Abstract
Reversible interactions of glycoconjugates on leukocytes with P- and E-selectin on endothelial cells mediate tethering and rolling of leukocytes in inflamed vascular beds, the first step in their recruitment to sites of injury. Although selectin ligands on hematopoietic precursors have been identified, here we review evidence that PSGL-1, CD44, and ESL-1 on mature leukocytes are physiologic glycoprotein ligands for endothelial selectins. Each ligand has specialized adhesive functions during tethering and rolling. Furthermore, PSGL-1 and CD44 induce signals that activate the β2 integrin LFA-1 and promote slow rolling, whereas ESL-1 induces signals that activate the β2 integrin Mac-1 in adherent neutrophils. We also review evidence for glycolipids, CD43, L-selectin, and other glycoconjugates as potential physiologic ligands for endothelial selectins on neutrophils or lymphocytes. Although the physiologic characterization of these ligands has been obtained in mice, we also note reported similarities and differences with human selectin ligands.
Introduction
The selectins mediate adhesion of hematopoietic cells to vascular surfaces and to each other.1 These interactions are important for host defense, hematopoiesis, immune cell surveillance, hemostasis, and inflammation. Each of the 3 selectins is a type I transmembrane protein with an N-terminal C-type lectin domain, an epidermal growth factor-like domain, a series of consensus repeats, a transmembrane domain, and a short cytoplasmic tail. L-selectin is constitutively expressed on most leukocytes. P-selectin is rapidly mobilized from secretory granules to the plasma membranes of platelets and endothelial cells on stimulation. E-selectin expression on endothelial cells is regulated at the transcriptional level by inflammatory mediators, such as tumor necrosis factor-α.
The rolling cell adhesion mediated by selectins is a dynamic process that requires rapid formation and breakage of bonds under flow.2 Rolling enables cells to receive signals that activate integrins, another class of adhesion receptors, which cause the cells to roll slower and to arrest.3 Here we discuss how leukocytes, particularly neutrophils, interact with endothelial selectins during inflammation. We review evidence that surprisingly few neutrophil glycoproteins are physiologic selectin ligands, defined by their ability to mediate rolling adhesion. Rolling can be studied with flow chambers in vitro or ex vivo and in transparent tissues or by epifluorescence in vivo.
The selectins are Ca2+-dependent lectins. The minimal glycan determinant for selectin binding is sialyl Lewis x (sLex; NeuAcα2,3Galβ1,4[Fucα1,3]GlcNAcβ1-R).1 The fucose moiety of sLex expressed on selectin ligands forms critical interactions with the Ca2+-coordination site on the lectin domain of selectins.2 Leukocytes from mice lacking the 2 α1,3-fucosyltransferases that add fucose to form sLex on hematopoietic cells cannot roll on P- and E-selectin.4 Because sLex can potentially cap N- and O-glycans on many proteins and also glycans on lipids, neutrophils might display many selectin ligands. However, α1,3-fucosylation occurs at limited sites on some proteins on human myeloid cells,5 and there is very little α1,3-fucosylation on murine myeloid cells.6 A subset of these glycoproteins might cluster sLex-capped glycans to increase avidity. As described in the next section, P-selectin binds with higher affinity to an N-terminal region of P-selectin glycoprotein ligand-1 (PSGL-1) through cooperative interactions with sulfated tyrosines and other amino acids and with an adjacent sLex-capped O-glycan. However, the affinity or avidity of a glycoprotein for a selectin in solution may not predict physiologic relevance. During rolling, selectins interact with their ligands under 2-dimensional conditions where force regulates off-rates.2 Rolling of a selectin-expressing cell on an isolated glycoprotein does not prove the latter's function in the context of the complex topography of the leukocyte surface (Figure 1A). Factors, such as the number of molecules per cell, molecular length, dimerization or oligomerization, clustering in lipid rafts or microvilli, or cytoskeletal anchorage, may be crucial determinants of function.2 Therefore, the physiologic roles of P- and E-selectin ligands require confirmation in primary leukocytes.
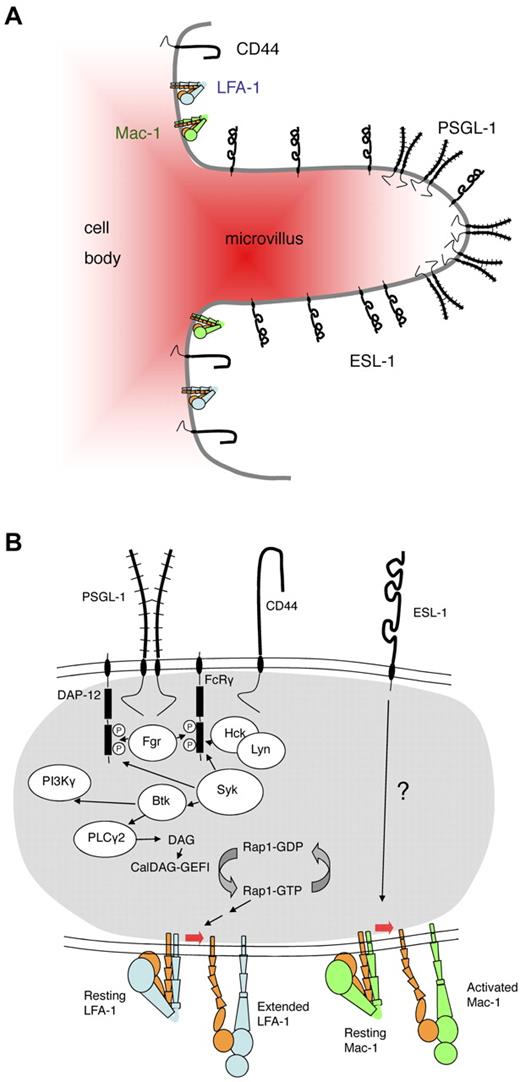
Topography and signaling pathways triggered by selectin ligands in neutrophils. (A) Topography of selectin ligands on neutrophils. Based on biochemical and electron microscopic evidence, PSGL-1 is thought to be concentrated in lipid rafts on the tips of microvilli.13 Electron microscopy places (some of) ESL-1 on microvilli, but not necessarily the tips,69 whereas CD44 is concentrated in the valleys between microvilli.86 LFA-1 and Mac-1 are thought to be mostly on the cell body. (B) Signaling pathways of selectin ligands in neutrophils. Engagement of PSGL-1 by P-selectin or E-selectin or engagement of CD44 by E-selectin induces activation of the SFKs Fgr, Hck, and Lyn.40,82 The activated SFKs phosphorylate the ITAM domains of DAP-12 and FcRγ, enabling them to recruit spleen tyrosine kinase Syk.82 Knocking out Fgr or knocking out both Hck and Lyn blocks this signaling pathway.40,82 Direct physical association between PSGL-1, CD44, and the SFKs has not been demonstrated. Syk activity is needed to activate Bruton tyrosine kinase (Btk), which leads to phospholipase C-γ2 (PLCγ2) activation, providing diacylglycerol (DAG) for the activation of CalDAG-GEFI, an exchange factor for the small G protein Rap-1.84 Rap-1 drives LFA-1 extension through other signaling intermediates (not shown). Engagement of ESL-1 by E-selectin has been shown to activate Mac-1,85 but the signaling pathway is unknown.
Topography and signaling pathways triggered by selectin ligands in neutrophils. (A) Topography of selectin ligands on neutrophils. Based on biochemical and electron microscopic evidence, PSGL-1 is thought to be concentrated in lipid rafts on the tips of microvilli.13 Electron microscopy places (some of) ESL-1 on microvilli, but not necessarily the tips,69 whereas CD44 is concentrated in the valleys between microvilli.86 LFA-1 and Mac-1 are thought to be mostly on the cell body. (B) Signaling pathways of selectin ligands in neutrophils. Engagement of PSGL-1 by P-selectin or E-selectin or engagement of CD44 by E-selectin induces activation of the SFKs Fgr, Hck, and Lyn.40,82 The activated SFKs phosphorylate the ITAM domains of DAP-12 and FcRγ, enabling them to recruit spleen tyrosine kinase Syk.82 Knocking out Fgr or knocking out both Hck and Lyn blocks this signaling pathway.40,82 Direct physical association between PSGL-1, CD44, and the SFKs has not been demonstrated. Syk activity is needed to activate Bruton tyrosine kinase (Btk), which leads to phospholipase C-γ2 (PLCγ2) activation, providing diacylglycerol (DAG) for the activation of CalDAG-GEFI, an exchange factor for the small G protein Rap-1.84 Rap-1 drives LFA-1 extension through other signaling intermediates (not shown). Engagement of ESL-1 by E-selectin has been shown to activate Mac-1,85 but the signaling pathway is unknown.
Each approach to identify physiologic selectin ligands on a leukocyte has strengths and limitations. Gene knockout or gene silencing (mRNA knockdown) in mice allows assessment of ligand activity under physiologic conditions, but loss of a glycoprotein might impair rolling through an indirect effect on cellular function rather than by eliminating a key selectin ligand. Definitive identification of a glycoprotein as a selectin ligand should ideally meet several criteria, including: (1) the capacity to support rolling of selectin-bearing cells or beads on isolated ligand; (2) gene depletion or silencing must impair selectin-mediated functions on intact cells in vitro and in vivo (eg, rolling or signaling); and (3) monoclonal antibodies (mAbs) against a specific glycoprotein must also impair selectin-mediated functions. To date, only mAbs to the unique N-terminal P-selectin-binding region of PSGL-1 fulfill the third requirement.1,2 Importantly, mAbs to glycoproteins that reproducibly block binding to E-selectin have not been described. This has significantly hindered testing of physiologic functions of candidate E-selectin ligands on primary human leukocytes, where gene knockout and gene silencing methods are less feasible. In mice, as we discuss in “Additional ligands for E-selectin,” the functional redundancy of E-selectin ligands has required simultaneous deletion of more than one glycoprotein to unmask their functions. Even when all 3 of the aforementioned conditions are met, the ability of mAb or knockout/knockdown approaches to abrogate leukocyte rolling on a selectin may not definitively identify all glycoprotein ligands. Other glycoproteins might be necessary but not sufficient without the targeted glycoproteins.
Despite these challenges, significant progress has been made in identifying physiologic selectin ligands on leukocytes that mediate not only rolling but also signaling, thus enabling integrins to stabilize interactions with endothelial cells and other blood cells. These advances offer the opportunity to identify new physiologic contributions of selectins and their ligands to homeostasis and disease. Here we review the evidence that 3 glycoproteins act as physiologic ligands for P- and/or E-selectin on mouse neutrophils, describe the specialized roles of each ligand for neutrophil recruitment during inflammation, discuss limitations of current data and some controversies, note other leukocyte glycoproteins and glycolipids that might be physiologic ligands, and suggest avenues for future research. The contributions of selectin ligands on hematopoietic precursors to in vivo trafficking are less well characterized and will not be discussed here.
Ligands for endothelial selectins
PSGL-1
PSGL-1 is a major selectin ligand on leukocytes. PSGL-1 binds to P-selectin,7 E-selectin,8,9 and L-selectin10 under flow conditions. It is the predominant physiologic ligand for P-selectin and L-selectin on leukocytes, and it cooperates with additional ligands to mediate leukocyte rolling on E-selectin. In addition to mediating leukocyte tethering and rolling, it transduces signals into rolling leukocytes and into leukocytes decorated with platelets.11
PSGL-1 is a type I membrane protein that is preferentially located in lipid rafts12 on the tips of microvilli13 (Figure 1A). It is expressed as a disulfide-linked homodimer; each subunit consists of an extracellular, transmembrane, and cytoplasmic domain14,15 (Figure 1B). The extracellular domain of PSGL-1 is rich in prolines, serines, and threonines, most of which are located in a series of decameric repeats (14-16 in humans and 15 in mice).16,17 Posttranslational modifications of PSGL-1 are important for optimal selectin binding.15 Protein O-glycosylation is initiated by a polypeptide N-acetylgalactosamine transferase (ppGalNAcT) that adds GalNAc to serine and threonine residues. Leukocytes from mice lacking the glycosyltransferase ppGalNAcT-1 roll poorly on P- and E-selectin in vitro.18 To bind to P-selectin, PSGL-1 requires an α2,3-sialylated and α1,3-fucosylated core 2 O-glycan attached to a specific N-terminal threonine.15 Three core 2 β1,6-N-acetylglucosaminyltransferase enzymes (C2GnT) transfer GlcNAc to the Galβ1,3GalNAc core 1 structure.19 Studies with knockout mice have established the contribution of C2GnT1 to leukocyte interactions with all 3 selectins.20-24 C2GnT1 is required for the trafficking of neutrophils and activated T cells to sites of inflammation.20,22 Sulfation of tyrosine residues near the N-terminus optimizes the binding of PSGL-1 to P-selectin.15,25 To bind to E-selectin, PSGL-1 requires core 2 α1,3-fucosylated and α2,3-sialylated O-glycans but not tyrosine sulfation.15,26 Interactions of chemokines with the N-terminal domain of PSGL-1 may enhance recruitment of specific leukocyte subsets into inflamed or secondary lymphoid tissues.27,28 Interestingly, PSGL-1 glycosylation, which promotes selectin binding, negatively affects chemokine binding.28
The sequences of the transmembrane and cytoplasmic domains of PSGL-1 are highly conserved. In the endoplasmic reticulum, cooperative interactions between transmembrane domains and between cytoplasmic domains facilitate the formation of PSGL-1 dimers.29,30 Each noncovalent dimer is then stabilized by a single juxtamembrane disulfide bond. An export signal in the cytoplasmic domain promotes transfer of PSGL-1 from the endoplasmic reticulum to the Golgi apparatus, where O-glycans are added en route to the cell surface.30 The cytoplasmic tail of PSGL-1 consists of 67 amino acids in mice and 69 amino acids in humans and may interact with different proteins.11 In vitro, the cytoplasmic domain binds to ezrin/radixin/moesin (ERM) proteins, which in turn interact with actin filaments.31,32 Because both ERM proteins and PSGL-1 move to the uropod on polarization, the PSGL-1-ERM interaction might play a role in later steps of the leukocyte adhesion cascade, such as intravascular crawling or transendothelial migration. Nef-associated factor 1 (Naf-1) forms a constitutive complex with the juxtamembrane region of the cytoplasmic tail of PSGL-1.33 The PSGL-1 tail also binds to selectin ligand interactor cytoplasmic-1 (human ortholog of the mouse sorting nexin 20), which binds phosphoinositides and targets PSGL-1 to endosomes in transfected cells. However, selectin ligand interactor cytoplasmic-1 does not participate in PSGL-1-mediated leukocyte adhesion and signaling in vivo.34
mAbs to the N-terminal region of human or murine PSGL-1 block P- and L-selectin binding and abolish leukocyte rolling on L-selectin and P-selectin in vivo.7,10,35,36 L- and P- selectin bind to the same or closely overlapping sites near the N-terminus of PSGL-1, whereas E-selectin appears to bind to at least one more site.15 In vivo, PSGL-1-deficient leukocytes have markedly impaired tethering to and rolling on P-selectin.37 They tether less well to E-selectin, but those that tether roll with normal velocities.8,38-40 These observations confirmed that PSGL-1 is the predominant ligand for P-selectin. They also demonstrated that PSGL-1, albeit an important E-selectin ligand, must cooperate with other physiologic ligands for E-selectin, as we discuss in the following 2 sections. In the absence of an inflammatory or infectious challenge, the phenotype of PSGL-1-deficient (Selplg−/−) mice is remarkably mild.8,37
CD44
CD44 is a class I transmembrane glycoprotein that is expressed on most vertebrate cells, including hematopoietic stem cells, monocytes, neutrophils, lymphocytes, and endothelial cells. CD44 is involved in many cellular processes, including growth, survival, differentiation, and motility. A glycoform of CD44 isolated from human, but not murine, hematopoietic progenitors binds to L- and E-selectin in vitro. This glycoform has been termed hematopoietic cell E-/L-selectin ligand.41,42 Whether hematopoietic cell E-/L-selectin ligand functions as a selectin ligand on intact primary cells in vitro or in vivo has not been established. CD44 on neutrophils and some lymphocytes is a physiologic E-selectin ligand, suggesting cell-specific posttranslational modifications of CD44.39,43 The binding activity of neutrophil-derived CD44 requires its decoration by sialylated, α1,3-fucosylated, N-linked glycans.39 Altered glycosylation of CD44 proteins may account for pathologic conditions. For example, CD44 is hypofucosylated in neutrophils from patients with leukocyte adhesion deficiency type II syndrome.39
CD44, although encoded by a single gene, has more than 40 isoforms.44 The heterogeneity results from posttranslational modifications, such as sulfation and glycosylation as well as alternative splicing. Cells can simultaneously express multiple CD44 isoforms. The expression profile of the isoforms is dependent on the type of tissue and differentiation stage.45,46 The “standard” form of CD44 is composed of an extracellular amino-terminal globular protein domain, a stem structure, a transmembrane region, and a cytoplasmic tail. Hematopoietic cells express this standard form (Figure 1B), but glycosylation of the standard form varies as cells differentiate.
The N-terminal globular domain of CD44 has motifs that function as docking sites for several components of the extracellular matrix (eg, hyaluronan, collagen, laminin, fibronectin, and glycosaminoglycans).47,48 Binding of hyaluronan by CD44 is tightly regulated by posttranslational modifications.49-51 Physiologic stimuli can alter these modifications, resulting in the induction of hyaluronan binding.50,52
The stem structure (46 amino acids) links the amino-terminal globular domain to the transmembrane domain, which consists of 23 hydrophobic amino acids and a cysteine residue. The transmembrane domain may be responsible for the association of CD44 proteins with lipid rafts.53 Although the cytoplasmic tail of CD44 has no intrinsic catalytic activity, it interacts with several intracellular signaling molecules, including Src family kinases (SFKs), Rho GTPase, Rho kinase, and protein kinase C.54 It is not known whether these interactions are direct or indirect or whether they have functional impact in leukocytes.
CD44-deficient mice develop normally but have altered immune responses.55 CD44 has hyaluronan-dependent and -independent functions. Intravital microscopy has documented CD44-dependent rolling of T-cell subsets on hyaluronan in vivo.56-58 In vivo experiments also demonstrated that CD44 and hyaluronan are required for T-cell recruitment into the inflamed peritoneal cavity.59 CD44 and hyaluronan may enhance neutrophil recruitment to sites of inflammation.60-62 However, neutrophils do not tether to and roll on hyaluronan,61,62 and this agrees with the normal recruitment of neutrophils to the inflamed peritoneal cavity of Cd44−/− mice.39 In contrast, sequestration of neutrophils within liver sinusoids has been shown to be CD44- and hyaluronan-dependent.62 CD44-deficient neutrophils show reduced adhesion to the inflamed endothelium and subsequently increased rolling flux60,63 and increased rolling velocities,39 suggesting that CD44 is important for adhesion and/or sequestration.
T-lymphocytes require CD44 for integrin α4β1-mediated firm adhesion to the endothelium; this function requires the cytoplasmic tail of CD44.64,65 CD44-dependent rolling of T-helper (Th1) and Th2 CD4 lymphocytes has been observed in a mouse model of tumor necrosis factor-α–induced inflammation.56 CD44 extracted from Th1 lymphocytes binds to soluble E-selectin in vitro and cooperates with PSGL-1 in vivo by controlling rolling velocities and promoting firm arrest.43 Competitive recruitment assays demonstrated that T cells lacking both CD44 and PSGL-1 have more severe defects in migration to inflamed sites than T cells lacking only PSGL-1.43
ESL-1
Deleting PSGL-1 and CD44 in murine neutrophils strongly reduces but does not eliminate rolling on E-selectin in vitro or in vivo.39 A third key glycoprotein ligand for E-selectin on murine neutrophils is E-selectin ligand-1 (ESL-1). ESL-1 (also called MGF-160 or CFR-1, encoded by the gene Glg1) is a type I transmembrane protein. It consists of 1148 amino acids with 16 conserved cysteine-rich repeats and 5 potential N-glycosylation sites in the extracellular domain, a 21-residue transmembrane domain, and a short 13-residue cytoplasmic tail66,67 (Figure 1B). Although ESL-1 primarily localizes in the Golgi apparatus, a minor portion of ESL-1 is also exported to the plasma membrane, perhaps because of differential processing of the C-terminal domain.68,69 ESL-1 is expressed in many cell types,70 but selectin-binding activity has only been demonstrated in myeloid cells and human metastatic prostate cancer cells.71-73 Available antibodies to ESL-1 do not detect the protein on leukocyte surfaces by flow cytometry. Biotinylation strategies have demonstrated surface expression of ESL-1 on murine neutrophils69 and lymphocytes (A.H., unpublished data, January 2007), but not on human neutrophils and lymphocytes (D. Vestweber, oral communication, Max-Planck Institute for Molecular Medicine, Münster, Germany, August 2011). Although we focus here on its role as a selectin ligand, ESL-1 may exert pleiotropic effects. It functions as a receptor for several members of the fibroblast growth factor family,70,74 and it modulates intracellular processing and secretion of TGF-β.75 Consequently, mice deficient in ESL-1 show growth retardation and skeletal dysplasia.70,75
The first evidence that ESL-1 could bind E-selectin was obtained by applying myeloid cell lysates to E-selectin affinity columns.73 ESL-1 requires appropriate modifications of N-glycans to bind to E-selectin,67,72,73 whereas O-glycosylation of the glycoprotein has not been described. As with all other selectin ligands, α1,3 fucosylation of ESL-1 is required for interactions with E-selectin. Neutrophils appear to use fucosyltransferase IV to modify ESL-1 and fucosyltransferase VII to modify PSGL-1.76 Although biochemical and cell-based interactions of ESL-1 with E-selectin were documented many years ago,67 a physiologic role for ESL-1 in mediating rolling of murine leukocytes on E-selectin under flow was only recently documented.
Knockdown of ESL-1 by a short hairpin RNA strategy demonstrated that binding of a recombinant soluble form of E-selectin is mildly reduced when ESL-1 alone is absent but is abrogated when both PSGL-1 and ESL-1 are absent.38 Intravital studies of leukocyte rolling in venules of inflamed tissues further showed that leukocyte tethering requires the combined presence of PSGL-1 and ESL-1, where PSGL-1 interacts with both P- and E-selectin and ESL-1 interacts only with E-selectin.
Selectin ligand-mediated signaling
Binding of selectins to their ligands on leukocytes induces the activation of different signaling pathways (Figure 1B). Neutrophils rolling on P-selectin partially activate integrin αLβ2, also known as lymphocyte-associated antigen-1 (LFA-1), which slows their rolling velocities by enhancing transient LFA-1 binding to intercellular cell adhesion molecule-1 (ICAM-1). E- or P-selectin binding induces LFA-1 extension in a Syk-dependent manner.77 The cytoplasmic tail of PSGL-1 is required for LFA-1 activation and slower rolling on ICAM-1.78 However, deleting the cytoplasmic tail of PSGL-1 does not change the rolling of neutrophils on P-selectin or its localization in microvilli, lipid rafts, and uropods.78
In transfected cells, PSGL-1 interacts with the p85 subunit of PI3K in the presence of Naf-1.33 Under nonflow conditions, stimulation of human neutrophils with a soluble P-selectin-Fc chimeric protein induces SFK-dependent phosphorylation of Naf-1, which recruits the phosphoinositide-3-OH kinase p85-p110δ (PI3Kδ) heterodimer and leads to leukocyte integrin activation.33 These conditions may occur as leukocytes adhere to activated platelets, which express P-selectin at high densities, but are less likely to occur as leukocytes roll on P-selectin expressed on activated endothelial cells. Indeed, murine neutrophils lacking PI3Kδ exhibit normal LFA-1-dependent slow rolling on P-selectin and ICAM-1.40
Three studies using flow chambers have shown that PSGL-1 participates in E-selectin–mediated slow rolling of murine neutrophils.40,78,79 In one study using unfractionated murine blood, PSGL-1-deficient neutrophils did not roll slower on E-selectin and ICAM-1 than on E-selectin alone. In contrast, CD44-deficient neutrophils rolled much slower on E-selectin and ICAM-1 than on E-selectin alone, although they rolled slightly faster than wild-type neutrophils.79 In another study using isolated murine leukocytes, reduced rolling velocity was abolished only in neutrophils that lacked both PSGL-1 and CD44.40 In flow chamber experiments using whole human blood, blocking the N-terminal P-selectin–binding site on PSGL-1 with monovalent Fab fragments of mAb PL1 was sufficient to prevent slow rolling on E-selectin and ICAM-1, suggesting that PSGL-1 engagement is necessary for effective signaling in human neutrophils.77 This surprising result implies that the N-terminal region of PSGL-1 must engage E-selectin to trigger signaling because PSGL-1 has more than one binding site for E-selectin and PL1 does not completely block binding of PSGL-1 to E-selectin.80 Four in vivo studies failed to find a rolling velocity difference between murine wild-type and PSGL-1–deficient neutrophils.10,38-40 However, in these experiments, rolling behavior of knockout and wild-type cells could not be compared in the same microvessels. Natural variations in wall shear stress, vessel diameter, and flow velocity may introduce some experimental noise that can make small differences difficult to detect in vivo.79 On the other hand, intravenous injection of a blocking mAb to β2 integrins significantly increased rolling velocities in wild-type mice81 or in mice lacking PSGL-1 or CD44,40 suggesting that either PSGL-1 or CD44 is sufficient to trigger slow rolling in vivo. At present, technical differences probably account for these apparent discrepancies, and the relative roles of PSGL-1 and CD44 in E-selectin–triggered signaling require further study. To date, no direct physical interaction of PSGL-1 or CD44 with downstream signaling molecules has been demonstrated.
E-selectin ligand engagement on neutrophils induces signals that partially activate LFA-1, which mediates slow rolling on ICAM-1.77,79,82 E-selectin–mediated signaling requires intact lipid rafts on neutrophils40 and an intact cytoplasmic domain of PSGL-1.40 E-selectin binding induces the phosphorylation of the SFKs Fgr, Hck, and Lyn40,82 and of the ITAM-containing adaptor proteins DAP12 and FcRγ, which subsequently interact with the tyrosine kinase Syk.82 PSGL-140,77,79 or CD4440 and the activation of Syk79 are required for E-selectin–mediated slow rolling (Figure 1B). DAP12 and Syk phosphorylation is absent in neutrophils from Fgr−/− mice and Lyn−/−/Hck−/− mice after E-selectin engagement.40,82 Likewise, elimination of both ITAM-containing adaptor proteins, DAP12 and FcRγ, abolishes Syk phosphorylation and slow rolling.40,82 The Tec family kinase Bruton tyrosine kinase acts downstream of Syk40,83 and regulates 2 pathways: one requires phospholipase C-γ2; the other may require PI3K-γ,83 although another study did not observe this requirement.40 Because the rolling velocity defect in PI3Kγ-deficient neutrophils is small, it may fall below the limit of detection in some assays. The small GTPase Rap1 is activated after E-selectin engagement, and blocking Rap1a in Pik3cg−/− mice by a dominant-negative TAT-fusion mutant completely abolishes E-selectin–mediated slow rolling.84 CalDAG-GEFI (gene name Rasgrp2) and p38 MAPK are key signaling intermediates between phospholipase C-γ2 and Rap1a. Interestingly, the extension of LFA-1 induced by E-selectin binding is only partially dependent on CalDAG-GEFI, whereas chemokine-triggered LFA-1 activation is completely defective in Rasgrp2−/− mice.84
ESL-1 also appears to contribute to the pro-adhesive action of E-selectin38,85 and probably cooperates with CD44 for this function as suggested by the observation that combined deficiency in both ligands results in elevated rolling flux fractions at the expense of reduced firm adhesion.38 These experiments also showed that slow leukocyte rolling requires ESL-1 to an extent similar to that described for CD44.38,39 Although there is evidence that signaling is defective when ESL-1 is not expressed,85 the precise function of ESL-1 in controlling slow rolling remains unknown. The rolling phenotype of ESL-1–deficient (as opposed to knocked-down, where silencing may be not fully specific) leukocytes has not been described. An interesting particularity of ESL-1 is its role in maintaining steady rolling kinetics by allowing continuous contact with the inflamed endothelium.38 Overall, these studies suggest that ESL-1 is a versatile ligand, capable of cooperating with PSGL-1 to mediate tethering on E-selectin while also contributing to steady rolling on endothelial cells once the leukocyte has tethered. Why ESL-1 is endowed with these functional properties is not well understood. One possibility is that its topologic distribution underlies this versatility: it is homogeneously expressed on the microvilli surface,69 whereas PSGL-1 expression is mostly restricted to the tips of these structures13 and CD44 is expressed in the planar cell body86 (Figure 1A).
Besides controlling rolling velocities, E-selectin ligand engagement also induces redistribution or “capping” of adhesion molecules on the cell surface of neutrophils.38,87 Interestingly, this effect appears to be exclusively mediated by CD44 and involves activation of p38 MAPK.38,87 Why receptor translation along the membrane is controlled by CD44 but not PSGL-1 is unknown. There is also evidence that engagement of PSGL-1 by P-selectin or E-selectin can trigger proliferative and differentiation signals in hematopoietic cells, including hematopoietic progenitors88 and dendritic cells,89 with functional consequences during inflammation.90 The signaling pathways controlling these processes have not been described.
Little is known about the possible pathophysiologic roles of ESL-1, but a potential contribution to vascular occlusion in sickle cell disease has been recently described.85 In a murine model of sickle cell disease after challenge with tumor necrosis factor-α, interactions between sickle-shaped erythrocytes and neutrophils generate intravascular cell aggregates that trigger the vaso-occlusive episodes characteristic of patients with this disease.85,91 In this murine model, ESL-1 transduces signals that activate integrin αMβ2 (also known as Mac-1) on neutrophils (Figure 1B), thus favoring interactions with circulating erythrocytes and promoting vaso-occlusion.85 PSGL-1 and CD44 do not contribute significantly to integrin activation in this model, which suggests ligand-specific signaling pathways. Indirect evidence with chemical inhibitors in vivo suggested that SFKs, but not p38 MAPK or Syk, are required for integrin activation downstream of ESL-1.85 Thus, although ESL-1 shares the SFK signaling pathway with PSGL-1 and CD44 to modulate integrin activation,33,40,82 it appears to have its own activating functions. One possible explanation is that each ligand has a temporally restricted signaling function: PSGL-1 and CD44 predominating during early (ie, tethering and rolling) phases of recruitment and ESL-1 at later stages (ie, during the crawling phase), but this hypothesis needs to be experimentally tested. How ESL-1 initiates signaling events on leukocytes also requires further study. Because ESL-1 is important for the processing and signaling of various growth factors,70,75 caution is needed to discriminate between effects that may be purely selectin-triggered and those related to other physiologic inputs.
Additional ligands for E-selectin
E-selectin can bind to multiple glycoconjugates. Loss of PSGL-1, CD44, and ESL-1 in murine neutrophils virtually eliminates rolling on E-selectin, suggesting that these 3 glycoproteins compose all physiologically relevant ligands for E-selectin on these cells.38 However, loss of core 1-derived O-glycans in murine neutrophils also virtually eliminates rolling on E-selectin, even though CD44 and ESL-1 from these cells (which require N-glycans to bind to E-selectin) still bind to E-selectin in biochemical assays.26 One possible explanation for the discrepant results is that neutrophils express at least one more glycoprotein ligand for E-selectin that requires specific O-glycosylation. In the absence of this putative ligand, the N-glycans on CD44 and ESL-1 are insufficient to support rolling. In the absence of PSGL-1, CD44, and ESL-1, the O-glycans on this putative ligand are also insufficient to support rolling. Another possibility is that loss of core 1 O-glycans indirectly impairs the functions of CD44 and ESL-1 by altering their cell-surface distributions or by other mechanisms. A third possibility is that loss of the other biologic functions of ESL-1 affects neutrophil properties that indirectly impair rolling on E-selectin.
There is experimental evidence that a different combination of glycoproteins, including PSGL-1, CD44, and CD43, functions in murine inflammatory T cells.43,92-94 Furthermore, human neutrophils may use L-selectin and glycolipids to mediate E-selectin binding95,96 (Table 1). In vitro, E-selectin binds to human CD66/carcinoembryonic antigen,97 integrin Mac-1,98 podocalyxin-like protein, or melanoma cell adhesion molecule,99 which may be relevant in specific cellular contexts. Thus, the identification of the full repertoire of ligands for E-selectin is still a matter of debate and active research. We briefly summarize additional glycoconjugates on hematopoietic cells with stronger evidence as bona fide ligands for E-selectin.
CD43
The high level of expression on leukocytes, extensive glycosylation, and molecular length of CD43 (also known as leukosialin) were proposed to favor both pro-adhesive or anti-adhesive roles.100,101 Biochemical and in vitro cellular studies as well as in vivo data support a role for CD43 as an E-selectin ligand on human and murine inflammatory T cells (Th1 type)93,102 but not on neutrophils.26,103 Delayed-type hypersensitivity models have been used to demonstrate the physiologic contribution of CD43 to skin inflammation during T cell–dependent responses. In all cases, ablation of PSGL-1 was required to unmask a role for CD43.92,94 Like PSGL-1, CD43 localizes to microvilli,13,104 but it is not known whether both receptors cooperate in mediating T-cell tethering. These findings, together with the observation that CD43 expression on murine neutrophils is not sufficient to support tethering, rolling, or recruitment in the absence of PSGL-1, CD44, and ESL-1,38 and the differential contributions of CD44 on murine T cells and neutrophils,43 are consistent with the proposal that lymphoid and myeloid cells use a different repertoire of selectin ligands.105
L-selectin
This selectin is exclusively expressed on leukocytes, where it directs the migration of naive and central memory T cells to lymph nodes through recognition of glycoproteins expressed on high endothelial venules.106 L-selectin–deficient mice exhibit impaired leukocyte recruitment to sites of inflammation,107 which may reflect the inability of L-selectin–deficient neutrophils to bind to adherent neutrophils and neutrophil fragments.10 Furthermore, L-selectin is expressed on the tips of microvilli and, on human neutrophils, is decorated with N-glycans capped with sLex.95,108 L-selectin from human (but not mouse) neutrophils binds to E-selectin affinity columns and supports the rolling of E-selectin–transfected cells96 (Table 1). Antibodies that recognize the lectin domain of human L-selectin partially inhibit in vitro neutrophil rolling on E-selectin,95,96 but this was later explained by inhibition of secondary neutrophil-neutrophil tethering.109-111 In vivo, the reduced recruitment of leukocytes in L-selectin–deficient mice appears to be the result of loss of secondary tethers between circulating leukocytes and those already attached to the endothelium, which are mediated by interactions between L-selectin and PSGL-1.10
Glycolipids
E-selectin binds to sialylated and fucosylated lactosylceramides extracted from human neutrophils,112 and immobilized lipids modified with sLex or sLea mediate tethering and rolling of E-selectin–expressing cells under flow.113 These early findings, performed mostly using human samples, conflict with the recent description that a limited array of glycoproteins accounts for the full repertoire of E-selectin ligand activity on mouse neutrophils.38 Given reported differences in the structure and function of selectin ligands between mouse and human neutrophils,6,96 it is conceivable that glycolipids play a more prominent role as E-selectin ligands in human neutrophils (Table 1). In agreement with this, sialylated glycosphingolipids containing several terminal repeats of N-acetyl-lactosamine with 2 or 3 fucose residues have been purified from human neutrophils. These glycosphingolipids support tethering and rolling of E-selectin–expressing cells at densities similar to those found on intact cells.114 Inhibition of glycosphingolipid synthesis on neutrophils partially abrogates E-selectin binding,114 although this finding could be explained by indirect effects through membrane stiffening.115 It has been proposed that the high density of glycolipids on the cell membrane compensates for their reduced accessibility compared with extended glycoproteins presented on microvilli.113 Thus, glycolipid-mediated interactions may be particularly important during the slow rolling phase, when the cell's body is in close proximity to the endothelial membrane. Notwithstanding these observations, the physiologic relevance of glycolipids for tethering and rolling of human neutrophils or other leukocyte subsets on E-selectin awaits definitive confirmation.
Future directions
More than 2 decades after the initial description of selectins, the complete repertoire of physiologic ligands that interact with endothelial selectins remains to be elucidated. The precise nature of all ligands, their contribution to leukocyte rolling and signaling, and the possible interspecies differences (Table 1) remain to be identified. At the same time, because the majority of research on selectin ligands has focused on myeloid cell lines and neutrophils, it will be important to establish whether the same repertoire of ligands functions in other leukocyte subsets, including inflammatory T cells, hematopoietic progenitors, or leukemic cells. Differences in the use of ligands among these cell types could be exploited to interfere with the extravasation of damaging subsets (eg, self-reactive lymphocytes, pro-atherogenic monocytes, or leukemic clones) without compromising homeostatic host defense.
From a mechanistic standpoint, signaling initiated by engagement of various selectin ligands is now well established. However, the complete sequence of events leading from selectin engagement of PSGL-1 and CD44 at the cell surface to integrin activation needs to be fully characterized (Figure 1B). A number of signaling intermediaries have been identified, but the potential contributions of Ca2+, diacylglycerol, protein kinase C, or PI3Kγ remain to be defined. It will also be important to define the exact signaling mechanisms by which ESL-1 contributes to leukocyte recruitment. The continuous advances in this field are rapidly reshaping our perception of selectin ligands as specialized signal transducers in immune cells; this perception should open new therapeutic avenues for the treatment of vascular and immune disorders.
Acknowledgments
The authors thank Dr A. Urzainqui for helpful comments on the manuscript.
This work was supported by the Interdisciplinary Clinical Research Center (IZKF, Münster, Germany) and the German Research Foundation (A.Z.), the National Institutes of Health (K.L. and R.P.M.), a Ramón y Cajal fellowship, the Spanish Ministry of Science and Innovation, and the FP7-People-IRG Program (A.H.). The Centro Nacional de Investigaciones Cardiovasculares is supported by the Spanish Ministry of Science and Innovation and the Pro-CNIC Foundation.
National Institutes of Health
Authorship
Contribution: All authors wrote and edited the manuscript and designed the figures.
Conflict-of-interest disclosure: R.P.M. has interest in Selexys, a company that is developing inhibitors of selectins and selectin ligands. The remaining authors declare no competing financial interests.
Correspondence: Andrés Hidalgo, Department of Epidemiology, Atherothrombosis and Imaging, Centro Nacional de Investigaciones Cardiovasculares, Melchor Fernandez Almagro 3, Madrid 28039, Spain; e-mail: ahidalgo@cnic.es.