Abstract
Previous studies have revealed various extrinsic stimuli and factors involved in the regulation of hematopoiesis. Among these, Notch-mediated signaling has been suggested to be critically involved in this process. Herein, we show that conditional inactivation of ADAM10, a membrane-bound protease with a crucial role in Notch signaling (S2 cleavage), results in myeloproliferative disorder (MPD) highlighted by severe splenomegaly and increased populations of myeloid cells and hematopoietic stem cells. Reciprocal transfer of bone marrow cells between wild-type and ADAM10 mutant mice revealed that ADAM10 activity in both hematopoietic and nonhematopoietic cells is involved in the development of MPD. Notably, we found that MPD caused by lack of ADAM10 in nonhematopoietic cells was mediated by G-CSF, whereas MPD caused by ADAM10-deficient hematopoietic cells was not. Taken together, the present findings reveal previously undescribed nonredundant roles of cell-autonomous and non–cell-autonomous ADAM10 activity in the maintenance of hematopoiesis.
Introduction
Notch signaling plays crucial roles in cell fate decision in diverse tissues.1 Previous studies have shown the potential involvement of Notch receptors and their ligands in the maintenance of hematopoietic stem cells (HSCs) in bone marrow (BM).2,3 However, despite intensive studies in the past decade, the physiologic relevance of Notch signaling in HSC maintenance remains controversial. Gain-of-function experiments both in vitro and in vivo suggest that Notch signaling in HSCs increases self-renewal and suppresses differentiation of HSCs.4-7 Conversely, others have shown that Notch signaling in HSCs is dispensable for their maintenance.8,9
The metalloprotease responsible for S2 cleavage of Notch also remains controversial. Initial studies identified ADAM17/TACE, a membrane-bound metalloprotease belonging to the ADAM gene family, as the sheddase for Notch.10 However, findings that Adam17 mutant mice did not exhibit any Notch-related developmental defects raised questions about the relevance of ADAM17 for Notch processing in vivo.11,12 On the other hand, emerging evidence suggests that another family member, ADAM10, is the major protease responsible for Notch processing.13-16
To clarify these issues, we generated conditional ADAM10-deficient mice and found that conditional inactivation of ADAM10 by the inducible Mx1-Cre transgene results in myeloproliferative disorder (MPD), which resembles that observed in Notch signaling–defective mutant mice.17-20 Reciprocal BM transplantation experiments revealed that lack of ADAM10 in either hematopoietic cells or the host environment leads to the MPD phenotype. Furthermore, we found that MPD caused by lack of ADAM10 in the host environment, but not in hematopoietic cells, is dependent on G-CSF. Thus, the present study reveals previously undescribed nonredundant roles of cell-autonomous and non–cell-autonomous ADAM10 activity in the regulation of hematopoiesis.
Methods
A detailed description of the gene targeting and generation of Adam10flox/flox mice is provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Adam10flox/flox mice exhibited no apparent defects (data not shown) and were used as control animals for the experiments (henceforth referred to as control mice). Adam10flox/flox mice were crossed with Mx1-Cre transgenic mice21 to generate inducible conditional ADAM10-knockout mice (Adam10flox/flox/Mx1-Cre+, henceforth referred to as Adam10/Mx1 mice). Conditional excision of the floxed allele was achieved by intraperitoneal injection of polyinosinic:polycytidylic acid. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Keio University School of Medicine. All other experimental methods are described in supplemental Methods.
Images of the sections were acquired with ACT-1 Version 2.62 software (Nikon) via a digital camera (DXM1200; Nikon) mounted on a BX50 microscope (Olympus). The objectives used were 4×/0.16, 10×/0.40, and 20×/0.70 UPlanApo (Olympus). Images were processed with Adobe Photoshop CS4 (Adobe Systems).
Results and discussion
Conditional inactivation of ADAM10 results in MPD
To elucidate the roles of ADAM10 in hematopoiesis and its potential involvement in Notch signaling, we generated a mutant line (Adam10/Mx1) in which temporal and systemic deletion of Adam10 was achieved by the Mx1-Cre transgene.21 Notably, Adam10/Mx1 mice developed severe splenomegaly characterized by expansion of the red pulp (Figure 1A-B, Ci-iv). Infiltration of myeloid cells was also observed in the liver of Adam10/Mx1 mice (Figure 1Cv-vi). The metatarsal BM, which is usually replaced by fat cells in adult mice, was filled with hematopoietic cells (Figure 1Cvii-viii). The complete blood counts revealed decreases in hemoglobulin and hematocrit and an increase in the number of white blood cells (Figure 1D). Flow cytometric analysis of peripheral blood cells showed a relative increase in neutrophils (CD11b+ Ly6Cint), as well as a decrease in a T-cell population (CD3e+; Figure 1E).
Conditional inactivation of ADAM10 results in MPD. (A) Gross morphology of spleen from 8-week-old control and Adam10/Mx1 mice injected with polyinosinic:polycytidylic acid at 4 weeks before euthanasia (scale bar represents 1 cm). (B) Spleen/body weight ratios and total cell counts in the spleen. Bars indicate mean ± SD (n = 5). (C) H&E staining of sections of the spleen (i-iv; bars represent 200 μm [i and ii] and 50 μm [iii and iv]), liver (v and vi; bar represents 100 μm), and metatarsi (vii and viii; bar represents 250 μm) from 12-week-old control and Adam10/Mx1 mice injected with polyinosinic:polycytidylic acid at 8 weeks before euthanasia. The spleen of Adam10/Mx1 mice shows an increase in the number of myeloid cells, as well as megakaryocytes (arrowheads) in the red pulp (i-iv). In addition, granulocytic cells are present in the liver of Adam10/Mx1 mice, which indicates extramedullary hematopoiesis (v and vi arrowheads). The metatarsi of the mutant mice are filled with hematopoietic cells, whereas the BM cavity of control animals is filled with adipose cells (vii and viii arrowheads). (D) Complete blood counts for control and Adam10/Mx1 mice. Bars indicate mean ± SD (n = 4); HCT, hematocrit; HGB, hemoglobin; RBC, red blood cells; WBC, white blood cells; and PLT, platelets. (E) Flow cytometric analysis of peripheral blood. Bars indicate mean ± SD (n = 4). (F) Flow cytometric analysis of CD3e+, and B220+ cells in the spleen and Gr-1+ CD11b+ cells in the BM from control and Adam10/Mx1 mice (boxed areas). (G) Absolute cell numbers of Gr-1+ CD11b+ cells in the BM and CD3e+ and Gr-1+ CD11b+ cells in the spleen. Bars indicate mean ± SD (n = 4). (H) Flow cytometric analysis of the KSL cell populations in the BM and spleen of control and Adam10/Mx1 mice. Boxed areas indicate KSL cells. (I) Absolute numbers of KSL cells in the BM and spleen. Bars indicate mean ± SD (n = 3). (J) Numbers of colony-forming units (CFUs) per 1 × 105 splenocytes and 1 × 104 BM cells, respectively. Bars indicate mean ± SD (n = 5). The flow cytometric analysis shown here is a representative result of 3 independent experiments. (K) Transcript levels of Hes1, Hey1, and Hey2 in the spleen of control and Adam10/Mx1 mice. t test: *P < .05, **P < .005.
Conditional inactivation of ADAM10 results in MPD. (A) Gross morphology of spleen from 8-week-old control and Adam10/Mx1 mice injected with polyinosinic:polycytidylic acid at 4 weeks before euthanasia (scale bar represents 1 cm). (B) Spleen/body weight ratios and total cell counts in the spleen. Bars indicate mean ± SD (n = 5). (C) H&E staining of sections of the spleen (i-iv; bars represent 200 μm [i and ii] and 50 μm [iii and iv]), liver (v and vi; bar represents 100 μm), and metatarsi (vii and viii; bar represents 250 μm) from 12-week-old control and Adam10/Mx1 mice injected with polyinosinic:polycytidylic acid at 8 weeks before euthanasia. The spleen of Adam10/Mx1 mice shows an increase in the number of myeloid cells, as well as megakaryocytes (arrowheads) in the red pulp (i-iv). In addition, granulocytic cells are present in the liver of Adam10/Mx1 mice, which indicates extramedullary hematopoiesis (v and vi arrowheads). The metatarsi of the mutant mice are filled with hematopoietic cells, whereas the BM cavity of control animals is filled with adipose cells (vii and viii arrowheads). (D) Complete blood counts for control and Adam10/Mx1 mice. Bars indicate mean ± SD (n = 4); HCT, hematocrit; HGB, hemoglobin; RBC, red blood cells; WBC, white blood cells; and PLT, platelets. (E) Flow cytometric analysis of peripheral blood. Bars indicate mean ± SD (n = 4). (F) Flow cytometric analysis of CD3e+, and B220+ cells in the spleen and Gr-1+ CD11b+ cells in the BM from control and Adam10/Mx1 mice (boxed areas). (G) Absolute cell numbers of Gr-1+ CD11b+ cells in the BM and CD3e+ and Gr-1+ CD11b+ cells in the spleen. Bars indicate mean ± SD (n = 4). (H) Flow cytometric analysis of the KSL cell populations in the BM and spleen of control and Adam10/Mx1 mice. Boxed areas indicate KSL cells. (I) Absolute numbers of KSL cells in the BM and spleen. Bars indicate mean ± SD (n = 3). (J) Numbers of colony-forming units (CFUs) per 1 × 105 splenocytes and 1 × 104 BM cells, respectively. Bars indicate mean ± SD (n = 5). The flow cytometric analysis shown here is a representative result of 3 independent experiments. (K) Transcript levels of Hes1, Hey1, and Hey2 in the spleen of control and Adam10/Mx1 mice. t test: *P < .05, **P < .005.
Flow cytometric analysis of splenocytes from Adam10/Mx1 mice revealed an increase in the population of Gr-1+CD11b+ myeloid cells (Figure 1F-G), whereas no significant difference was observed in the BM of the femur. There was also a significant increase in HSCs (c-Kit+Sca-1+Lin−; KSL cells22 ) in Adam10/Mx1 mice (Figure 1H-I). Consistent with these findings, there were increases in the numbers of colony-forming units in the BM and spleen of Adam10/Mx1 mice compared with control mice (Figure 1J). Taken together, these observations show that conditional inactivation of ADAM10 leads to MPD. In addition, we found that T-cell development was impaired at an early stage of differentiation (supplemental Figure 2), similar to the case for conditional Notch1–knock-out mice.23 In accordance with this, the transcript levels of the Notch target-genes (Hes1, Hey1, and Hey2) were significantly reduced in Adam10/Mx1 mice (Figure 1K).
Both cell-autonomous and non–cell-autonomous ADAM10 activity affects granulopoiesis
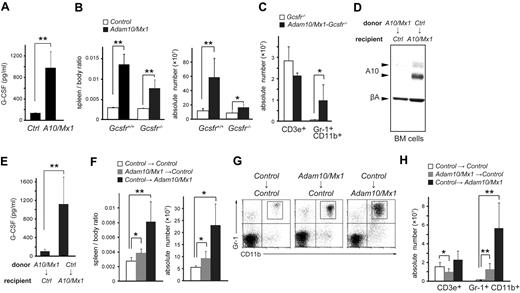
We next performed a multiplex analysis of serum cytokines using antibody arrays and found that several cytokines, including G-CSF, were up-regulated in Adam10/Mx1 mice (Figure 2A; supplemental Figure 3). Because G-CSF is a highly potent cytokine for stimulating granulopoiesis and progenitor cell proliferation, we hypothesized that aberrant production of G-CSF was the primary cause of MPD, as has been shown in different Notch signaling–defective mutant mice.19 To test this hypothesis, we mated Adam10/Mx1 mice with Gcsfr−/− mice24 to block G-CSF receptor signaling. Unexpectedly, although the degree of splenomegaly and increase in granulopoiesis were significantly lessened, Adam10/Mx1-Gcsfr−/− double-mutant mice still developed splenomegaly (Figure 2B-C), which indicates that MPD in Adam10/Mx1 mice was caused by G-CSF–dependent and G-CSF–independent mechanisms. We next performed reciprocal BM transfer experiments to determine the source of G-CSF. A nearly complete lack of ADAM10 expression in the BM cells in control recipient mice and positive ADAM10 expression in the BM cells in Adam10/Mx1 recipient mice were confirmed at the time of euthanasia (Figure 2D). The serum level of G-CSF was up-regulated in Adam10/Mx1 recipient mice reconstituted with control BM cells, whereas that in control mice reconstituted with Adam10/Mx1 BM cells remained unaffected (Figure 2E). These findings indicate that a lack of ADAM10 in the host environment, but not in hematopoietic cells, leads to aberrant production of G-CSF. Furthermore, we found that not only Adam10/Mx1 recipient mice reconstituted with control BM cells but also control recipient mice reconstituted with Adam10/Mx1 cells developed splenomegaly and MPD (Figure 2F-H). These observations suggest that a lack of ADAM10 activity in the host environment leads to aberrant G-CSF production and ultimately results in MPD, whereas MPD caused by ADAM10-defective hematopoietic cells is not dependent on G-CSF. Therefore, ADAM10 has both cell-autonomous and non–cell-autonomous functions in granulopoiesis.
Dimorphic effects of ADAM10 on granulopoiesis. (A) Serum G-CSF levels in control (Ctrl) and Adam10/Mx1 (A10/Mx1) mice evaluated by ELISA. Bars indicate mean ± SD (n = 5). (B) Spleen/body weight ratios and total cell counts in the spleen of control and Adam10/Mx1 mice in either Gcsf+/+ or Gcsfr−/− genetic background. Bars indicate mean ± SD (n = 4). (C) Numbers of CD3e+ cells and Gr-1+ CD11b+ splenocytes in Gcsfr−/− and Adam10/Mx1-Gcsfr−/− mice. Bars indicate mean ± SD (n = 5). (D) Western blot analysis of ADAM10 (A10) and β-actin (βA) in BM cells from control (Ctrl) recipient mice reconstituted with Adam10/Mx1 (A10/Mx1) BM cells and Adam10/Mx1 recipient mice reconstituted with control BM cells. (E) Serum G-CSF levels in control (Ctrl) recipient mice reconstituted with Adam10/Mx1 (A10/Mx1) BM cells and Adam10/Mx1 recipient mice reconstituted with control BM cells. (F) Spleen/body weight ratios and total cell counts in the spleen of control recipient mice reconstituted with control BM cells, control recipient mice reconstituted with Adam10/Mx1 BM cells, and Adam10/Mx1 recipient mice reconstituted with control BM cells. Bars indicate mean ± SD (n = 10). Flow cytometric analysis (G) and total cell counts (H) of Gr-1+ CD11b+ cells and CD3e+ cells in the spleen of control recipient mice reconstituted with control BM cells, control recipient mice reconstituted with Adam10/Mx1 BM cells, and Adam10/Mx1 recipient mice reconstituted with control BM cells. Note that there was also a decrease in a T-cell population (CD3e+ cells) in the spleen of control recipient mice reconstituted with Adam10/Mx1 cells (H), consistent with previous findings that the effect of Notch signaling in T-cell development is cell autonomous.23 Bars indicate mean ± SD (n = 10). The flow cytometric analysis shown here is a representative result of 3 independent experiments. t test: *P < .05, **P < .005.
Dimorphic effects of ADAM10 on granulopoiesis. (A) Serum G-CSF levels in control (Ctrl) and Adam10/Mx1 (A10/Mx1) mice evaluated by ELISA. Bars indicate mean ± SD (n = 5). (B) Spleen/body weight ratios and total cell counts in the spleen of control and Adam10/Mx1 mice in either Gcsf+/+ or Gcsfr−/− genetic background. Bars indicate mean ± SD (n = 4). (C) Numbers of CD3e+ cells and Gr-1+ CD11b+ splenocytes in Gcsfr−/− and Adam10/Mx1-Gcsfr−/− mice. Bars indicate mean ± SD (n = 5). (D) Western blot analysis of ADAM10 (A10) and β-actin (βA) in BM cells from control (Ctrl) recipient mice reconstituted with Adam10/Mx1 (A10/Mx1) BM cells and Adam10/Mx1 recipient mice reconstituted with control BM cells. (E) Serum G-CSF levels in control (Ctrl) recipient mice reconstituted with Adam10/Mx1 (A10/Mx1) BM cells and Adam10/Mx1 recipient mice reconstituted with control BM cells. (F) Spleen/body weight ratios and total cell counts in the spleen of control recipient mice reconstituted with control BM cells, control recipient mice reconstituted with Adam10/Mx1 BM cells, and Adam10/Mx1 recipient mice reconstituted with control BM cells. Bars indicate mean ± SD (n = 10). Flow cytometric analysis (G) and total cell counts (H) of Gr-1+ CD11b+ cells and CD3e+ cells in the spleen of control recipient mice reconstituted with control BM cells, control recipient mice reconstituted with Adam10/Mx1 BM cells, and Adam10/Mx1 recipient mice reconstituted with control BM cells. Note that there was also a decrease in a T-cell population (CD3e+ cells) in the spleen of control recipient mice reconstituted with Adam10/Mx1 cells (H), consistent with previous findings that the effect of Notch signaling in T-cell development is cell autonomous.23 Bars indicate mean ± SD (n = 10). The flow cytometric analysis shown here is a representative result of 3 independent experiments. t test: *P < .05, **P < .005.
It has been shown that several Notch signaling–defective mice17-20,25 develop a similar MPD phenotype as observed in the present study (supplemental Table 1). Although the targeted genes in these mutant lines are different in their participation in the Notch signaling cascade and also have functions outside Notch signaling, they share a common feature in the MPD phenotype: that it is primarily derived from a non–cell-autonomous mechanism and is potentially dependent on aberrant production of G-CSF.19 In this respect, a recent study showing that hematopoietic-specific inactivation of nicastrin, a component of the γ-secretase complex, results in a cell-autonomous, but not in non–cell-autonomous, MPD appears contradictory.25 This discrepancy could be attributed to various factors, including the differences in the functions of the targeted genes and the temporal and spatial differences in gene disruption among these mutant lines. Analysis and comparison of the serum cytokine profiles (especially those involved in hematopoiesis, including G-CSF) among these mutant mice should provide a clue as to whether these Notch signaling–defective mice share a common mechanism behind the MPD phenotype.
In conclusion, the present study further supports ADAM10 as the relevant sheddase for Notch in vivo and reveals dimorphic effects of ADAM10 on granulopoiesis, in which ADAM10 in nonhematopoietic cells acts on granulopoiesis through regulation of G-CSF production, whereas ADAM10 in hematopoietic cells acts in a cell-autonomous and G-CSF–independent manner. In addition, because disruption of Adam10 can potentially block all Notch signaling, the Adam10flox/flox mice described herein may serve as a useful tool for studying the functions of Notch signaling in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Shizue Tomita, Kaori Sue, and Yuko Hashimoto for their technical assistance.
This work was supported in part by the Takeda Science Foundation, the Nakatomi Foundation, Keio University Kanrinmaru project, MEXT KAKENHI (21390424 and 23592230), and a grant from the Japanese Ministry of Health, Labor, and Welfare (H20-Nanchi-Ippan-032).
Authorship
Contribution: M.Y. performed research and analyzed data; T. Kimura and Y.O. performed histologic analysis; T.T., S.U., T. Koba, J.T., and H.M. performed parts of the research; M.M., K.C., and Y.T. supervised the project; D.C.L. provided Gcsfr−/− mice; and K.H. designed research, performed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Keisuke Horiuchi, The Department of Orthopedic Surgery, School of Medicine, Keio University, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail: horiuchi@z3.keio.jp.

![Figure 1. Conditional inactivation of ADAM10 results in MPD. (A) Gross morphology of spleen from 8-week-old control and Adam10/Mx1 mice injected with polyinosinic:polycytidylic acid at 4 weeks before euthanasia (scale bar represents 1 cm). (B) Spleen/body weight ratios and total cell counts in the spleen. Bars indicate mean ± SD (n = 5). (C) H&E staining of sections of the spleen (i-iv; bars represent 200 μm [i and ii] and 50 μm [iii and iv]), liver (v and vi; bar represents 100 μm), and metatarsi (vii and viii; bar represents 250 μm) from 12-week-old control and Adam10/Mx1 mice injected with polyinosinic:polycytidylic acid at 8 weeks before euthanasia. The spleen of Adam10/Mx1 mice shows an increase in the number of myeloid cells, as well as megakaryocytes (arrowheads) in the red pulp (i-iv). In addition, granulocytic cells are present in the liver of Adam10/Mx1 mice, which indicates extramedullary hematopoiesis (v and vi arrowheads). The metatarsi of the mutant mice are filled with hematopoietic cells, whereas the BM cavity of control animals is filled with adipose cells (vii and viii arrowheads). (D) Complete blood counts for control and Adam10/Mx1 mice. Bars indicate mean ± SD (n = 4); HCT, hematocrit; HGB, hemoglobin; RBC, red blood cells; WBC, white blood cells; and PLT, platelets. (E) Flow cytometric analysis of peripheral blood. Bars indicate mean ± SD (n = 4). (F) Flow cytometric analysis of CD3e+, and B220+ cells in the spleen and Gr-1+ CD11b+ cells in the BM from control and Adam10/Mx1 mice (boxed areas). (G) Absolute cell numbers of Gr-1+ CD11b+ cells in the BM and CD3e+ and Gr-1+ CD11b+ cells in the spleen. Bars indicate mean ± SD (n = 4). (H) Flow cytometric analysis of the KSL cell populations in the BM and spleen of control and Adam10/Mx1 mice. Boxed areas indicate KSL cells. (I) Absolute numbers of KSL cells in the BM and spleen. Bars indicate mean ± SD (n = 3). (J) Numbers of colony-forming units (CFUs) per 1 × 105 splenocytes and 1 × 104 BM cells, respectively. Bars indicate mean ± SD (n = 5). The flow cytometric analysis shown here is a representative result of 3 independent experiments. (K) Transcript levels of Hes1, Hey1, and Hey2 in the spleen of control and Adam10/Mx1 mice. t test: *P < .05, **P < .005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/26/10.1182_blood-2011-06-357210/4/m_zh89991183470001.jpeg?Expires=1769157752&Signature=VZ34pU8qgVbtcYbxz8mmwpcEShXyRB0lIbDa-TI9hjnCF03C5lhLrnMHESN0-ttro9BWGqa6rug-9H-RU5c~2Ck0IKgBpDz4kF1ZU-mGVq~eBQcmYoIG-yE3-uuZQHTRju6ntIFko3W4Pu5U6~mfyroF-R4w498U3gowygVEsdlkI90tPfrDLAmHEaAjaSZ2N4Ipin0fl2syQBzm8n2wiD-tqeRHLTxPNJ3-VNLBkirh20~8eViHxrzsoIOfOUdBQ2QxghrlCfVQE60EezwOfdSl9vOnSbhRUKlht~8fpY8eMD1jD9jPTVJRXSQgE~PwuAOl-JYbk5lc-QdkWIMApg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)