To the editor:
A recent study by Pilch-Cooper et al1 refuting the existence of intracellular pools of CCR5 in human T cells challenged some of our observations reported in Blood.2 Although these authors observed the same intracellular accumulation of CCR5 using our cell permeabilization and fixation conditions before FACS analysis, they concluded that this intracellular labeling was likely nonspecific binding. This was based on the observations that our experimental conditions in their hands led to nonspecific signals in osteosarcoma cells, which do not express CCR5, and that confocal immunofluorescence (IF) microscopy studies in untransfected Chinese hamster ovary (CHO) cells showed an off-target nuclear signal.1
Correct permeabilization conditions are indeed critical to observe intraluminal epitopes while avoiding background. However, we do not believe that these results challenge our previous conclusions. First, antibody combinations showing background, such as fluochrome-conjugated anti-CCR5 2D7 antibodies used in FACS studies or nonconjugated 1/85a antibody in IF experiments1 were not used in Achour et al.2 In addition, our multiple controls were not considered. In our Figure 1A2 the strong signal obtained in permeabilized T lymphocytes with 2 different anti-CCR5 antibodies was not found for the anti-CD4 antibody, excluding a general nonspecific signal.2 Our confocal IF studies in T cells and THP-1 monocytes, revealed abundant intracellular CCR5, whereas no signal was visible in control CCR5-negative Jurkat T cells (Figure 1B)2 and IF studies on CCR5-expressing CHO cells did not reveal any nuclear staining with the CCR5 antibody (supplemental Figure 2).2 Moreover, the authors apparently misinterpreted their own confocal IF experiments, which clearly showed abundant intracellular CCR5 (supplemental Figure 1 top panels in Pilch-Cooper et al1 ).
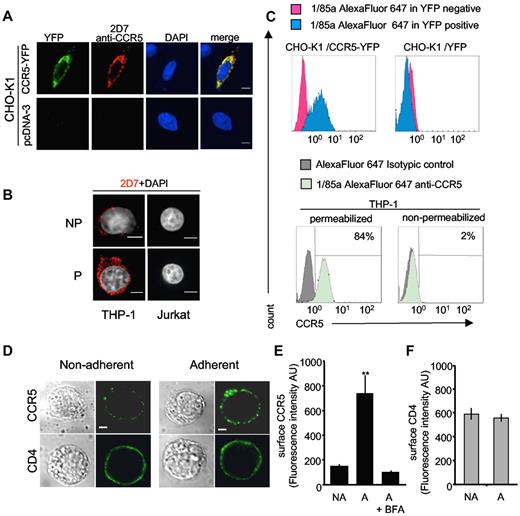
Specificity of antibodies under “mild” permeabilization conditions andcell-surface expression of endogenous CCR5 after adhesion of THP-1 cells. (A-C) Specificity of antibodies used in Achour et al2 under “mild” permeabilization conditions. (A) CHO-K1 cells transfected with CCR5-YFP or a control pcDNA3 plasmid were fixed with 4% paraformaldehyde (PFA) for 20 minutes at 4°C. After quenching with 50mM NH4Cl in PBS for 15 minutes, cells were washed with phosphate-buffered saline (PBS) and then incubated with PBS-bovine serum albumin (BSA) 1% containing 0.1% saponin for 20 minutes. After 1 hour incubation with the 2D7 anti-CCR5 antibody they were washed several times and incubated with Cy3-conjugated anti-mouse IgG in the same buffer. After extensive washing, slides were mounted in DAPI-containing medium. Confocal microscopy images (obtained with a Leica spinning-disk microscope equipped with a CoolSnap HQ2 CCD camera) were processed with ImageJ 1.43u software. Scale bar. 6 μm. (B) THP-1 cells analyzed for the expression of endogenous CCR5 as in panel A; saponin was omitted in nonpermeabilized cells (NP); (P):permeabilized. Control CCR5-negative Jurkat T cells are shown for comparison (C) FACS experiments in permeabilized CHO-K1 and THP-1 cells. Top panels: CHO-K1 cells were transfected with CCR5-YFP (left) or control YFP (right) plasmids. After fixation in 2% PFA for 20 minutes, cells were washed with PBS-1% BSA, 10mM HEPES, and 0.5mM EDTA, followed by 2 additional washes in the same buffer containing 0.1% saponin. Cells were then incubated in the same permeabilization buffer with the 1/85a Alexa Fluor 647–conjugated anti-human CCR5 antibody (1:50) for 45 minutes. After 2 washes, 1/85a Alexa Fluor 647 antibody signals gated on YFP-positive (blue histograms) or -negative (red histograms) cell populations were analyzed. Bottom: panels: after fixation, THP-1 cells were washed 2 times with PBS-0.2% BSA, 10mM HEPES, 0.5mM EDTA containing (permeabilized) or not (nonpermeabilized) 0.1% saponin. Cells were then incubated for 45 minutes in 100 μL of the appropriate buffer, containing (or not) 0.1% saponin and the 1/85a Alexa Fluor 647–conjugated anti-human CCR5 antibody (1:50) or the isotypic control (same antibodies as in Achour et al2 ). 1/85a Alexa Fluor 647 antibody signal (green histograms) or isotypic control signal (gray histograms) are shown. The percentage of positive cells is also indicated. FACS experiments were also conducted with the 2D7 antibody and gave similar results (not shown). (D-F) Increasing cell-surface expression of endogenous CCR5 after adhesion of monocytic THP-1 cells. (D) THP-1 cells were left untreated (left panels) or were incubated on fibronectin-coated (5 μg/mL Superfibronectin, S5171, Sigma-Aldrich) glass coverslips for 10 minutes (right panels), fixed (without permeabilization) and stained for surface CCR5 (top panels) or CD4 (bottom panels), using mouse 2D7 anti–human CCR5 or mouse OKT4 anti–human CD4, respectively. After incubation with the anti-mouse Alexa Fluor 488–conjugated antibody, cells were examined with a confocal microscope. Scale bar, 10 μm. (E) Average values of CCR5 surface expression calculated in 40 cells analyzed in panel D. NA indicates nonadherent; A, adherent. Fluorescence quantification (in arbitrary units [AUs[) was performed using ImageJ Version 1.38X software, with background subtraction. Statistical significance (**P < .01) using Student t test. BFA: cells incubated with 2 μg/mL brefeldin A (Sigma-Aldrich) overnight before the experiment. (F) Quantization of CD4 surface expression as described in panel E.
Specificity of antibodies under “mild” permeabilization conditions andcell-surface expression of endogenous CCR5 after adhesion of THP-1 cells. (A-C) Specificity of antibodies used in Achour et al2 under “mild” permeabilization conditions. (A) CHO-K1 cells transfected with CCR5-YFP or a control pcDNA3 plasmid were fixed with 4% paraformaldehyde (PFA) for 20 minutes at 4°C. After quenching with 50mM NH4Cl in PBS for 15 minutes, cells were washed with phosphate-buffered saline (PBS) and then incubated with PBS-bovine serum albumin (BSA) 1% containing 0.1% saponin for 20 minutes. After 1 hour incubation with the 2D7 anti-CCR5 antibody they were washed several times and incubated with Cy3-conjugated anti-mouse IgG in the same buffer. After extensive washing, slides were mounted in DAPI-containing medium. Confocal microscopy images (obtained with a Leica spinning-disk microscope equipped with a CoolSnap HQ2 CCD camera) were processed with ImageJ 1.43u software. Scale bar. 6 μm. (B) THP-1 cells analyzed for the expression of endogenous CCR5 as in panel A; saponin was omitted in nonpermeabilized cells (NP); (P):permeabilized. Control CCR5-negative Jurkat T cells are shown for comparison (C) FACS experiments in permeabilized CHO-K1 and THP-1 cells. Top panels: CHO-K1 cells were transfected with CCR5-YFP (left) or control YFP (right) plasmids. After fixation in 2% PFA for 20 minutes, cells were washed with PBS-1% BSA, 10mM HEPES, and 0.5mM EDTA, followed by 2 additional washes in the same buffer containing 0.1% saponin. Cells were then incubated in the same permeabilization buffer with the 1/85a Alexa Fluor 647–conjugated anti-human CCR5 antibody (1:50) for 45 minutes. After 2 washes, 1/85a Alexa Fluor 647 antibody signals gated on YFP-positive (blue histograms) or -negative (red histograms) cell populations were analyzed. Bottom: panels: after fixation, THP-1 cells were washed 2 times with PBS-0.2% BSA, 10mM HEPES, 0.5mM EDTA containing (permeabilized) or not (nonpermeabilized) 0.1% saponin. Cells were then incubated for 45 minutes in 100 μL of the appropriate buffer, containing (or not) 0.1% saponin and the 1/85a Alexa Fluor 647–conjugated anti-human CCR5 antibody (1:50) or the isotypic control (same antibodies as in Achour et al2 ). 1/85a Alexa Fluor 647 antibody signal (green histograms) or isotypic control signal (gray histograms) are shown. The percentage of positive cells is also indicated. FACS experiments were also conducted with the 2D7 antibody and gave similar results (not shown). (D-F) Increasing cell-surface expression of endogenous CCR5 after adhesion of monocytic THP-1 cells. (D) THP-1 cells were left untreated (left panels) or were incubated on fibronectin-coated (5 μg/mL Superfibronectin, S5171, Sigma-Aldrich) glass coverslips for 10 minutes (right panels), fixed (without permeabilization) and stained for surface CCR5 (top panels) or CD4 (bottom panels), using mouse 2D7 anti–human CCR5 or mouse OKT4 anti–human CD4, respectively. After incubation with the anti-mouse Alexa Fluor 488–conjugated antibody, cells were examined with a confocal microscope. Scale bar, 10 μm. (E) Average values of CCR5 surface expression calculated in 40 cells analyzed in panel D. NA indicates nonadherent; A, adherent. Fluorescence quantification (in arbitrary units [AUs[) was performed using ImageJ Version 1.38X software, with background subtraction. Statistical significance (**P < .01) using Student t test. BFA: cells incubated with 2 μg/mL brefeldin A (Sigma-Aldrich) overnight before the experiment. (F) Quantization of CD4 surface expression as described in panel E.
Nevertheless, we conducted new FACS and IF experiments in THP-1 and CHO cells (Figure 1), using the “mild” permeabilization procedure recommended by the authors.1 Confirming the specificity of CCR5 labeling, YFP-tagged CCR5 expressed in CHO cells was labeled by antibodies, and no off-target signal was visible by confocal IF, including in CHO cells transfected with control plasmid. Under these conditions, intracellular endogenous CCR5 was visible in THP-1 cells and again no staining was observed in CCR5-negative Jurkat cells. After mild permeabilization, FACS studies performed with the antibody combinations used in our previous study did not show any background in CHO cells and confirmed the presence of endogenous intracellular CCR5 in THP-1 cells (Figure 1C).
The existence of abundant intracellular stores of CCR5 is consistent with similar observations for many G protein-coupled receptors, the family of receptors CCR5 belongs to.3 In particular, intracellular stores of the chemokine receptor CXCR4 were reported in human T lymphocytes.4 Interestingly, L-selectin stimulation of T cells induced cell surface mobilization of CXCR4 within 10 minutes. Because such a fast effect, incompatible with receptor neosynthesis, represents major evidence for the existence of internal stores, we performed a similar experiment using THP-1 monocytes (Figure 1D-F). Adhesion of THP-1 cells on fibronectin-coated slides for 10 minutes was sufficient to increase 5-fold the fluorescence signal of surface CCR5, whereas surface CD4 was not affected. Golgi apparatus disassembly, induced by pretreating cells with brefeldin A, inhibited the phenomenon. These experiments were conducted without cell permeabilization. This finding and our previous data2 confirm that intracellular pools of CCR5 do exist in blood cells.
Rapidly mobilizable stores of chemokine receptors may represent a physiologic mechanism to escape desensitization on sustained activation and/or to facilitate blood cell extravasation into peripheral tissues. Internal pools of CCR5 also represent a challenging problem to solve for the development of anti-HIV drugs targeting this coreceptor. Studying the regulation of these intracellular pools is of primary importance to accelerate the development of such drugs.
Authorship
Acknowledgment: Supported by funds of the French Agency for AIDS research (ANRS-2009).
Contribution: H.S., L.A., and A.T. performed research and analyzed data; and M.G.H.S., C.L.-J., G.B., and S.M. designed the research, analyzed data, and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefano Marullo, Institut Cochin, 27 rue du Faubourg Saint Jacques, Paris 75014, France; e-mail: stefano.marullo@inserm.fr.