Abstract
Transient myeloproliferative disorder (TMD), restricted to newborns with trisomy 21, is a megakaryocytic leukemia that although lethal in some is distinguished by its spontaneous resolution. Later development of acute myeloid leukemia (AML) occurs in some. Prospective enrollment (n = 135) elucidated the natural history in Down syndrome (DS) patients diagnosed with TMD via the use of uniform monitoring and intervention guidelines. Prevalent at diagnosis were leukocytosis, peripheral blast exceeding marrow blast percentage, and hepatomegaly. Among those with life-threatening symptoms, most (n = 29/38; 76%) received intervention therapy until symptoms abated and then were monitored similarly. Organomegaly with cardiopulmonary compromise most frequently led to intervention (43%). Death occurred in 21% but only 10% were attributable to TMD (intervention vs observation patients: 13/14 vs 1/15 because of TMD). Among those solely observed, peripheral blasts and all other TMD symptoms cleared at a median of 36 and 49 days from diagnosis, respectively. On the basis of the diagnostic clinical findings of hepatomegaly with or without life-threatening symptoms, 3 groups were identified with differing survival: low risk with neither finding (38%), intermediate risk with hepatomegaly alone (40%), and high risk with both (21%; overall survival: 92% ± 8%, 77% ± 12%, and 51% ± 19%, respectively; P ≤ .001). Among all, AML subsequently occurred in 16% at a median of 441 days (range, 118-1085 days). The trial is registered at http://www.clinicaltrials.gov as NCT00003593.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 6996.
Disclosures
The authors; Associate Editor Crystal L. Mackall; and the CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe clinical characteristics and presenting features of TMD among newborns with trisomy 21, based on the prospective COG study
Describe outcomes in patients with TMD, based on the prospective COG study
Describe clinical findings that can be used to predict prognosis in patients with TMD, based on the prospective COG study.
Release date: December 22, 2011; Expiration date: December 22, 2012
Introduction
Neonates with Down syndrome (DS) have a unique predilection to develop transient myeloproliferative disorder (TMD), a rare clonal myeloproliferation1,2 characterized by peripheral leukocytosis indistinguishable at presentation from acute megakaryocytic leukemia, FAB M7, or acute myeloid leukemia (AML) with minimal differentiation, FAB M0. Its predilection for DS neonates coupled with its unique characteristics of a relative paucity of leukemic blasts within the marrow, variable pancytopenia, a propensity for mild to life-threatening hepatic infiltration, and typically a spontaneous regression without any intervention help to clinically distinguish this entity.3
Between 4% and 10% of newborn infants with DS are thought to develop TMD.4-6 Until recently, most attempts to describe this unique leukemia have come from single institutions or surveys.5-8 Derived from these early reports and more recently registries was that in addition to its typical spontaneous regression within 3-7 months of life9 without intervention,10 it appeared to have a highly fatal form, and among those who survived there was up to a 20%-30% risk of subsequent leukemia.11,12
The Children's Oncology Group (COG) A2971 study, reported here, is the largest study to date designed to define the natural history of clinically diagnosed TMD via the use of prospectively uniform observation and treatment guidelines. To achieve this goal, this study for the first time identified infants with TMD by the use of uniform (1) clinical diagnostic criteria, (2) intervention guidelines where needed, and (3) monitoring guidelines after resolution of the TMD for physicians to follow. This trial, although not a population prevalence study, sought to better describe the breadth of clinical TMD presentations, and among those clinically diagnosed with TMD (ie, without the use of GATA1 mutation analysis), its natural course toward spontaneous remission, its complication and case-fatality rates, and the subsequent risk of acute leukemia later in early childhood. DS children who later developed AML and those who developed AML without a known history of previous TMD were treated on a separate arm of this study and are reported separately.
Methods
Eligible patients (n = 135) with TMD were enrolled between 1999 and 2004 from participating COG institutions (n = 115) with institutional research board approvals for COG A2971. The study was conducted in accordance with the Declaration of Helsinki. The study protocol was reviewed by the ethics committee or institutional review board at each participating center. All patients' parents provided written informed consent, according to institutional regulations, before enrollment and the administration of chemotherapy.
Broad eligibility criteria were used within this study to capture the full spectrum of clinical TMD. Children were eligible if found to have trisomy 21 as a constitutional finding, a mosaic distribution, or a finding limited to the hematopoietic or leukemic cells. Diagnosis and eligibility were made if they were < 3 months of age at presentation with any nonerythroid blasts in the peripheral blood coupled with any of the 5 following criteria: verification of blasts with a second sample, > 5% nonerythroid BM blasts, hepatomegaly or splenomegaly, lymphadenopathy, or cardiac or pleural effusions. Nonstandardized institutional complete blood counts were used, and correction for nucleated RBC was not defined in this trial. Organomegaly or adenopathy were determined by the clinician's physical examination and did not have strict criteria to make these diagnoses. DS infants without peripheral blasts were also eligible if they had biopsy-proven or cytology-proven blasts in an affected organ, or in sampled fluid (pericardial, pleural, or peritoneal). A marrow aspirate was advised but not required at the time of enrollment and depended on the patient's condition and family's consent. This trial began before reports of an association between GATA1 and TMD and did not collect banked leukemia samples to determine mutation prevalence in this trial.
Most patients were referred to a COG center and seen while still with TMD, although a small percentage of patients (1.5%) were enrolled after the TMD had resolved. Patients were enrolled within 14 days of diagnosis by a hematologist or within 72 hours of starting chemotherapy if used.
Patients were seen at specified frequencies until the resolution of TMD, as well as subsequently to the time to development of AML, or for a period of 5 years to determine leukemia-free survival (LFS; see supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Patients were enrolled to either the observation or intervention arms of the study on the basis of the severity of presenting signs and symptoms. Most patients (72%) had no life-threatening symptoms (LTS) at any time and were observed. LTS, the sole criteria for intervention, were prospectively defined by one or more of the following: signs of hyperviscosity, blast count > 100,000/μL, hepatosplenomegaly causing respiratory compromise, heart failure (ejection fraction < 47% or shortening fraction < 27%) not directly the result of a congenital heart defect, hydrops fetalis, renal or hepatic dysfunction, or disseminated intravascular coagulation (DIC) with bleeding. Criteria without a specific exceeded value were determined by the judgment of the physician. The goal of intervention was to reduce symptoms to tolerable levels (defined by the treating physician) because spontaneous resolution was expected.
Because of high white blood cell (WBC) counts, most patients with LTS (n = 29/38) were treated with exchange transfusion or leukapheresis (ExT/L) or chemotherapy consisting of continuous infusion cytarabine at 3.33 mg/kg/24 hours for 5 days. Patients with significant organ compromise were treated with chemotherapy that could be repeated every 14 days after count recovery for no more than 3 courses. ExT/L had no minimum interval between procedures. Patients whose LTS did not resolve after these interventions and before TMD remission were to be transferred to the AML arm of this study (n = 0). Once LTS resolved with intervention no longer required, patients were seen at set frequencies similar to those not requiring intervention (supplemental Table 1). Patients initially observed were permitted to later receive intervention as needed if they were < 90 days of age (n = 2).
Complete remission (CR) of TMD was defined as resolution of peripheral blasts, evidence of trilineage recovery, disappearance of effusions, and resolution of organomegaly (OM) on 2 consecutive occasions > 7 days apart. Hematologic CR was assessed to adjust for the hepatic fibrosis known to persist after peripheral blast resolution and count recovery in many patients and did not require the resolution of hepatomegaly. Marrow remission determination was not required for either definition. Patients in CR > 8 weeks, who were older than 90 days, and who had ≥ 30% marrow blasts (on the basis of French-American-British AML criteria) were to be diagnosed with AML and treated appropriately for DS AML. Patients who were > 90 days and who had ≥ 5% marrow blasts in the presence of myelodysplasia were to be diagnosed to have myelodysplastic syndromes (MDS) and treated similarly to those diagnosed with AML.
Statistical analysis
At the time of this report, the study was current as of July 7, 2008. The significance of observed differences in proportions was tested by use of the χ2 and Fisher exact tests when data were sparse. The Mann-Whitney U test was used to determine the significance between differences in medians. The Kaplan-Meier method was used to calculate estimates of overall survival (OS), event-free survival (EFS), and TMD-related mortality. Estimates are reported with their Greenwood standard errors. Differences in these estimates were tested for significance using the log-rank statistic.13 OS was defined as time from study entry until death. EFS was defined as time to TMD recurrence, death, or development of either AML, MDS, or acute lymphoblastic leukemia (ALL). TMD-related mortality was defined as time to death related to TMD where deaths unrelated to TMD and patients who developed AML/ALL/MDS were censored.
Modified event-free survival (mEFS) and LFS were estimated by methods of competing risks.14 Modified EFS was defined similarly to the aforementioned definition of EFS except that patients having non-TMD–related deaths were classified as competing events. LFS is defined as time from resolution to development of AML/ALL/MDS, where patients who died before development were considered competing events. Gray's P value was used to compare groups of patients for modified EFS and LFS analyses. Children lost to follow-up were censored at their date of last known contact or at a cutoff 6 months before July 7, 2008. We also used Cox regression models for univariate and multivariate analyses, comparing differences between characteristic groups defined by WBC count, BM blasts, platelets, hepatomegaly, splenomegaly, or other characteristics taken from the time of diagnosis.15
Results
Patient characteristics
There were 139 patients enrolled from 1999 to 2004, of whom 4 were ineligible (either receiving treatment and not having a marrow examination performed before enrollment, having AML and not TMD, or having an incomplete registration), which left 135 analyzed patients with a median follow-up of 1153 days (range, 0-2857 days) from diagnosis. Presenting characteristics and intervention assignment are listed in Table 1 and the supplemental Table 2. Most presented within the first week of life. Trisomy 21 was confirmed in all eligible DS patients, with mosaicism for trisomy 21 found in 16% of these. Most were full-term (67%), although those in need of intervention were more frequently premature (50%).
Selected TMD patient characteristics
| . | All TMD patients . | TMD: observation only . | TMD: intervention . | Observation vs intervention, P . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | Median (range) . | n . | % . | Median (range) . | n . | % . | Median (range) . | ||
| Total enrolled | 139 | |||||||||
| No. ineligible | 4 | 3 | ||||||||
| No. eligible | 135 | 97 | 106 | 78.5 | 29 | 21.5 | ||||
| Treatment arm at time of registration | ||||||||||
| Observation | 108 | 80.0 | 106 | 100 | 2 | 6.9 | – | |||
| Received TMD intervention later | 2 | 1.9 | – | |||||||
| Leukopheresis/exchange transfusion | 5 | 3.7 | 5 | 17.2 | – | |||||
| Chemotherapy (Ara-C) | 22 | 16.3 | 22 | 75.9 | – | |||||
| Age at diagnosis, d | 5 (0-58) | 6 (0-52) | 2 (0-58) | .061 | ||||||
| Age at study entry, d | 13 (1-66) | 14 (2-66) | 8 (1-61) | .004 | ||||||
| Follow-up time, d | 1153 (0-2857) | 1151 (0-2857) | 1196 (42-1923) | .978 | ||||||
| Time to development of AML/MDS, d | 21 | 16 | 441 (118-1085) | 17 | 16 | 444 (118-1085) | 4 | 14 | 280 (202-596) | .244 |
| Constitutional trisomy 21 | 113 | 83.7 | 87 | 82.1 | 26 | 89.7 | .407 | |||
| Trisomy 21 mosaicism | 22 | 16.3 | 19 | 17.9 | 3 | 10.3 | ||||
| Hepatomegaly | ||||||||||
| Not enlarged | 57 | 42.2 | 53 | 50.0 | 4 | 13.8 | < .001 | |||
| Enlarged but not below umbilcus | 63 | 46.7 | 49 | 46.2 | 14 | 48.3 | .845 | |||
| Below umbilicus | 15 | 11.1 | 4 | 3.8 | 11 | 37.9 | < .001 | |||
| Liver dysfunction (symptomatic) | ||||||||||
| Yes | 17 | 12.7 | 5 | 4.7 | 12 | 42.9 | < .001 | |||
| No | 117 | 87.3 | 101 | 95.3 | 16 | 57.1 | ||||
| Unknown | 1 | 0 | 1 | |||||||
| Organomegaly (liver or spleen) with respiratory compromise | ||||||||||
| Yes | 13 | 9.7 | 1 | 0.9 | 12 | 42.9 | < .001 | |||
| No | 121 | 90.3 | 105 | 99.1 | 16 | 57.1 | ||||
| Unknown | 1 | 0 | 1 | |||||||
| Diagnostic laboratory examination | ||||||||||
| WBC (× 103/μL) | 32.8 (4.8-259) | 26.9 (4.8-253) | 74.9 (9.4-259) | < .001 | ||||||
| A total blast count of ≥ 100,000/μL | ||||||||||
| Yes | 16 | 11.9 | 5 | 4.7 | 11 | 37.9 | < .001 | |||
| No | 119 | 88.1 | 101 | 95.3 | 18 | 62.1 | ||||
| Blasts in peripheral blood, % | 25 (0-92) | 22 (0-87) | 56 (0-92) | .002 | ||||||
| BM blasts, % | 15 (0-95) | 14 (0-78) | 27 (1-95) | .029 | ||||||
| HGB, g/dL | 14.8 (5-22.5) | 15.6 (5.0-22.5) | 11.9 (5.9-20.5) | < .001 | ||||||
| Platelet count (× 103/μL) | 125 (10-958) | 112 (10-958) | 182 (36-670) | .033 | ||||||
| . | All TMD patients . | TMD: observation only . | TMD: intervention . | Observation vs intervention, P . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | Median (range) . | n . | % . | Median (range) . | n . | % . | Median (range) . | ||
| Total enrolled | 139 | |||||||||
| No. ineligible | 4 | 3 | ||||||||
| No. eligible | 135 | 97 | 106 | 78.5 | 29 | 21.5 | ||||
| Treatment arm at time of registration | ||||||||||
| Observation | 108 | 80.0 | 106 | 100 | 2 | 6.9 | – | |||
| Received TMD intervention later | 2 | 1.9 | – | |||||||
| Leukopheresis/exchange transfusion | 5 | 3.7 | 5 | 17.2 | – | |||||
| Chemotherapy (Ara-C) | 22 | 16.3 | 22 | 75.9 | – | |||||
| Age at diagnosis, d | 5 (0-58) | 6 (0-52) | 2 (0-58) | .061 | ||||||
| Age at study entry, d | 13 (1-66) | 14 (2-66) | 8 (1-61) | .004 | ||||||
| Follow-up time, d | 1153 (0-2857) | 1151 (0-2857) | 1196 (42-1923) | .978 | ||||||
| Time to development of AML/MDS, d | 21 | 16 | 441 (118-1085) | 17 | 16 | 444 (118-1085) | 4 | 14 | 280 (202-596) | .244 |
| Constitutional trisomy 21 | 113 | 83.7 | 87 | 82.1 | 26 | 89.7 | .407 | |||
| Trisomy 21 mosaicism | 22 | 16.3 | 19 | 17.9 | 3 | 10.3 | ||||
| Hepatomegaly | ||||||||||
| Not enlarged | 57 | 42.2 | 53 | 50.0 | 4 | 13.8 | < .001 | |||
| Enlarged but not below umbilcus | 63 | 46.7 | 49 | 46.2 | 14 | 48.3 | .845 | |||
| Below umbilicus | 15 | 11.1 | 4 | 3.8 | 11 | 37.9 | < .001 | |||
| Liver dysfunction (symptomatic) | ||||||||||
| Yes | 17 | 12.7 | 5 | 4.7 | 12 | 42.9 | < .001 | |||
| No | 117 | 87.3 | 101 | 95.3 | 16 | 57.1 | ||||
| Unknown | 1 | 0 | 1 | |||||||
| Organomegaly (liver or spleen) with respiratory compromise | ||||||||||
| Yes | 13 | 9.7 | 1 | 0.9 | 12 | 42.9 | < .001 | |||
| No | 121 | 90.3 | 105 | 99.1 | 16 | 57.1 | ||||
| Unknown | 1 | 0 | 1 | |||||||
| Diagnostic laboratory examination | ||||||||||
| WBC (× 103/μL) | 32.8 (4.8-259) | 26.9 (4.8-253) | 74.9 (9.4-259) | < .001 | ||||||
| A total blast count of ≥ 100,000/μL | ||||||||||
| Yes | 16 | 11.9 | 5 | 4.7 | 11 | 37.9 | < .001 | |||
| No | 119 | 88.1 | 101 | 95.3 | 18 | 62.1 | ||||
| Blasts in peripheral blood, % | 25 (0-92) | 22 (0-87) | 56 (0-92) | .002 | ||||||
| BM blasts, % | 15 (0-95) | 14 (0-78) | 27 (1-95) | .029 | ||||||
| HGB, g/dL | 14.8 (5-22.5) | 15.6 (5.0-22.5) | 11.9 (5.9-20.5) | < .001 | ||||||
| Platelet count (× 103/μL) | 125 (10-958) | 112 (10-958) | 182 (36-670) | .033 | ||||||
AML indicates acute myeloid leukemia; HGB, hemoglobin; MDS, myelodysplastic syndromes; TMD, transient myeloproliferative disorder; and WBC, white blood cell.
Congenital anomalies other than trisomy 21 phenotypic stigmata were found in 68% of children. Congenital cardiac or gastrointestinal abnormalities (including duodenal atresia) were seen most often, 57% and 10%, respectively, with no significant difference between those needing intervention and those not. Median WBC at diagnosis was elevated, whereas hemoglobin was generally normal and platelets were only slightly reduced (7/135 were > 150 000/μL). Peripheral blast percentage equaled or exceeded the marrow blast percentage in 69% of patients with marrow examinations (74/107). Sixteen (12%) children had an initial WBC > 100 000/μL; however, only 11 received treatment. Hepatomegaly was the most prevalent symptom of organ infiltration present in 58%.
Pericardial effusions and congestive heart failure (CHF) were more likely to occur in those with underlying cardiac defects versus those without cardiac defects (effusion: 16% vs. 2%; CHF: 8% vs 2%). Patients were assigned by their clinicians to observation (n = 106) or intervention (n = 29) arms on the basis of the aforementioned prospectively designated clinical severity criteria detailed. Among the 108 initially observed, 2 later had LTS and required intervention, and 9 others had at least one LTS at diagnosis but were not treated (5 because of resolving symptoms at enrollment, 2 where the clinician chose not to intervene, 1 where the LTS were thought to be because of coexisting biliary atresia, and 1 in which AML therapy was used rather than TMD; see “Outcomes and prognostic factors”).
Patients requiring intervention
Those with LTS (n = 38) and in whom the treating physician elected to pursue intervention (n = 29) constituted 22% of the TMD patients. Patients required intervention because of hyperviscosity (11%), blast count > 100 000/μL (25%), OM with respiratory compromise (43%), CHF (11%), hydrops fetalis (21%), liver dysfunction (43%), renal dysfunction (14%), or DIC (25%). All patients with symptoms of hyperviscosity, CHF not caused by a congenital heart defect, and renal dysfunction did go on to an intervention. Among the other symptoms that could trigger intervention, 69% (11/16) with WBC > 100 000/μL, 92% (12/13) with respiratory compromise because of OM, 75% (6/8) with hydrops fetalis, 71% (12/17) with hepatic dysfunction, and 88% (7/8) with DIC received therapy.
Those receiving intervention for symptoms that define life-threatening disease were significantly younger at diagnosis, more likely to be premature, and found with a lower hemoglobin count at diagnosis. Mosaic DS children were as likely to require intervention as fully constitutional DS children (14% vs 23%, P = .407). Among the intervention patients, most had complete trisomy of chromosome 21 (n = 26) rather than mosaic trisomy 21 (n = 3). Nine (31%) received ExT/L, and 24 (83%) received low-dose cytarabine for OM, organ dysfunction causing respiratory compromise, or continued symptoms after ExT/L. Four children required subsequent cytarabine after initial management with ExT/L. Two additional patients at 5 and 39 days after diagnosis (9 and 54 days of life, respectively) required transfer from the observation arm to the intervention arm when LTS first arose after diagnosis (ExT/L, n = 1; ExT/L and cytarabine, n = 1), both of whom survived the episode and required no further therapy for their TMD.
Among patients receiving ExT/L (n = 10), the TMD was controlled sufficiently in 2 patients to avoid further intervention; 2 died after receiving 1 course of intervention. Six required further intervention for severe TMD symptoms: 1 received a second course (ExT/L), and 5 subsequently were given cytarabine. Among those 24 receiving cytarabine, only 1 received an additional course for ongoing LTS as determined by the treating physician. Among the 24 patients initially requiring cytarabine, 96% had at least one grade 3 or 4 toxicity, the most frequent of which was myelosuppression (anemia, 38% [n = 9], leucopenia, 58% [n = 14], or thrombocytopenia, 83% [n = 20]). Ultimately, 15 were able to be followed without further intervention, whereas 13 succumbed to their TMD or treatment-related complications, and 1 died because of complications unrelated to their TMD. No patient transferred to the other arm of the trial for traditional AML therapy to specifically further treat their TMD. Several ultimately were treated for later occurrences of AML and are discussed in “Subsequent AML/MSD risk.”
Patients not requiring intervention
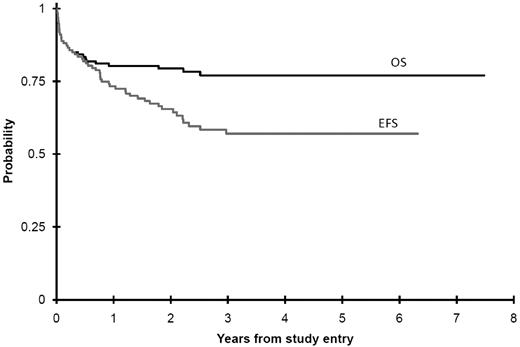
Of 108 observation arm patients, 106 achieved a spontaneous remission, and 2 were transferred to the intervention arm because of delayed LTS occurrence (described previously). Median time to TMD resolution from diagnosis was 49 days (range, 5-745 days; Figure 1; supplemental Table 3). Peripheral blast resolution was attained in a median of 36 days (range, 2-126 days). The difference, particularly in those in whom resolution of TMD was quite prolonged (> 180 days, n = 7), was entirely because of prolonged hepatomegaly. The time for peripheral blast resolution was decreased among those receiving intervention, whereas there was no impact on resolution of OM with intervention.
Time to TMD resolution from diagnosis for all patients enrolled.
Time to TMD resolution from diagnosis for all patients enrolled.
Outcomes and prognostic factors
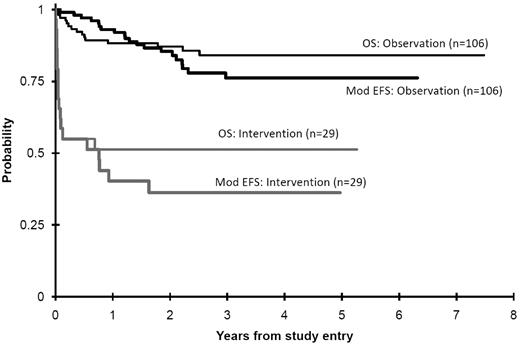
Among all 135 patients (Table 2), the 3-year OS was 77% ± 8% (± 2 SD), EFS was 57% ± 10%, and mEFS was 68% ± 9% (Figures 2 and 3). Twenty-nine deaths were recorded (14 related, 14 unrelated to TMD or therapy, 1 unknown). There was an overall case-fatality rate (death because of TMD) of 10%, whereas total mortality was 21%. Of 14 deaths in the intervention arm patients, 13 were determined to be TMD related. Of 15 deaths in the observation arm patients, only 1 patient had unresolved TMD at the time of death. This patient was initially observed despite a white count of 135 000, and when progressive hepatic dysfunction developed, the physician chose to treat off study with this trial's AML therapy. This infant died because of neutropenic Staphylococcus epidermidis sepsis complicated by massive ascites and hepatic dysfunction. As such, there was a significantly greater case-fatality rate in the intervention group (13/29, 45%) compared with those not requiring intervention (1/106, 1%).
TMD outcomes
| . | All patients . | Observation patients . | Intervention patients . | Observation vs intervention, P . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | 3-year % . | ± 2 SE% . | n . | 3-year % . | ± 2 SE% . | n . | 3-year % . | ± 2 SE% . | ||
| OS from study entry | 135 | 77 | 8 | 106 | 84 | 8 | 29 | 51 | 19 | < .001 |
| EFS from study entry | 135 | 57 | 10 | 106 | 64 | 11 | 29 | 33 | 18 | < .001 |
| mEFS from study entry | 135 | 68 | 9 | 106 | 76 | 10 | 29 | 36 | 18 | < .001 |
| LFS (from resolution) for patients whose TMD resolved | 107 | 80 | 9 | 92 | 78 | 10 | 15 | 71 | 25 | .327 |
| . | All patients . | Observation patients . | Intervention patients . | Observation vs intervention, P . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | 3-year % . | ± 2 SE% . | n . | 3-year % . | ± 2 SE% . | n . | 3-year % . | ± 2 SE% . | ||
| OS from study entry | 135 | 77 | 8 | 106 | 84 | 8 | 29 | 51 | 19 | < .001 |
| EFS from study entry | 135 | 57 | 10 | 106 | 64 | 11 | 29 | 33 | 18 | < .001 |
| mEFS from study entry | 135 | 68 | 9 | 106 | 76 | 10 | 29 | 36 | 18 | < .001 |
| LFS (from resolution) for patients whose TMD resolved | 107 | 80 | 9 | 92 | 78 | 10 | 15 | 71 | 25 | .327 |
EFS indicates event-free survival; LFS, leukemia-free survival; mEFS, modified event-free survival; and TMD, transient myeloproliferative disorder
OS and mEFS (Mod EFS) from study entry comparing observation to intervention patients. Deaths that are not TMD related are competing events in modified EFS (1 − cumulative incidence).
OS and mEFS (Mod EFS) from study entry comparing observation to intervention patients. Deaths that are not TMD related are competing events in modified EFS (1 − cumulative incidence).
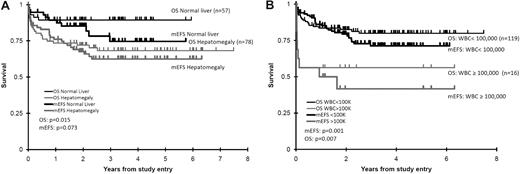
Examination of clinical characteristics at the time of TMD diagnosis were evaluated by univariate Cox analysis for their impact on OS, EFS, and mEFS (supplemental Table 4). For OS, hepatomegaly (hazard ratio [HR] 3.06, P = .015), hyperleukocytosis (defined as > 100 000 WBC/μL; HR 3.25, P = .007), and black (and nonwhite) race (HR 4.36, P = .003 for black vs not black and HR 2.46, P = .024 for white vs not white) were associated with increased overall mortality. Hepatomegaly (Figure 4A) and hyperleukocytosis (Figure 4B) among these 3 factors had a significant or near-significant impact on mEFS (survival until the time of TMD remission), Finally, when we considered all events before and after TMD resolution, we found that EFS was significantly impacted by all 3 risk factors. These risk factors for these survival analyses (OS, EFS, and mEFS) maintained or trended toward significance in multivariate modeling (supplemental Table 5). Platelet count, peripheral compared with marrow blast percentage, gestational age, splenomegaly, cardiac lesion, congenital anomaly or Trisomy 21 mosaicism, did not impact survival in univariate Cox analyses.
OS and modified EFS (mEFS) based on diagnostic hepatomegaly and WBC values. (A) Diagnostic hepatomegaly. (B) WBC values. These illustrate the univariate impact upon OS and modified EFS of these 2 risk factors.
OS and modified EFS (mEFS) based on diagnostic hepatomegaly and WBC values. (A) Diagnostic hepatomegaly. (B) WBC values. These illustrate the univariate impact upon OS and modified EFS of these 2 risk factors.
As patients with severe TMD were guided to intervention, a further comparison of symptoms and signs between the patients receiving intervention who died and those who did not was pursued. Only age at diagnosis (median 1 day of age among those dying vs 6 days of age among surviving, P = .013) and the presence of renal dysfunction at diagnosis (found in 31% of those who later died vs 0% among those who survived, P = .035) reached statistical significance for risk of mortality. WBC > 100 000/μL (39% vs 13%, P = .198), elevated ALT (77% vs 40%, P = .067), and congenital heart disease (100% vs 71%, P = .137) were more prevalent among those patients who died but did not achieve statistical significance. Whereas black race was a univariate prognostic factor among all causes of mortality, when restricted to the intervention patients of whom all but 2 died of TMD-related causes, it was no longer prognostic (P = .583). Also gestational age, CHF, OM causing respiratory compromise, median WBC, or bilirubin or hepatic dysfunction failed to exhibit differences between those who died and lived after or during intervention.
Categorizing survival on the basis of hepatomegaly and/or LTS presence
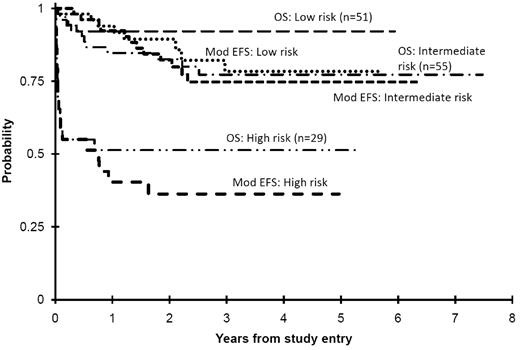
A survival risk classification was developed to identify children in need of intervention at diagnosis, particularly because of the high prevalence of hepatomegaly. Dividing patients into 3 risk groups (Table 3) resulted in classifying TMD patients as “low risk” if they had no evidence of LTS or hepatomegaly and thus no need for intervention (n = 51, 38%); “intermediate risk” if they had any degree of hepatomegaly with or without hepatic dysfunction but not determined to be severe enough to require intervention (n = 55, 41%); and “high risk” if they presented with LTS from cardiorespiratory compromise, hepatic dysfunction, or hyperleukocytosis requiring intervention (n = 29, 21%). The OS for low-, intermediate-, and high-risk groups was 92% ± 8%, 77% ± 12%, and 51% ± 19%, and mEFS (Figure 5) was 78% ± 14%, 75% ± 13%, and 36% ± 18%, respectively (P ≤ .001 for low- vs high-risk and intermediate- vs high-risk groups for OS and mEFS). The 3-year LFS among those who attained TMD CR was 79% ± 15%, 77% ± 14%, and 71% ± 25%, respectively.
Outcome by risk groups
| . | Risk group . | Log-rank P value . | ||||
|---|---|---|---|---|---|---|
| Low . | Intermediate . | High . | Low vs intermediate . | Low vs high . | Intermediate vs high . | |
| n | 51 | 55 | 29 | |||
| Event-free survival, year 3 estimate* | 72.4% ± 15% | 56.5% ± 15% | 32.6% ± 18% | .103 | < .001 | < .001 |
| Overall survival, year 3 estimate | 92.1% ± 8% | 77.2% ± 12% | 51.3% ± 19% | .089 | < .001 | .001 |
| TMD-related mortality, year 3 estimate | 2% ± 4% | 0% ± 0% | 45.0% ± 18% | .303 | < .001 | < .001 |
| . | Risk group . | Log-rank P value . | ||||
|---|---|---|---|---|---|---|
| Low . | Intermediate . | High . | Low vs intermediate . | Low vs high . | Intermediate vs high . | |
| n | 51 | 55 | 29 | |||
| Event-free survival, year 3 estimate* | 72.4% ± 15% | 56.5% ± 15% | 32.6% ± 18% | .103 | < .001 | < .001 |
| Overall survival, year 3 estimate | 92.1% ± 8% | 77.2% ± 12% | 51.3% ± 19% | .089 | < .001 | .001 |
| TMD-related mortality, year 3 estimate | 2% ± 4% | 0% ± 0% | 45.0% ± 18% | .303 | < .001 | < .001 |
TMD indicates transient myeloproliferative disorder.
The development of AML is considered an event.
OS and modified EFS (Mod EFS) for the 3 risk groups of TMD patients. This illustrates that the intermediate risk group, that is, those with hepatomegaly alone, had minimal TMD-related problems but did have significant mortality from other causes.
OS and modified EFS (Mod EFS) for the 3 risk groups of TMD patients. This illustrates that the intermediate risk group, that is, those with hepatomegaly alone, had minimal TMD-related problems but did have significant mortality from other causes.
Subsequent AML/MDS risk
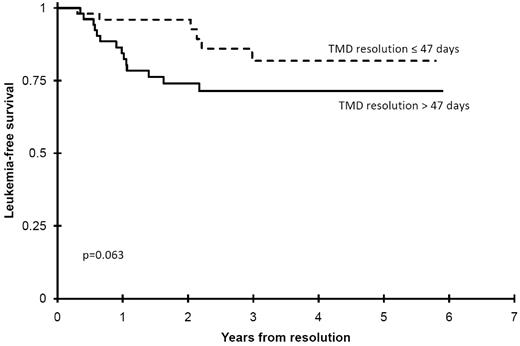
Once TMD had resolved, patients were systematically seen at a set frequency to monitor for the recurrence of TMD as well as later acute leukemia. To date, 21 patients (16%), including 4 previous intervention patients of whom 3 received cytarabine, have developed AML/MDS at a median time of 441 days (range, 118-1085 days; Table 1). There was no significant difference in incidence of subsequent AML between those receiving cytarabine (3/24, 13%) vs those not (18/111, 16%; P = .766). Among all clinical factors at diagnosis or during the clinical course, only time to TMD resolution approached a significant correlation with the risk of later development of AML. Dividing the patients into 2 groups depending on whether their TMD resolved sooner or later than the median (47 days from diagnosis) found that those with TMD resolution shorter or equal to the median time had a 3 years LFS of 82% ± 14% compared with 71% ± 13% (P = .063) for those with longer resolution times (Figure 6). In addition, 2 patients developed acute lymphoblastic leukemia at 727 and 851 days of life.
Impact of TMD resolution upon later risk of developing AML. This illustrates that those children whose resolution of their TMD exceeded the median resolution time were at greater risk of later developing AML.
Impact of TMD resolution upon later risk of developing AML. This illustrates that those children whose resolution of their TMD exceeded the median resolution time were at greater risk of later developing AML.
Discussion
In this prospective trial for children with DS clinically diagnosed with TMD we have been able to ascertain the natural history of a heretofore enigmatic disorder that, despite its well-known and unique characteristic of spontaneous resolution, was also considered to have a relatively high risk of mortality before it resolved.11,16 In addition, on the basis of this mortality risk, this trial provided guidelines for intervention and used a moderately low dose of cytarabine to control the myeloproliferation, if life-threatening, until spontaneous regression began. By the establishment of these prospective monitoring and intervention guidelines, a natural history among those clinically diagnosed with TMD with less confounding variability has been elucidated.
In previous case series,9,17 TMD was found to have a high case fatality rate, despite the generally accepted approach of supportive care until the disease's spontaneous resolution. This has led to significant confusion and unease among clinicians as to the proper route of action when a patient with TMD presents. In this trial, 78% of children with TMD had mild symptoms and spontaneous resolution of their disease without intervention, similar to a recent report from the BFM (84%).17 This ranged from those who only had transient blasts in the peripheral blood (31%) to those with mild OM, such as hepatomegaly (58%), abnormal liver function studies (41%), and splenomegaly (36%), similar to other recent reports.11,17
In comparison, those patients who presented in moribund condition or with LTS were likely to lead to mortality if intervention is not implemented. Organ infiltration, primarily hepatic, may be severe, progressive, and fatal.9,18,19 Hayashi et al found 10 of 15 TMD patients died within the first few months of DIC, hepatic failure, or renal failure.9 Zipursky et al identified 7 of 13 severe TMD patients in the literature died, of whom 5 were stillborn, and 2 died later.17 Hydrops fetalis was a predominant symptom in these patients, with prominent blast infiltration of the heart and liver found at autopsy with associated fibrosis, whereas none of these findings were found in the marrow.17 Three of the 4 reviewed patients died within 24 hours of birth.
Subsequently, Al-Kasim et al further described the central role of hepatic involvement in those with a fatal outcome.18 Nine of 48 patients enrolled had life-threatening disease, 7 with hepatic fibrosis and 2 with cardiorespiratory failure. Without intervention all died, whereas 3 children in whom short courses of low-dose cytarabine were administered survived. More recently, Klusmann et al reported the BFM registry experience, which identified that high-risk patients (high WBC, prematurity, ascites, and failure of TMD resolution) had an improved outcome if intervention was given (72% vs 24%, P = .001).16 Intervention clearly appears to have a role in supporting these children through a critical period of their disease.
As seen in the previous 2 reports, where hepatic fibrosis was pathologically diagnosed, all have died. Whether hepatomegaly alone is a criterion for intervention is a frequently asked question among clinicians. It was a prominent feature in this (58%) and other trials and is presumably the site of TMD origin.11,17 However, it alone does not portend a high mortality risk because it was equally present in both those who required intervention or did not require (48% and 46%, respectively). In those in whom LTS were present, as predefined by the protocol for intervention, massive hepatomegaly (ie, below the umbilicus) was more often present and presumed to be one of the primary causes of these symptoms. This association with other LTS was found in 24% of enrolled patients. In this intervention group, massive hepatic enlargement was observed in 38% compared with only 4% in the cohort who did not meet the threshold for intervention. In this trial, hepatic complications of TMD were apparent in all children at the time of death who died of TMD-related causes except one, who died of hydrops-induced multiorgan failure.
Because of this wide range of presentations and their incumbent mortality risks, it is important to better ascertain who may require intervention before spontaneous resolution. This study proposes a mortality risk-based classification system to stratify the treatment of TMD patients. We identified 3 distinct TMD risk groups. High-risk patients have early evidence of LTS and a TMD-associated mortality rate of 55% at 3 years. Most infants with TMD (41%) belong to the intermediate-risk group, specifically those with hepatomegaly although without LTS. These infants rarely die from acute complications of TMD; however, they have a 23% 3-year mortality rate. Deaths were because of congenital heart defects (n = 5), SIDS-like events (n = 2), tracheal stenosis (n = 1), subsequent AML and relapse (n = 1), and for unknown reasons outside the country (n = 1). This is double the overall mortality rate of the low risk group and is disproportionately greater than the mortality rates of DS infants without TMD.19-22 The rest (38%) are the classic “low-risk” patients in whom the disease spontaneously resolves without therapeutic intervention. Low-risk patients have less than a 2.3% chance of dying from acute complications of TMD.
Overall mortality in our population was 21.5%, which is consistent with reports of 16%-23% in the literature.11,18 However, TMD-related mortality was 11% because most deaths were non-TMD related in this cohort of children known to have a significant incidence of other life-threatening congenital anomalies. For those with LTS, there is a high case-fatality rate (45%).
The most appropriate dose, administration, and scheduling of low-dose cytarabine for TMD patients is unknown. In this study, those with LTS were treated with continuous infusion cytarabine (CI cytarabine) at 3.33 mg/kg/24 hours over the course of 5 days, similar to the protocol's AML induction dose. Virtually all (23/24, 96%) cytarabine-treated patients experienced grade 3/4 myelosuppression. All deaths among those requiring cytarabine were related to TMD and its sequelae, although prolonged neutropenia may have contributed to the poor outcome of those who died of infectious complications. Only one of the 24 infants who required cytarabine received more than one course. Despite additional intervention this infant succumbed from TMD-associated complications. This suggests that lower doses of cytarabine with less significant hematologic toxicity need to be investigated. The use of lower subcutaneous cytarabine dosing (0.5-1.5 mg/kg) for 3-12 days has been reported.11,17,19
A better understanding of the natural history of clinically diagnosed TMD is derived from this trial and provides a clearer understanding as clinicians compare their own patients to the full spectrum of TMD patients. All patients resolved their TMD by 745 days with a median time to resolution of 47 days (range, 5-745 days). Peripheral blasts cleared at an earlier median of 33 days (range, 2-126 days) than hepatomegaly resolution (45.5 days; range, 0-745 days).
Although time to complete resolution is of interest, the greatest concerns are 2-fold. Which patients may still develop LTS in whom intervention is needed, and which may truly be a child with AML that does not spontaneously resolve? Only 2 patients initially believed not to have LTS subsequently required intervention. The reasons for both were increasing peripheral blast counts that eventually exceeded 100 000. Intervention occurred on days 5 and 39 from diagnosis (or 9 and 54 days from birth), respectively. Thus, beyond 39 days from diagnosis, no patient subsequently required intervention.
Because intervention may have confounded the determination of who did have true AML, one must examine other characteristics (other than age) believed to distinguish AML from TMD in the DS population. As has been recognized previously, hepatic involvement has been more often seen in TMD than in AML patients in whom marrow involvement is more prevalent. Examination in those in whom a marrow examination was performed at the time of their TMD diagnosis showed 31% (33/107) had more blasts in the marrow than in the periphery. Within the observation group who did not receive intervention, this degree of marrow involvement was not associated with failure to eventually resolve their TMD nor was it associated with later development of AML (3-year OS, marrow blasts > PB blasts, 79% ± 13% vs 79% ± 11%, P = .875; 3-year EFS, 57% ± 17% vs 63% ± 13% respectively, P = .711).
Later development of AML is known to occur in TMD patients. Zipursky et al,8,23 first in a literature review and later in a survey, identified that 26%-30% went on to develop leukemia in the first 3 years of life. More recently, the POG and the BFM cooperative groups identified 19%-23% who later developed acute leukemia, primarily myeloid.11,17 To date, 21 patients among the 135 enrolled (16%), including 4 treated with cytarabine, developed AML/MDS at a median time of 441 days (range, 118-1085 days). Among those who achieved TMD remission, the 3-year cumulative incidence of subsequent AML was 20% ± 9% from remission, and among those surviving to age 6 months, 21/111 (18.9%) later developed AML. Examining risk factors for later AML development, only the time to resolution was predictive. When the median time to resolution of 47 days from diagnosis for the entire cohort is used, those that took < 47 days to TMD resolution had a better 3-year LFS (82% vs 71%). Can the early use of chemotherapy in TMD prevent later occurrence of AML? This was not found to be beneficial in our group because 4 of 24 (17%) cytarabine recipients later developed AML, comparable with 14 of 111 (13%) who did not receive chemotherapy for TMD (P = .527). This was similarly found in the BFM study (27% and 23% [P = .46], respectively).17
This study is not a population-based study, and thus, the incidence of TMD within DS patients cannot be directly ascertained. There is likely a group of children with DS with milder manifestations of TMD that goes undiagnosed before its spontaneous resolution. Analysis of 590 Guthrie cards from infants with DS born in New York State revealed a GATA1 mutation incidence of 3.8% (22/585 evaluable).24 Nevertheless, this study sought to examine the full range of patients with TMD who are diagnosed either because of TMD-related symptoms or serendipitously during other examinations in the newborn and infant period. As such, this trial prospectively defined TMD to include all potential patients as long as there were verified blasts on 2 separate examinations or symptoms or signs of TMD in addition to the identification of blasts on a single laboratory examination. Because this trial was designed before the discovery of the GATA1 association and did not determine the presence of this mutation (nor were there banked specimens to retrospectively determine this), it is possible that some of the most mild presentations of TMD in our cohort may have simply represented a leukemoid reaction. However, only 8 of the patients in whom this might be considered were found with the most minimal of symptoms (eg, transient peripheral blasts alone that cleared within 7 days). Nevertheless, in this way, this study likely represents the full spectrum of TMD manifestations even though an incidence cannot be derived from this analysis.
This portion of the COG trial, A2971, further clarifies the natural history of clinically diagnosed TMD, identifies risk groups for survival and development of subsequent AML, and provides baseline comparisons for upcoming COG and other trials of TMD. A current COG trial, AAML08B1, is focusing on the biologic characteristics of TMD, including molecular mutations such as GATA-1 known to occur in the majority if not all TMD patients, the correlates with acute morbidity, particularly fibrosis, as well as the biologic analyses in each patient who both has TMD and later develops AML.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Presented in abstract form at the 48th annual meeting of the American Society of Hematology, Orlando, FL, December 11, 2006.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the COG institutional principal investigators as well as the numerous clinical research associates, nurses, and physicians who oversaw the care and study performance for these patients. The authors are indebted to the families and children who consented to participate in this clinical trial.
This work was supported by grants from the National Institutes of Health (U10 CA98543 and U10 CA98413).
National Institutes of Health
Authorship
Contribution: A.S.G. designed and performed research, analyzed and interpreted data, and wrote the manuscript; T.A.A. designed and performed research, analyzed and interpreted data, performed statistical analysis, and edited the manuscript; R.B.G. analyzed and interpreted data, performed statistical analysis, and edited the manuscript; J.M.H. and F.O.S. designed and performed research and edited the manuscript; A.D.S. performed research and edited the manuscript; M.K. analyzed and interpreted data and edited the manuscript; and T.W.L., R.J.A., D.B., J.D., G.M., J.P., Y.R., and J.T. performed research and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan S. Gamis, MD, MPH, Children's Mercy Hospitals & Clinics, 2401 Gillham Rd, Kansas City, MO 64108; e-mail: agamis@cmh.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal