiments (C). Purified infected cells with indicated genotypes were incubated in HSPC medium for 24 hours, followed by annexin V and 7-AAD staining for cell death analysis (D). **Significant reduction compared with MSCV-GFP–infected control group (P < .01). $Significant increase compared with Tak1fx/fx group ($P < .05; $$P < .01). ##Significant increase compared with Tak1fx/fx Tnfr2−/− group (P < .01). &Significant increase compared with Tak1fx/fx Tnfr1−/− group (&P < .05; &&P < .01).
Both Tnfr1 and Tnfr2 are involved in mediating the TNF-α–induced death of Tak1−/− HSPCs. (A) Schematic diagram of the experimental design. c-kit+ HSPCs were isolated from BM of mice with indicated genotypes. These HSPCs were infected with MSCV-Cre-GFP virus to induce Tak1 deletion in vitro. Infected cells were cultured in HSPC medium with medium changes every day. MSCV-GFP–infected Tak1fx/fx HSPCs were studied in parallel as controls (group 1 in B-C). (B) The percentages of infected cells (GFP+) from each sample were examined by flow cytometry at indicated time points and normalized to the percentage of MSCV-GFP–infected control group cells at corresponding time points. (C-D) Infected cells were purified by FACS and seeded for CFU assay. Exactly 30 000 GFP+ cells from each genotype were sorted and seeded into 3 plates. Numbers of colonies for each plate were counted on day 10 after cell seeding. Data shown in the figure are representative of 3 independent experiments (C). Purified infected cells with indicated genotypes were incubated in HSPC medium for 24 hours, followed by annexin V and 7-AAD staining for cell death analysis (D). **Significant reduction compared with MSCV-GFP–infected control group (P < .01). $Significant increase compared with Tak1fx/fx group ($P < .05; $$P < .01). ##Significant increase compared with Tak1fx/fx Tnfr2−/− group (P < .01). &Significant increase compared with Tak1fx/fx Tnfr1−/− group (&P < .05; &&P < .01).
To study whether TNF-α signaling inactivation can rescue functional HSPCs from Tak1 deletion-induced depletion, we used FACS to purify the GFP+ cells from the MSCV-Cre-GFP–infected BM HSPCs with indicated genotypes 16 hours after infection. Purified cells were seeded into methylcellulose medium for CFU assay. Because exactly the same number of infected cells was sorted by FACS in each sample for CFU assay, the accuracy of experimental results (accuracy of cell numbers seeded) was assured. We found that deletion of Tnfr1, r2, or both, as well as Cre overexpression, did not affect the colony-forming ability of Tak1-intact HSPCs (supplemental Figure 3). However, deletion of Tnfr1 and r2 was able to rescue 20% to 30% and 5% to 10% of the colony-forming capacity of Tak1−/− HSPCs, respectively. Such rescue was further enhanced by the codeletion of both Tnfr1 and r2 (30%-40% of MSCV-GFP-infected control HSPCs; Figure 3C). The phenomenon of prevention of cell death in Tak1−/− HSPCs by inactivated TNF-α signaling is mainly accomplished through the repression of cell death, as shown by annexin V staining (Figure 3D).
Tak1−/−Tnfr1−/−r2−/− HSPCs have long-term engraftment ability and multipotent differentiation capacity
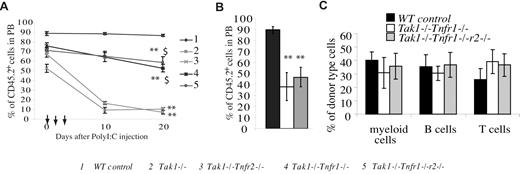
To study whether HSPCs from Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− mice have multipotent differentiation and long-term engraft-ment capacities, BM MNCs were isolated from Tak1−/−Tnfr2−/−, Tak1−/−Tnfr1−/−, and Tak1−/−Tnfr1−/−r2−/− mice (CD45.2+) before Tak1 deletion was induced. Cells for each group were mixed at a 10:1 ratio with support BM MNCs from WT mice (CD45.1+). These mixed BM cells were transplanted into lethally-irradiated recipient mice, each mouse receiving 2 × 106 mutant MNCs and 2 × 105 WT support MNCs. BM MNCs from WT (Cre−) littermates and Tak1−/− mice were transplanted in parallel as positive and negative controls, respectively. One month after transplantation, the contribution of donor-derived cells (CD45.2+) in the PB of recipient mice was examined. Recipient mice were then injected with poly I:C (every other day) for a total of 3 injections to induce Tak1 gene deletion. The percentages of donor-derived cells in thePB of recipient mice were examined at the indicated time points (Figure 4A).
Long-term engraftment ability and multipotent differentiation capacity of compound-mutant HSCs. BM MNCs (CD45.2+) were collected separately from each genotype of mice before Tak1 deletion was induced. These BM cells were mixed with competitor BM MNCs (CD45.1+) in a 10:1 ratio and transplanted into lethally-irradiated recipient mice (CD45.1+). Each recipient mouse received 2 × 106 BM MNCs of the indicated genotype and 2 × 105 competitor BM cells. One month after transplantation, the contribution of donor cells to hematopoiesis in recipient mice was examined by analyzing the percentage of CD45.2+ cells in their PB. Mice were then injected with poly I:C every other day for a total of 3 injections to induce Tak1 deletion. The donor cell contribution to recipients' PB composition was analyzed on day 10 and day 20 (A), as well as 3 months (B) after poly I:C injections. (C) The myeloid and lymphocyte differentiation of Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− HSPCs was analyzed 3 months after the induction of Tak1 deletion. **Significant reduction compared with WT control group (P < .01). $$Significant increase compared with Tak1−/− group (P < .01).
Long-term engraftment ability and multipotent differentiation capacity of compound-mutant HSCs. BM MNCs (CD45.2+) were collected separately from each genotype of mice before Tak1 deletion was induced. These BM cells were mixed with competitor BM MNCs (CD45.1+) in a 10:1 ratio and transplanted into lethally-irradiated recipient mice (CD45.1+). Each recipient mouse received 2 × 106 BM MNCs of the indicated genotype and 2 × 105 competitor BM cells. One month after transplantation, the contribution of donor cells to hematopoiesis in recipient mice was examined by analyzing the percentage of CD45.2+ cells in their PB. Mice were then injected with poly I:C every other day for a total of 3 injections to induce Tak1 deletion. The donor cell contribution to recipients' PB composition was analyzed on day 10 and day 20 (A), as well as 3 months (B) after poly I:C injections. (C) The myeloid and lymphocyte differentiation of Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− HSPCs was analyzed 3 months after the induction of Tak1 deletion. **Significant reduction compared with WT control group (P < .01). $$Significant increase compared with Tak1−/− group (P < .01).
As was expected, the percentage of donor cells in WT control-transplanted mice remained consistent at > 90%, whereas this percentage was significantly reduced in mice receiving Tak1−/− BM cells. We found that donor-derived cells in mice receiving Tak1−/−Tnfr1−/−, Tak1−/−Tnfr2−/−, and Tak1−/−Tnfr1−/−r2−/− BM cells were also reduced significantly compared with WT control transplantation. However, this reduction in the Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− groups was significantly less than that for Tak1−/−transplanted animals, whereas the Tak1−/−Tnfr2−/− group data were comparable with those for the Tak1−/− group. The reduction in donor-derived cells observed in all experimental groups compared with WT controls before poly I:C injection was due to the spontaneous deletion of the Tak1 gene caused by Mx1Cre leakage. This result further supports the suggestion of a partial rescue of the depletion of Tak1−/− HSPCs by inactivation of TNF-α signaling. The donor-derived cells of Tak1−/−Tnfr1−/− and Tak1−/−Tnfr1−/−r2−/− compound-mutant mice were maintained for more than 3 months in recipient mice, at which time the Tak1−/− and Tak1−/−Tnfr2−/− transplantations were no long detectable (Figure 4B). Cell surface marker staining suggested that the compound-mutant HSCs had multipotent differentiation capacity with a minor T lymphocyte bias (Figure 4C).
Fas inactivation partially prevents the apoptosis of Tak1−/− HSPCs in vitro but not in vivo
To search for other factors that might potentially be involved in inducing depletion of Tak1−/− HSPCs, we examined the expression levels of receptors specific for other inflammatory-related factors, including Tnfr2, Tlr-1(Toll-like receptor-1), Tlr-4, Tlr-5, Tlr-9, Dr-5 (TRAIL receptor) and IFN-γ–receptor, in normal and Tak1−/−Tnfr1−/− hematopoietic cells to define which of these are expressed in normal HSPCs and which are induced in Tak1−/−Tnfr1−/− HSPCs. We found that the expression levels of Tnfr2, Tlr-1, Tlr-4, Tlr-5, Tlr-9, Dr-5, and IFN-γ-r are higher in HSPCs and CPs than in lineage+ mature blood cells. We also found that the levels of these receptors are elevated in Tak1−/−Tnfr1−/− HSPCs compared to normal HSPCs. In addition, we found that, although Fas (CD95) is not expressed in normal HSPCs, it is expressed in Tak1−/−Tnfr1−/− HSPCs (supplemental Figure 4).
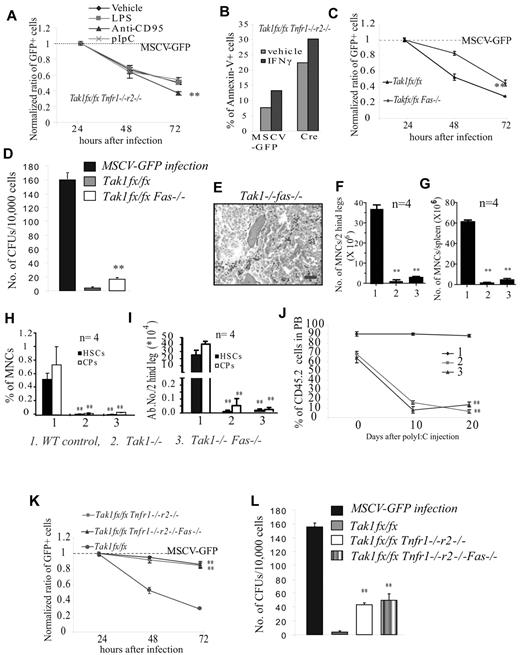
To study whether these inflammatory-related factors are also involved in inducing HSPC death and BMF in Tak1−/− mice, c-kit+ HSPCs were isolated from Tak1fx/fxTnfr1−/−r2−/− mice and infected with MSCV-Cre-GFP retrovirus. Infected cells were treated with these factors individually to test which of them could further enhance the elimination of Tak1−/−Tnfr1−/−r2−/− HSPCs by examining the ratio of reduction of GFP+ cells. MSCV-GFP viral infection was studied in parallel as a control. We found that, among all the factors we tested, only FasL further enhanced the death of Tak1−/−Tnfr1−/−r2−/− HSPCs (Figure 5A; supplemental Figure 5). IFN-γ, on the other hand, induced the death of both WT and Tak1−/−Tnfr1−/−r2−/− HSPCs, which seems to be independent of Tak1 (Figure 5B). To confirm this finding further, c-kit+ HSPCs were isolated from Tak1fx/fxFas−/− mice and infected with MSCV-Cre-GFP or MSCV-GFP retrovirus separately. Infected cells were cultured in HSPC culture medium. The effects of Fas deletion on the survival of Tak1−/− HSPCs were evaluated by examining the dynamic ratio of changes in GFP+ cells or by CFU assay. We found that Fas deletion could also partially prevent the depletion of Tak1−/− HSPCs in vitro (Figure 5C-D). Interestingly, we found that the depletion of HSPCs and the BMF phenotype of Tak1−/− mice could not be protected by Fas−/− in vivo (Figure 5E-J). This difference in Fas−/− phenotype in Tak1−/− HSPCs in vitro and in vivo suggests that there might be an additional in vivo factor, which can induce the depletion of the same population of cells, rendering the effects of Fas signaling undetectable. This notion was confirmed by a comparative study of Tak1−/−Tnfr1−/−r2−/− HSPCs with Tak1−/−Tnfr1−/−r2−/−Fas−/− HSPCs in vitro. We found that Fas−/− did not further improve the survival of Tak1−/−Tnfr1−/−r2−/− HSPCs, suggesting that Fas and TNF-α were operating on the same population of cells. In vivo, the death induced by high levels of TNF-α obscures the effects of Fas signaling (Figure 5K-L).
Fas inactivation can partially prevent the depletion of Tak1−/−HSPCs in vitro but not in vivo. (A-B) c-kit+ HSPCs were isolated from Tak1fx/fxTnfr1−/−r2−/− mice. (A) These cells were infected with MSCV-Cre-GFP virus to induce Tak1 deletion and cultured in HSPC medium with or without inflammatory cytokine treatment. MSCV-GFP infection was studied in parallel as a control. The ratios of GFP+ cells (normalized to MSCV-GFP infection) were compared among different treatment groups. **Significant reduction compared with vehicle-treated group (P < .01). (B) These infected cells were purified by FACS and cultured in HSPC medium with or without IFN-γ treatment. Cell death was examined by annexin V staining. Data shown in this figure are representative of 3 independent experiments. (C-D) c-kit+ HSPCs were isolated from Tak1fx/fx and Tak1fx/fxFas−/− mice. These cells were infected with MSCV-Cre-GFP virus to induce Tak1 deletion and cultured in HSPC medium to examine dynamic changes in the ratios of infected (GFP+) cells (C) or seeded for CFU assay (D). MSCV-GFP infections were studied in parallel as a control. Normalized ratios of GFP+ cells were compared between Tak1fx/fx and Tak1fx/fxFas−/− HSPCs. **Significant increase compared with Tak1fx/fx group (P < .01). (E-J) Tak1−/−Fas−/− mice were injected with poly I:C every other day for a total 3 injections; BM and spleens were collected 8 days hence. WT and Tak1−/− mice were treated and studied in parallel as controls. BM histology (20 × 0.7 air; bar represents 100 μm in length; E), MNCs in BM (F), MNCs in spleen (G), percentages (H), and absolute numbers (I) of HSCs and CPs in BM were compared among WT, Tak1−/−, and Tak1−/−Fas−/− groups. J. As described in Figure 4, BM MNCs from Tak1−/−Fas−/− (CD45.2+) mice (before Tak1 deletion was induced) were mixed with WT (CD45.1+) competitor cells (10:1 ratio) and transplanted into lethally-irradiated recipient mice (CD45.1+). After examining the percentages of donor-derived cells in PB of recipient mice, these mice were injected with poly I:C every other day for a total of 3 injections to induce Tak1 deletion. BM MNCs from WT and Tak1−/− mice were transplanted and studied in parallel as controls. The percentages of donor-derived cells in PB of the recipients were examined 10 and 20 days later. (F-J) **Significant reduction compared with WT control (P < .01). (K-L) c-kit+ HSPCs were collected from Tak1fx/fx Tnfr1−/−r2−/−, Tak1fx/fxTnfr1−/−r2−/−Fas−/−, and Tak1fx/fx mice and infected with MSCV-Cre-GFP to induce Tak1 deletion. Infected cells were incubated in either HSPC medium to examine the dynamic changes in ratios of infected cells (K), or sorted for CFU assay (L). MSCV-GFP–infected Tak1fx/fx HSPCs were studied in parallel as controls. (K-L) **Significant increase compared with Tak1fx/fx group (P < .01).
Fas inactivation can partially prevent the depletion of Tak1−/−HSPCs in vitro but not in vivo. (A-B) c-kit+ HSPCs were isolated from Tak1fx/fxTnfr1−/−r2−/− mice. (A) These cells were infected with MSCV-Cre-GFP virus to induce Tak1 deletion and cultured in HSPC medium with or without inflammatory cytokine treatment. MSCV-GFP infection was studied in parallel as a control. The ratios of GFP+ cells (normalized to MSCV-GFP infection) were compared among different treatment groups. **Significant reduction compared with vehicle-treated group (P < .01). (B) These infected cells were purified by FACS and cultured in HSPC medium with or without IFN-γ treatment. Cell death was examined by annexin V staining. Data shown in this figure are representative of 3 independent experiments. (C-D) c-kit+ HSPCs were isolated from Tak1fx/fx and Tak1fx/fxFas−/− mice. These cells were infected with MSCV-Cre-GFP virus to induce Tak1 deletion and cultured in HSPC medium to examine dynamic changes in the ratios of infected (GFP+) cells (C) or seeded for CFU assay (D). MSCV-GFP infections were studied in parallel as a control. Normalized ratios of GFP+ cells were compared between Tak1fx/fx and Tak1fx/fxFas−/− HSPCs. **Significant increase compared with Tak1fx/fx group (P < .01). (E-J) Tak1−/−Fas−/− mice were injected with poly I:C every other day for a total 3 injections; BM and spleens were collected 8 days hence. WT and Tak1−/− mice were treated and studied in parallel as controls. BM histology (20 × 0.7 air; bar represents 100 μm in length; E), MNCs in BM (F), MNCs in spleen (G), percentages (H), and absolute numbers (I) of HSCs and CPs in BM were compared among WT, Tak1−/−, and Tak1−/−Fas−/− groups. J. As described in Figure 4, BM MNCs from Tak1−/−Fas−/− (CD45.2+) mice (before Tak1 deletion was induced) were mixed with WT (CD45.1+) competitor cells (10:1 ratio) and transplanted into lethally-irradiated recipient mice (CD45.1+). After examining the percentages of donor-derived cells in PB of recipient mice, these mice were injected with poly I:C every other day for a total of 3 injections to induce Tak1 deletion. BM MNCs from WT and Tak1−/− mice were transplanted and studied in parallel as controls. The percentages of donor-derived cells in PB of the recipients were examined 10 and 20 days later. (F-J) **Significant reduction compared with WT control (P < .01). (K-L) c-kit+ HSPCs were collected from Tak1fx/fx Tnfr1−/−r2−/−, Tak1fx/fxTnfr1−/−r2−/−Fas−/−, and Tak1fx/fx mice and infected with MSCV-Cre-GFP to induce Tak1 deletion. Infected cells were incubated in either HSPC medium to examine the dynamic changes in ratios of infected cells (K), or sorted for CFU assay (L). MSCV-GFP–infected Tak1fx/fx HSPCs were studied in parallel as controls. (K-L) **Significant increase compared with Tak1fx/fx group (P < .01).
Inhibition of RIP-1 mimics the effects of inactivation of TNF-α signaling and partially represses the death of Tak1−/− HSPCs
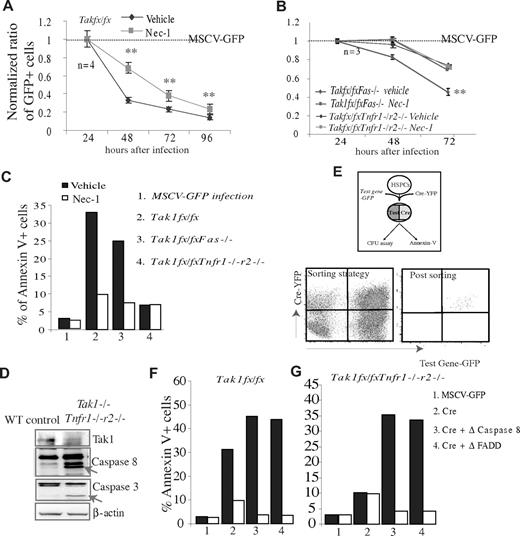
It was known that TNF-α and Fas-induced death signaling is mediated by FADD/caspase-8 (known as the traditional extrinsic apoptotic pathway) and RIP-1/RIP-3 (know as the necroptotic pathway), as shown by many cell studies.27-32 We asked which of these 2 pathways transduces TNF-α and Fas-induced death signaling in Tak1−/− HSPCs. We found that the death of Tak1−/− HSPCs was significantly repressed by treatment with the RIP-1 specific inhibitor Nec-1. Interestingly, Nec-1 can only partially prevent the death of Tak1−/− and Tak1−/−Fas−/− HSPCs; it failed to further improve the survival of Tak1−/−Tnfr1−/−r2−/− HSPCs (Figure 6A-C). These results suggest that the RIP-1 pathway mediates TNF/Fas-induced death signaling in Tak1−/− HSPCs.
The death of Tak1−/− HSPCs is nearly completely prevented by the inhibition of both RIP-1 and caspase-8 activity. (A-C) c-kit+ HSPCs were isolated from Tak1fx/fx, Tak1fx/fxTnfr1−/−r2−/−, and Tak1fx/fxFas−/− mice and separately infected with MSCV-Cre-GFP and MSCV-GFP virus. (A-B) Infected cells were incubated in HSPC medium with or without addition of 30 μg/mL Nec-1. The dynamic changes in GFP+ cell ratios were examined at the indicated time points. Data were normalized to MSCV-GFP infected HSPCs. Dashed line represents the normalized data for MSCV-GFP infected HSPCs. **Significant difference between Nec-1–treated groups and vehicle-only groups. (C) Infected HSPCs were purified by FACS 16 hours after infection and incubated in HSPC medium with or without addition of Nec-1. Death of infected cells was analyzed 24 hours later by annexin V staining. (D) WT and Tak1−/−Tnfr1−/−r2−/− mice were injected with poly I:C on days 1 and 2 to induce Tak1 deletion. BM MNCs were isolated on day 3 to examine the activity of caspase-8 and caspase-3 by Western blotting. The bands indicated by arrows are active forms of caspase-8 or caspase-3. (E-G) c-kit+ HSPCs were isolated from Tak1fx/fx and Tak1fx/fxTnfr1−/−r2−/− mice and coinfected with MSCV-Cre-YFP and MSCV-Δ-caspase-8-GFP or MSCV-Δ-FADD-GFP virus. Coinfected cells were purified by FACS to sort for GFP+YFP+ cells (D) and incubated in HSPC medium for 24 hours with or without Nec-1 treatment. Death of coinfected cells was analyzed by annexin V staining (F-G). MSCV-GFP and MSCV-Cre-YFP infections were studied in parallel as controls. (C,F-G) Data are a representative of 3 independent experiments.
The death of Tak1−/− HSPCs is nearly completely prevented by the inhibition of both RIP-1 and caspase-8 activity. (A-C) c-kit+ HSPCs were isolated from Tak1fx/fx, Tak1fx/fxTnfr1−/−r2−/−, and Tak1fx/fxFas−/− mice and separately infected with MSCV-Cre-GFP and MSCV-GFP virus. (A-B) Infected cells were incubated in HSPC medium with or without addition of 30 μg/mL Nec-1. The dynamic changes in GFP+ cell ratios were examined at the indicated time points. Data were normalized to MSCV-GFP infected HSPCs. Dashed line represents the normalized data for MSCV-GFP infected HSPCs. **Significant difference between Nec-1–treated groups and vehicle-only groups. (C) Infected HSPCs were purified by FACS 16 hours after infection and incubated in HSPC medium with or without addition of Nec-1. Death of infected cells was analyzed 24 hours later by annexin V staining. (D) WT and Tak1−/−Tnfr1−/−r2−/− mice were injected with poly I:C on days 1 and 2 to induce Tak1 deletion. BM MNCs were isolated on day 3 to examine the activity of caspase-8 and caspase-3 by Western blotting. The bands indicated by arrows are active forms of caspase-8 or caspase-3. (E-G) c-kit+ HSPCs were isolated from Tak1fx/fx and Tak1fx/fxTnfr1−/−r2−/− mice and coinfected with MSCV-Cre-YFP and MSCV-Δ-caspase-8-GFP or MSCV-Δ-FADD-GFP virus. Coinfected cells were purified by FACS to sort for GFP+YFP+ cells (D) and incubated in HSPC medium for 24 hours with or without Nec-1 treatment. Death of coinfected cells was analyzed by annexin V staining (F-G). MSCV-GFP and MSCV-Cre-YFP infections were studied in parallel as controls. (C,F-G) Data are a representative of 3 independent experiments.
The death of Tak1−/− HSPCs can be nearly completely prevented by the inhibition of both RIP-1 and caspase-8 activities
To study whether the remaining Tak1−/− HSPCs died of apoptosis, we examined whether caspases are activated in Tak1−/− cells and whether inhibition of caspase activity can prevent the death of these cells. We found that the activities of caspase-8 and caspase-3 are increased in Tak1−/− and Tak1−/−Tnfr1−/−r2−/− BM MNCs compared with WT counterparts, as shown by Western blotting (Figure 6D, data from Tak1−/−Tnfr1−/−r2−/− BM MNCs only are shown; similar results were observed in Tak1−/− cells). However, inhibition of FADD/caspase-8 signaling by transduced overexpression of dominant-negative (Δ)-FADD or Δ-caspase-8 did not repress but rather promoted the death of Tak1−/− and Tak1−/−Tnfr1−/−r2−/− HSPCs (necroptosis occurs faster than apoptosis). Interestingly, inhibition of both caspase-8 and RIP-1 activities can nearly completely prevent the death of Tak1−/− and Tak1−/−Tnfr1−/−r2−/− HSPCs (Figure 6F-G). These data suggested that the death of the remaining Tak1−/− HSPCs could be attributed to caspase-8–dependent apoptosis because it can be converted into RIP-1–dependent necroptosis by the inhibition of caspase-8.27-32
It was known that active caspase-8 can promote either type I apoptosis by directly inducing the activation of executive caspase-3 or type II apoptosis by inducing mitochondrial-related caspase-9 activation.45 The increased percentages of Tak1−/− and Tak1−/−Tnfr1−/−r2−/− BM MNCs with reduced mitochondrial membrane potential (Δψm) and the activation of caspase-9 in the same cells suggested that at least a subset of Tak1−/− HSPCs died of type II apoptosis (supplemental Figure 6A-B). This notion was further supported by a partial prevention of the apoptosis of Tak1−/− and Tak1−/−Tnfr1−/−r2−/− HSPCs by the overexpression of Bcl-xl or Δ-caspase-9, as shown by both annexin V staining and CFU assays (supplemental Figure 6C,E).
Antioxidant treatment fails to prevent the apoptosis of HSC/Ps and BMF in Tak1−/− mice
It has been shown that ROS is the major cause of apoptotic death in intestinal enterocytes and skin keratinocytes in Tak1−/− mice.34,36 To study whether ROS-induced cell death also contributes to the BM damage observed in Tak1−/− mice, we first examined whether ROS levels are increased in Tak1−/− HSPCs using CM-H2DCFDA staining. We found that ROS levels in Tak1−/− HSPCs are comparable to those in WT counterparts (supplemental Figure 7A). In addition, we found that repression of ROS by treatment of cells with ROS scavengers, such as N-acetyl-L-cysteine or butylated hydroxyanisole, or by overexpression of superoxide dismutase, did not protect the death of Tak1−/− HSC/Ps in vitro (supplemental Figure 7B-E). Moreover, we found that treatment of in vivo with N-acetyl-L-cysteine can significantly prevent liver damage but not the BMF phenotype in Tak1−/− mice (supplemental Figures 7F-I and 8). We concluded that ROS-induced cell damage might not be significantly involved in the Tak1−/− deletion-induced apoptotic death of HSPCs.
Discussion
Studies have suggested that proinflammatory factors are the major negative regulators of hematopoiesis in the BM microenvironment, inducing the depletion of HSPCs. However, studies also suggested that, under certain conditions, most of these inflammatory factors can enhance the growth of HSPCs.16-19 The molecular mechanism underlying this phenomenon has not been explored.
TNF-α is a major proinflammatory cytokine produced by activated macrophages and lymphocytes. Physiologic levels of TNF-α can be detected in the BM microenvironment.46 Classically, TNF-α has been understood to be an inhibitor of growth and an inducer of apoptosis in HSPCs.5,10,43 However, increased evidence suggests that TNF-α can also have a positive stimulatory effect on hematopoiesis and is required for the self-renewal and BM engraftment of HSPCs. The significant reduction in both competitive reconstitutive capacity and success in secondary transplantation of Tnfr1−/− HSPCs suggests that a certain physiologic baseline level of this inflammatory factor is crucial for the maintenance of proper HSC function.47
The bidirectional effects of TNF-α on HSPCs observed in previous experiments have been speculated to be the result of the concentration used, effects mediated by different receptors, or other cytokines generating effects at subsequent stages of differentiation.18,48 Recent evidence suggests that the hematopoietic system is actually maintained by multiple HSC subtypes, each possessing distinct functional characteristics. Based on the ratio of myeloid to lymphoid cells they produced in recipient animals, HSCs can be further distinguished into myeloid-biased or lymphoid-biased HSC categories.49 Studies indicated that different subsets of HSCs might respond differently to stimulation by various environmental factors. For example, TGF-β stimulates the proliferation of myeloid-biased HSCs while repressing the proliferation of lymphoid-biased HSCs.50 This might explain why certain lineages of hematopoietic cells become selectively activated and expand measurably in certain disease situations, as well as during the aging process.50
Our studies suggested that HSPCs are also heterogeneous in terms of their responses to death signal stimulation. HSPCs can be clearly separated into 2 distinct populations based on their response to TNF-α/Fas-induced death signaling (Figure 7). TNF-α/Fas-induces a RIP-1–dependent necroptosis in 30% to 40% of Tak1−/− HSPCs. Whether TNF-α–RIP-1 signaling selectively induces the death of lymphoid-biased HSCs or myeloid-biased HSCs needs further study. In addition, our studies suggest that the remaining Tak1−/− HSPCs died of traditional extrinsic apoptotic pathway-mediated FADD/caspase-8 cascade–dependent apoptosis. The apoptosis-to-necroptosis conversion of Tak1−/− HSPCs by inhibition of caspase-8 activity supports the mutual inhibition concept of FADD/caspase-8 and RIP-1/RIP-3 signaling.27-32
Schematic model of the mechanism of Tak1 protection of HSPC survival. Two distinct populations of HSPCs exist in BM hematopoietic tissue based on their response to TNF-α/Fas-induced death signaling. TNF-α/Fas, acting through RIP-1, stimulates necroptosis in 30% to 40% of HSPCs, whereas (an) unknown factor(s) induce(s) caspase-8–dependent type I or II apoptosis in the remaining HSPCs. Tak1-mediated survival signaling, stimulated by proinflammatory cytokines, prevents both types of cell death.
Schematic model of the mechanism of Tak1 protection of HSPC survival. Two distinct populations of HSPCs exist in BM hematopoietic tissue based on their response to TNF-α/Fas-induced death signaling. TNF-α/Fas, acting through RIP-1, stimulates necroptosis in 30% to 40% of HSPCs, whereas (an) unknown factor(s) induce(s) caspase-8–dependent type I or II apoptosis in the remaining HSPCs. Tak1-mediated survival signaling, stimulated by proinflammatory cytokines, prevents both types of cell death.
The factor(s) which induce(s) the apoptosis of these remaining cells need(s) further investigation. Nevertheless, our studies demonstrate that Fas-induced signaling is definitely not the cause. Our data suggest that Fas might be operative in the same population of hematopoietic cells on which TNF-α acts. Inflammatory factors, such as IL-1β, TRAIL, and LPS, also induce death receptor-related apoptosis and Tak1-mediated survival signaling, as demonstrated in studies of other tissues.33,37 These factors have also been found to repress hematopoiesis and are implicated in the pathogenesis of some BMF syndromes.11,19 Although we found that the addition of IL-1β, TRAIL, poly I:C, or LPS in vitro did not further enhance the death of Tak1−/−Tnfr1−/−r2−/− HSPCs, there is a possibility that Tak1−/− HSPCs might be super-sensitive to the apoptosis induced by these factors. Even a low level of these factors in the culture medium might be sufficient to induce the maximum elimination of Tak1−/− HSPCs. Therefore, we cannot completely exclude the contribution of death signals induced by these factors to the apoptosis of Tak1−/− HSPCs.
Inflammatory factors, such as IFN-α and IFN-γ, have been found to inhibit the growth of HSPCs and promote the proliferation of quiescent HSCs; this scenario has been implicated in many BMF syndromes.5 However, these factors do not induce death receptor-related signaling, nor do they stimulate Tak1 activity. We found that IFN-α has no significant effect on the apoptosis of WT or Tak1−/− HSPCs, whereas IFN- γ was able to induce the death of both WT and Tak1−/− HSPCs. This suggests that IFN-γ might induce HSPC death through a mechanism that is independent of Tak1 and TNF-α/Fas. The significant increase of IFN-γ concentration in Tak1−/−Tnfr1−/−r2−/− mice suggests that Tak1 might repress the expression of IFN-γ. The mechanism behind this phenomenon needs further investigation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the Department of Comparative Medicine at Loyola University Medical Center for excellent animal care services, Ms Patricia Simms for flow cytometric sorting of HSCs and progenitors, and Drs Manuel Diaz, Nancy Zeleznik-Le, and Andrew Dingwall for ongoing professional collaboration and scientific suggestions and discussions that improved the present studies.
This work was supported by Department of Defense (grant PR080447 through Loyola University Chicago), the National Natural Science Foundation of China (project 81071774), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning through Shanghai Normal School. P.B. is supported in part by the Jimmy Burns Foundation.
Authorship
Contribution: Y.X., H.L., Jun Zhang, A.V., Shubin Zhang, W.W., Shanshan Zhang, and P.B. performed the research, analyzed the data, and wrote the paper; and Jiwang Zhang designed and performed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for H.L. is Department of Hematology/Oncology, Gansu People's Hospital, Gansu, People's Republic of China. The current affiliation for Jun Zhang is Department of Biology, College of Life and Environment Science, Shanghai Normal University, Shanghai, People's Republic of China.
Correspondence: Peter Breslin, Oncology Institute, Cardinal Bernardin Cancer Center, Loyola University Medical Center, 2160 South 1st Ave, Maywood, IL 60153; e-mail: pbresli@luc.edu; and Jiwang Zhang, Oncology Institute, Cardinal Bernardin Cancer Center, Loyola University Medical Center, 2160 South 1st Ave, Maywood, IL 60153; e-mail: jzhang@lumc.edu.