Case presentation
Ms TS is a 66-year-old woman who receives warfarin for prevention of systemic embolization in the setting of hypertension, diabetes, and atrial fibrillation. She had a transient ischemic attack about 4 years ago when she was receiving aspirin. Her INR control was excellent; however, over the past few months it has become erratic, and her average dose required to maintain an INR of 2.0 to 3.0 appears to have decreased. She has had back pain over this same period and has been taking acetaminophen at doses as large as 650 mg four times daily, with her dose varying based on her symptoms. You recall a potential interaction and wonder if (1) her acetaminophen use is contributing to her loss of INR control, and (2) does this interaction place her at increased risk of warfarin-related complications?
Warfarin has remained the most commonly prescribed vitamin K antagonist (VKA) since its introduction into clinical practice approximately 60 years ago. VKAs exert their effect by inhibiting the cyclic replenishment of reduced vitamin K, an obligate cofactor in the γ-carboxylation of the biologically inactive procoagulant factors II, VII, IX, and X, as well as the anticoagulant factors protein C, protein S, and protein Z.1 The resultant anticoagulant effect is measured by the international normalized ratio (INR), which for most indications is targeted between 2.0 and 3.0. Thromboembolism, major hemorrhage, and death have all been strongly linked to the proportion of time spent in this therapeutic range.2,3 Despite its efficacy in preventing and treating thromboembolic disease, warfarin has several limitations that challenge its effectiveness in clinical practice, including a narrow therapeutic index, variable dose-response, and importantly the potential for important interactions with numerous commonly used medications.4
Reports of an interaction between warfarin and acetaminophen first appeared in the literature in 1968.5 Acetaminophen is part of the class of drugs known as “aniline analgesics”; it is the only such drug still in use today.6 Acetaminophen is used worldwide as an analgesic and antipyretic. Because aspirin and other nonsteroidal anti-inflammatory drugs inhibit platelet function and can cause injury to the gastric mucosa, acetaminophen is the analgesic of choice for patients receiving oral anticoagulant therapy. Establishing the validity of this interaction is critically important as acetaminophen is currently the recommended first-line therapy for pain control in older adults, the group at highest risk of hemorrhage and concomitant use of VKAs.7 The objectives of this focused review are to summarize the observational and randomized data investigating this interaction, to provide insights into possible biologic mechanisms, and to suggest clinical practice recommendations for patients receiving both VKAs and acetaminophen.
Observational data
Early case reports demonstrated a temporal increase in the INR among persons taking warfarin after acetaminophen exposure, suggesting an interaction.8,9 Subsequent observational studies investigated the relationships between acetaminophen and INR and acetaminophen and hemorrhage among persons prescribed warfarin. In a case-control study, 93 consecutive patients with an INR greater than 6 were compared with 196 randomly selected control patients with an INR in the range of 1.7 to 3.3. Participants were interviewed within 24 hours of the INR measurement, and pertinent exposures within the previous 7 days were recorded. Acetaminophen was independently associated in a dose-dependent fashion with an INR greater than 6.0. For persons taking acetaminophen 9.1 g/week or more, the odds of an INR greater than 6.0 were increased 10-fold. This dose-response relationship persisted after controlling for other factors known to potentiate warfarin.10
Another case-control study enrolled 53 patients with an INR greater than 4.5 and 106 control patients with an in-range INR. Amiodarone (9.4% vs 0%, P < .004), acetaminophen (18.9% vs 0.9%, P < .001), tramadol (5.6% vs 0%, P < .04), ofloxacine (11.3% vs 1.9%, P < .001), and lactulose (11.3% vs 0%, P < .001) were associated with INR elevation. Other factors included fever, malnutrition, dehydration, and acute diarrhea.11 Bleeding complications occurred in 19.2% of cases versus 3.9% of the controls.
Shalansky et al prospectively studied 171 warfarin-treated patients to assess the risk of bleeding and elevated INR associated with the use of complementary and alternative medicines.12 Patients kept a diary of selected exposures for 16 weeks. Pharmacy, laboratory, and medical records were subsequently queried for evidence of bleeding or elevations in INR. Acetaminophen was associated with increased risk of bleeding (OR = 1.42; 95% confidence interval [CI], 1.05-1.90) and INR elevation, although the latter did not achieve statistical significance (OR = 1.76; 95% CI, 0.85-3.63, P = .13).12 Warfarin use of less than 3 months' duration was the only statistically significant risk factor identified for increased INR.
In a retrospective study of a postmortem toxicology database, Launiainen et al13 reported an association of combination therapy with fatal hemorrhage. Of the 328 patients who were taking warfarin at the time of death, a potentially interacting drug was present in one-third, and acetaminophen was the most common (50%). Concomitant use of acetaminophen and warfarin was associated with a 4.6 and 2.7 times higher risk of fatal bleeding than either acetaminophen or warfarin alone, respectively.13
No association between acetaminophen and INR was found in a retrospective study of 54 persons prescribed nonwarfarin VKAs, acenocoumarol, or phenprocoumon. However, 9 patients in the acetaminophen group required a reduction in coumarin dose after acetaminophen exposure compared with one in the control group, which may have blunted any increase in the INR.14
Observational studies investigating the interaction between acetaminophen and warfarin are limited in their ability to ascertain duration, dose, and cumulative dose of acetaminophen given the nonprescription or “over-the-counter” status of acetaminophen. The temporal relationship between acetaminophen exposure and INR elevation mandates standardized INR measurement at baseline and at prespecified intervals among persons already stabilized on warfarin. These limitations and the concern for residual confounding mandate a randomized assessment.
Intervention and randomized data
To investigate an observed increase in INR after a course of acetaminophen, a 74-year-old man was rechallenged with acetaminophen 1 g 4 times per day for 3 consecutive days. On rechallenge, the INR increased from 2.4 to 6.3, factor VII activity decreased from 29.4% to 15.5%, and there was no significant change in warfarin plasma concentration (1.54 μg/mL vs 1.34 μg/mL). These findings argued against a pharmacokinetic basis for the interaction and, instead, suggested a pharmacodynamic mechanism for the elevation in INR.15
Five randomized trials have been performed to evaluate the effect of acetaminophen on INR in patients treated with warfarin (Table 1). Four of the 5 studies were positive. The one negative study, published in 1969, randomized 20 patients with stable prothrombin times to 2 doses of acetaminophen 650 mg or matching placebo given at 8 am and 12 noon. Prothrombin times were measured at 8 am, 10 am, 12 noon, 2 pm, and 4 pm on the day of study drug administration, and at 8 am on the following 2 days. This study found no significant difference in prothrombin times between acetaminophen and placebo during the 48-hour period after study drug administration. This study demonstrated either no effect or no measurable effect of low-dose acetaminophen given for a very short duration.16
In 2004, Mahé et al randomized 11 stable warfarin patients to 4 grams per day of acetaminophen or placebo for 14 days.18 This was a prospective, double-blind, placebo-controlled trial. The mean observed INR was significantly increased after 4 days and throughout the study period in the acetaminophen group, whereas no differences were observed in the placebo group (P = .001, Table 1).17 In a follow-up study 2 years later, the authors conducted a double-blind, placebo-controlled, randomized, crossover study in 20 patients with stable INR. Participants were randomized to receive placebo or acetaminophen 1 g 4 times daily for 14 days. INR and clotting factor activities were measured before the first drug administration and then on days 2, 4, 7, 9, 11, and 14. The authors demonstrated that the mean INR rose rapidly after the start of acetaminophen and was significantly increased after 1 week of acetaminophen intake compared with placebo (P = .0002). Significant reductions in the vitamin K-dependent clotting factors II, VII, IX, and X accompanied this increase in INR.18
In 2007, Parra et al performed another randomized, double-blind, placebo-controlled trial testing the effect of different doses of acetaminophen versus placebo on INR in patients stabilized on warfarin.19 Patients received acetaminophen 2 g/day (n = 12) or 4 g/day (n = 12) or matching placebo 4 times/day (n = 12) for 4 weeks. More than 50% of the patients receiving acetaminophen exceeded the upper limit of the therapeutic INR range compared with 17% in the placebo group. At week 4, no differences were observed in alanine aminotransferase and aspartate aminotransferase between either of the acetaminophen groups or placebo. Patients receiving 4 g/day had significantly higher alanine aminotransferase at week 2 compared with placebo, suggesting a modest and temporary hepatic effect associated with the higher dose of acetaminophen. In more than 80% of the patients who developed an elevated INR and did not have their dose adjusted, the INR returned to normal when acetaminophen was stopped.19
Finally and more recently, the largest trial to date randomized 45 patients to one of 3 arms: acetaminophen 2 g/day, acetaminophen 3 g/day, and placebo for 10 consecutive days. Both doses of acetaminophen were associated with an increase in INR compared with placebo. The maximum INR increase was independently associated with a decrease in factor II (P < .001) and factor VII (P < .001) activities with an increase in acetaminophen plasma concentrations (P < .001).20
Biologic plausibility and mechanistic insights
A number of investigators have performed carefully designed experiments to evaluate the possibility that acetaminophen and/or its metabolites affect warfarin pharmacokinetics. Given that warfarin is a racemic mixture of R- and S-enantiomers with substantially differing pharmacokinetics and potency, these investigations have examined both enantiomers and have been adequately powered to exclude any major interactions. Given the lack of evidence for a pharmacokinetic interaction, a pharmacodynamic mechanism was hypothesized.21 The concept that acetaminophen might interact with warfarin by potentiating its inhibition of components of the vitamin K cycle was initially raised by Thijssen et al.22 These investigators drew attention to the recent finding that acetaminophen overdose had been associated with elevation of INR and diminution of vitamin K-dependent factors VII and IX levels (and not factor VIIIc) in the absence of other indices of acetaminophen-induced hepatotoxicity as evidence that acetaminophen and/or its metabolites might inhibit vitamin K function.23
These investigators therefore evaluated the effects of acetaminophen and its metabolite N-acetyl-p-benzoquinone-imine (NAPQ1) on the activity of 2 key enzymes of the vitamin K cycle, vitamin K-dependent carboxylase and vitamin K-epoxide reductase (VKOR), in washed microsomal preparations. Acetaminophen did not affect activities of either enzyme. However, NAPQI oxidized vitamin K-hydroquinone (KH2), the “active” form of the vitamin. In addition, NAPQI directly inhibited vitamin K-dependent carboxylation. Furthermore, VKOR activity was inhibited by NAPQI. Therefore, NAPQI disrupted the vitamin K cycle, potentially at 3 sites. Although the potency of NAPQI as an inhibitor at any one point of the cycle appeared limited, the interactions are important as they may be synergistic and dependent on localized intracellular increases in concentrations of NAPQI under certain circumstances.
Implications of variable production of NAPQI with therapeutic acetaminophen ingestion
The production of NAPQI as a toxic metabolite of acetaminophen has received considerable scientific attention in the context of acetaminophen overdose. NAPQI production reflects largely or entirely metabolism of acetaminophen by cytochrome P4502E1 (CYP2E1) with substantially increased generation of CYP2E1 occurring during overdose.24-26 Furthermore, there is considerable evidence that NAPQI depletes tissue sulfhydryls, including glutathione, and is covalently protein-bound.27
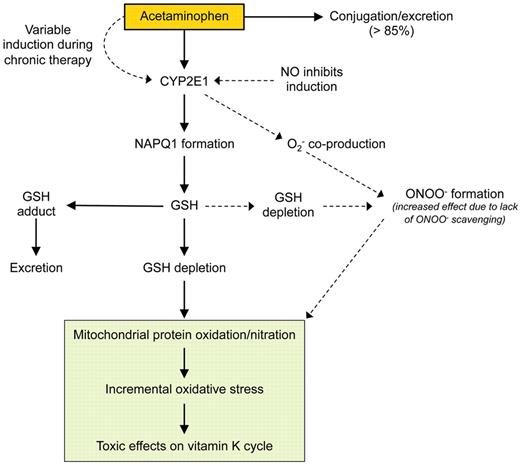
A number of metabolic pathways for acetaminophen have been delineated, including conjugation with glucuronic acid and subsequent elimination of the nontoxic conjugate. NAPQI generation, catalyzed by CYP2E1 in the presence of nicotinamide adenine dinucleotide phosphate (NADPH), does not inevitably lead to its accumulation, given that NAPQI can be rapidly cleared by conjugation with glutathione. The potential for NAPQI accumulation and toxicity therefore results from induction of CYP2E1 and/or depletion of glutathione. It is important to emphasize that variable generation of NAPQI reflects not only tissue acetaminophen concentrate but also induction of CYP2E1. Acetaminophen itself increases expression of CYP2E1, and this may occur with subtoxic doses of acetaminophen.28,29 Other factors that may potentially induce CYP2E1 include ethanol and diabetes mellitus/hyperglycemia.30,31 Other sources of variability in CYP2E1 activity include the Dra I polymorphism of the CYP2E1 gene, which may potentiate activity of CYP2E1 in response to inducing agents, and the inhibitory effect of nitric oxide (Figure 1).31,32
Determinants of variable induction of oxidative stress by acetaminophen. GSH indicates glutathione; NO, nitric oxide; and ONOO−, peroxynitrite.
Determinants of variable induction of oxidative stress by acetaminophen. GSH indicates glutathione; NO, nitric oxide; and ONOO−, peroxynitrite.
The effects of NAPQI in inactivating vitamin K-dependent γ-carboxylase and VKOR are not its only enzymatic interactions. NAPQI may also inhibit components of the mitochondrial electron transport chain.33 However, detailed evaluation of the full extent of direct toxic effects of NAPQI has been limited to date.
Potential downstream effectors of CYP2E1 activation/NAPQI production by acetaminophen
The potential ramifications of CYP2E1 activation and NAPQI production on the vitamin K cycle are extensive and include: (1) inactivation of VKOR via oxidation of essential cysteine moieties, (2) impairment of reductive reactivation of VKOR, and (3) impairment of VKOR-supported activation of vitamin K γ-carboxylase. It is probable that, although the extent of CYP2E1 activation is critical to impairment of the vitamin K cycle in the presence of acetaminophen, NAPQI is not the only effector of the extensive oxidative changes that underlie this impairment. One such potential effector is peroxynitrite, a reactive species produced via the reaction of superoxide anion with nitric oxide. There is an extensive literature suggesting that peroxynitrite modulates the development of acetaminophen hepatotoxicity.34 Release of reactive oxygen species via CYP2E1 activation has been shown to deplete sulfhydryl sources, such as reduced glutathione in mitochondria and endoplasmic reticulum.30 Similarly, there is some evidence that scavenging of peroxynitrite may limit the cytotoxic effects of acetaminophen.35
The impact of CYP2E1 up-regulation is also potentially modified in other ways. The role of nitric oxide is particularly complex, as nitric oxide is both involved in peroxynitrite formation as well as, apparently, via its activation of soluble guanylate cyclase, able to limit CYP2E1-related toxicity by limiting its expression.31,36 There is also considerable evidence that the transcription factor Nrf2, which controls antioxidant defense in part via increased glutathione synthesis, limits CYP2E1 toxicity.37
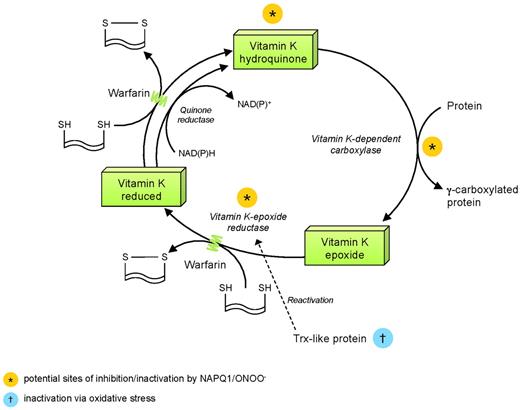
The activity of VKOR, vital to the integrity of the vitamin K cycle, is physiologically inhibited by oxidation of key cysteine moieties.38,39 Thus, any form of oxidative stress, via NAPQI, peroxynitrite, or both, could inactivate VKOR, as originally demonstrated by Thijssen et al.22 Indeed, depletion of glutathione and other sulfhydryl molecules is a common modality of peroxynitrite toxicity. Equally important is the susceptibility of VKOR reactivation to oxidative stress. Although the molecules reactivating VKOR have not been identified conclusively, there is considerable evidence that they are thioredoxin-like.39 As activity of thioredoxin is itself impaired in the presence of oxidative stress and/or via its physiologic antagonist thioredoxin-interacting protein, it seems likely, although as yet unexplored, that this represents a further site of the acetaminophen-vitamin K interaction. It has also recently been demonstrated that thioredoxin activity drives the role of VKOR in supporting vitamin K-dependent γ-carboxylation.39 Furthermore, the activity of vitamin K-dependent carboxylase is inhibited by oxidation of sulfhydryl groups, although these lie outside the catalytic site of the molecule.40 Therefore, the observations of Thijssen et al22 may reflect interplay of CYP2E1 activation, NAPQI and peroxynitrite production, and thioredoxin inactivation, as outlined in Figure 2.
Points of potential disruption of vitamin K cycle and potentiation of warfarin effect by NAPQ1 and ONOO−. NAD(P)H indicates reduced nicotinamide adenine dinucleotide phosphate; NAD(P)+, oxidized nicotinamide adenine dinucleotide phosphate; ONOO−, peroxynitrite; and SH, sulfhydryl group.
Points of potential disruption of vitamin K cycle and potentiation of warfarin effect by NAPQ1 and ONOO−. NAD(P)H indicates reduced nicotinamide adenine dinucleotide phosphate; NAD(P)+, oxidized nicotinamide adenine dinucleotide phosphate; ONOO−, peroxynitrite; and SH, sulfhydryl group.
General recommendations
The requisite features of causality exist for a warfarin/acetaminophen interaction: temporal relationship, measurable effect with dechallenge and rechallenge, dose-response, exclusion or accounting of other possible etiologic factors, and biologic plausibility. The strength of our clinical practice recommendations is low because, although the evidence of an important warfarin/acetaminophen interaction that results in INR variation is strong, there are no prospective management studies to indicate that the recommendations we make would reduce patient-important events, such as major bleeding or thrombosis.
In warfarin-treated patients who will use more than or equal to 2 g/day of acetaminophen for at least 3 consecutive days, we suggest that the INR should be tested 3 to 5 days after the first acetaminophen dose (grade 2C). In warfarin-treated patients with otherwise unexplained INR variability, acetaminophen use should be considered as a possible contributing factor (grade 2C).
On a wider scale, acetaminophen may disrupt, not only the production of the vitamin K-dependent proteins of the coagulation cascade, but all vitamin K-dependent proteins, such as those that normally function as inhibitors of calcification and modulate signal transduction and cell growth. Evaluation of long-term effects of acetaminophen ingestion with these changes in mind seems appropriate.
Acknowledgments
This work was supported by the Duke Clinical Research Institute, Durham, NC.
Authorship
Contribution: R.D.L. conducted the literature search; R.D.L. and J.D.H. wrote the first draft of the manuscript; and all authors provided clinical input and critical review of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Renato D. Lopes, Duke Clinical Research Institute, Box 3850, 2400 Pratt St, Rm 0311, Terrace Level, Durham, NC 27705; e-mail: renato.lopes@duke.edu.