Abstract
Lentiviruses such as HIV have a daunting challenge in gaining access to a new host predominantly through the penile, rectal, or vaginal/cervical mucosal tissue after sexual exposure. Multiple mechanisms have evolved to help prevent such infections, including anatomical barriers, innate inhibitors, and adaptive immune responses. For lentiviruses, it appears that in naive or even conventionally vaccinated hosts, typical adaptive immune responses are generally too little and too late to prevent infection. Nevertheless, a combination of anatomical barriers and innate immune responses may limit transmission, especially in patients without predisposing conditions such as mucosal lesions or preexisting sexually transmitted infections. Furthermore, when infection does occur, most often the primary viremia of the acute infection can be traced back genetically to a single founder virus. Unfortunately, even a single virion can establish an infection that will ultimately lead to the demise of the host. This review seeks to describe the biology of and barriers to establishment of systemic, disseminated productive infection with HIV after sexual exposure and to discuss the possible mechanisms leading to infection by a single viral variant. Understanding the initial events of infection, before systemic spread, could provide insights into strategies for reducing acquisition or ameliorating clinical outcome.
The transmitted virus
One of the greatest obstacles in preventing HIV transmission is the enormous sequence diversity both within an individual potential donor and globally. In an individual patient infected with HIV, viral genetic diversity increases during the course of infection due to viral infection and spread through a replication mechanism that uses an error-prone reverse transcriptase. The resulting generation of viral variation provides the substrate for natural selection in the face of environmental pressures, including host immune responses, and the resulting immune evasion facilitating viral persistence. Of importance, however, during transmission this considerable diversity is severely reduced because the infecting virus undergoes a genetic bottleneck that results from the biology of mucosal transmission. Recently developed methods for viral sequence analysis, often designated as single-genome amplification (SGA), have both documented and permitted a greater understanding of the genetic restriction during sexual transmission of HIV, providing an unprecedented view into early viral evolution.1-5
SGA is simply a limiting dilution protocol in which DNA or complementary DNA is serially diluted so only a single template is PCR amplified and directly sequenced.6 The advantages of SGA over standard population sequencing methodologies include (1) proportionality of sequences, (2) no Taq-induced mutations, and (3) no in vitro recombination.3,5 When patients are sampled during acute viremia, the number of variants establishing infection can be enumerated with this approach. It was discovered in cohorts self-identified as heterosexual that the number of transmitted variants can be traced back to a single founder variant in roughly 80% of infections.1-5 Men who have sex with men and intravenous drug user cohorts generally have increased risk for both infection and the number of transmitted variants.7,8 The unambiguous identification and subsequent cloning of the actual transmitted/founder Env allowed for comparisons with Envs obtained during chronic infection in phenotypic analyses and for coreceptor usage and sensitivity to blocking antibodies and entry inhibitors.3 To date, transmitted Envs have all been shown to be functional and capable of mediating entry via CD4, using CCR5 as the coreceptor in most cases.3
Furthermore, when full-length transmitted/founder viruses were cloned, each showed significant replication in CD4+ T cells but did not replicate in monocyte-derived macrophages,7 findings that are consistent with CD4+ T cells being identified as the earliest productively infected cell type in vivo in nonhuman primate (NHP) studies and the predominant cellular substrate for viral replication in early SIV infection.9 The lack of replication in macrophages was confirmed in 2 studies in which the authors tested infection of transmitted subtype C envs.10,11 Although this overall reduction in diversity is often referred to as a genetic bottleneck, the only reported selection of transmitted viruses involves the use of CCR5 as coreceptor, the capacity to replicate at a sufficient rate to cause systemic dissemination, and the lack of macrophage tropism.3,7,12 Additional signatures of transmission may yet be identified after detailed genetic and phenotypic analyses of transmitted/founder viruses.
Further detailed assessment of the Envs from transmitted/founder viruses in subtypes C and A infections have been reported to contain shorter and fewer potential N-linked glycosylation sites.13,14 These signatures may also hold for subtypes B and D,4,15 although perhaps more subtly. Identification of these and other potential signatures characteristic of transmitted/founder viruses raises the prospect that the genetic bottleneck during sexual transmission is selective by nature, with important implications for the design and evaluation of vaccines and other prophylactic intervention strategies.
The use of SGA methods to quantitatively characterize the barrier to mucosal transmission has documented the stringency of this blockade but does not clarify where the bottleneck occurs or the contributions of its various components to determining the nature of transmitted founder viruses. What is clear is that from a large, diverse inoculum, a small founder population is successful in establishing systemic infection. Single-variant infections and an overall low-per-coital-episode infection rate in most settings of potential heterosexual transmission16,17 indicate that these anatomical and physiologic barriers work well and often prevent infection. Thus, intervention modalities may require only modest incremental tipping of the balance in favor of the host to significantly reduce incidence.
Prospects for enhancing the effectiveness of these physiologic/innate barriers with preventive interventions will likely improve with a better understanding of the detailed biology of HIV transmission and early viral replication and a greater insight into the molecular mechanisms of the existing barriers to infection. Currently, logistical and practical constraints prevent the direct testing of the earliest events in humans during acute infection. On the basis in part of recent advances such as the application of SGA methods to characterize viral transmission via different mucosal routes, NHP models have been refined to more accurately recapitulate HIV transmission and early host-virus interactions, resulting in typical transmission of a single or only a few viral variants.
NHP models of transmission and early replication
Experimental SIV infections of rhesus macaques (Macaca mulatta; RM) have been used for decades to study the various aspects of the biology of primate lentivirus infection. However, the relevance of such models for many applications hinges on whether they accurately recapitulate the essential features of human mucosal transmission identified through the new approaches described previously. Historically, the initial development of RM models involved the identification of pathogenic viral isolates and the use of high-dose intravenous inoculation to reproducibly infect RM, cause viremia typical of human infection, and eventually lead to acquired immunodeficiency syndrome-like pathology. Next, a high-dose intrarectal challenge model was adopted for basic transmission and vaccine efficacy studies designed to achieve 100% infection rate while providing a relevant mucosal route of infection of broader clinical relevance. Given the small group sizes typical of NHP experiments in this era, challenge inocula were selected to provide as close to 100% infection of control animals as possible with a single challenge, given the severe consequences for statistical analysis of failing to infect all animals in the control groups. Although this improved model allowed for many important discoveries, with the knowledge that most human infections can be traced to a single founder sequence, the relevance of high-dose intrarectal challenge models for evaluation of vaccines was questioned on the basis that such challenges might pose an unrealistic and unnecessarily stringent standard for demonstrating potential efficacy, especially for less-than-optimal vaccines that might show only partial efficacy at best.18
In this setting, mucosal challenge models were further modified with a shift to repeated challenges with lower inocula, titered to more accurately simulate clinically relevant virus exposures; significant vaccine effects with partial, but often substantial, efficacy were reported with the use of these modified models.19-22 In these studies, the establishment of productive systemic infection was inhibited or controlled quickly with passively administered neutralizing antibodies,22 DNA/Ad5 immunization with homologous20 or heterologous challenge,19 or a recombinant rhesus cytomegalovirus vectored vaccine specifically designed to induce effector memory T cells to interact with infected cells at the portals of entry.21 However, in none of these studies did the authors compare directly the effects of high or low dose on vaccine efficacy.
The viruses used most widely for such studies were SIVmac251 and SIVsmE660, 2 different viral swarms with different origins and intermediate passage histories, which had been shown to produce pathogenic infection in a majority of inoculated SIV-naive RMs. Of note, the pair showed sufficient sequence differences (∼ 18% in Env) to represent a reasonable system for heterologous challenge. Although SIVmac251 and SIVsmE660 were not specifically generated for mucosal infections (and it can be argued that it would be desirable to identify additional mucosally transmitted virus isolates specifically for mucosal transmission studies), they each contain a swarm of virus with sufficient diversity (∼ 1%-2% max env diversity) that sequencing the env gene is sufficient to distinguish one variant from another.23-25
To further refine the transmission model, we sought to identify doses of different well-pedigreed challenge stocks, available in relative abundance, that would recapitulate single variant infections, which represent the typical outcome for mucosal infections of humans. Cell-free virus from both SIVmac251 and SIVsmE660 isolates were used in a repeat titered, rectal challenge model in RMs to determine the number of variants initiating infection.25 The results indicated that with appropriate titers of challenge inocula single-variant infections could be established in RMs.25 These results were expanded in a 24-animal study by the use of 4-log dilutions of SIVmac251 inoculum to determine the number of variants detectable in plasma 10 days after a single intrarectal challenge.23 Not surprisingly, the infection rate and the number of variants establishing the initial infection correlated to the challenge dose, with the lowest dose infecting animals with only a single variant, whereas the number of detectable variants increased in the greater doses. Interestingly, in this single experiment a low-dose challenge produced a lengthened eclipse phase while reducing innate immune activation compared with high-dose challenge.23
Intrarectal challenge models have been widely used because of the greater availability of male RMs, which are not in as much demand as breeding-age females, and the reproducibility of this challenge model, but vaginal transmissions represent the majority of new infections globally,26 and it is crucial to understand the biology of infection via this route. Studies of vaginal infections in NHP models have been problematic because of the extensive interanimal variability in establishing infection, even after uniform challenge conditions. Consequently, most investigators have used the greatest dose feasible to maximize the probability of a “take” of infection in all control animals.27 Although this approach has proven very useful in identifying important early events, vaccine efficacy studies may require a more physiologically relevant dose. By using inoculum titration and SGA-based sequence analysis, Stone et al24 identified a dose of virus that caused systemic infection with from 1 to 7 variants after a single challenge. Of importance, and consistent with previous experience, results for animals that received identical inocula were variable, and although there was a general dose effect, a strict dose-response relationship was not observed with regard to variable numbers of transmitted viruses. Factors contributing to this variability may include changes in epithelial thickness and mucus that vary as a function of menstrual cycle stage, access point of the virus (see “FGT transmission”), preexisting inflammation, or target cell availability. To refine this model and provide greater consistency, additional studies will be required to control sources of variation, including potentially timing menstrual cycles before challenge. The administration of exogenous hormones (ie, depot medroxyprogesterone acetate [Depo-Provera]) has been used as an approach in this direction, but the epithelial thinning mediated by this treatment, along with immunomodulatory effects of the exogenous hormones, renders it problematic for use in evaluation of vaccines and other applications.28
Finally, although it has been reported that cell-associated virus can also initiate infection,29 it is difficult to rigorously rule out the potential contributions to transmission of cell-free virus derived from freshly produced virus even in thoroughly washed inoculums of infected cells. Although it is thus difficult to unequivocally demonstrate a role for infected cells in mucosal transmission, it is unequivocally clear that cell-free SIV is sufficient in these models to recapitulate the infection outcomes observed in HIV infection. Although it is possible that compartmentalization of the virus within the donor could contribute to the overall genetic bottleneck seen at the time of transmission,30 NHP models have demonstrated that the extensive diversity present in a challenge inoculum can be reduced to a single infecting virus involved in establishing the initial disseminated infection within 1 week of infection after either vaginal or rectal challenge with cell free virus.
With recent progress, many aspects of human infection can now be recapitulated in a NHP model and are currently being used in basic research as well as vaccine efficacy trials. These models provide a unique opportunity to study early events in viral transmission and evolution, both systemically and within important tissue compartments. The ability to sample tissues before infection and immediately after challenge make NHP models a valuable tool for helping to elucidate the contributions of different anatomical barriers and innate immune mechanisms to preventing infection.
Distinct mucosal tissues are different
Mucosal surfaces play a major role in HIV transmission; however, it is essential to understand that each mucosal tissue is quite distinct in anatomy, physiology, and immunobiology. The unique characteristics of different mucosal tissues can dramatically influence the efficiency of HIV/SIV transmission. In this portion of the review, we attempt to highlight some of the key differences in the structural barriers and target cell composition and availability within the female and male genital tracts (FGT and MGT, respectively) and rectum, the mucosal sites that account for the majority of transmissions worldwide.16,26 Furthermore, we highlight the host defenses that may further contribute to the viral bottleneck that typifies mucosal transmission and discuss the importance of local mucosal viral expansion before systemic dissemination. Because the detailed analysis of tissues at early times postinoculation enabled by experimental infections in NHP models is the source of much of our knowledge about the interactions between the virus and host during the very early time points after mucosal transmission, much of the discussion is focused on these NHP models. In addition, this section will emphasize recent progress in understanding FGT transmission, both because of the importance of this route in the global pandemic and because of all the various routes of mucosal transmission, it is transmission across the female genital mucosae that is best understood.
FGT transmission
Although HIV penetration and infection have been demonstrated to occur from ex vivo tissue explants studies in all 3 mucosal environments in the human FGT (vagina, ectocerivx, and endocervix), the available direct in vivo evidence of viral penetration and mucosal infection comes exclusively from NHP models.27,31-33 However, the relative contributions of the vaginal, ectocervical, and endocervical mucosae to successful transmission remains somewhat controversial and debated. These controversies largely arise from data demonstrating HIV infection in a woman born without a cervix34 and SIV infection of RMs after surgical hysterectomy (including removal of the cervix),35 which contrasts with the results from high-dose intravaginal natural history studies in which the authors demonstrated predominant early SIV vRNA+ cell foci within the endocervix and transformation zone of RMs.27,36 However, rather than reflecting a real controversy about which mucosal surface HIV and SIV primarily use to traverse the FGT, in aggregate these data highlight the remarkable flexibility of HIV/SIV in exploiting any vulnerability within the FGT.
Each mucosal tissue in the FGT represents both significant challenges to overcome and unique opportunities for the virus to exploit to achieve productive infection (Figure 1A). For example, the vaginal and ectocervical mucosae are separated from the environment by a multilayered squamous epithelium that varies in thickness during the menstrual cycle, that is, between ∼ 200 and 300 μm mean thickness in humans37 and ∼ 80 and 250 μm mean thickness in RMs.38 These thick, tightly packed cells likely represent a significant barrier and challenge for the virus to cross before coming into contact with susceptible CD4+ target cells in the epithelium or superficial submucosae. In an RM SIV model of vaginal HIV transmission, it was shown that administration of exogenous hormones (Depo-Provera or estriol) caused changes in the thickness of the vaginal mucosae, which markedly affected SIV transmission rates, with a thicker vaginal epithelia strongly protecting against SIV vaginal transmission in contrast to increased infection when the vaginal mucosae was dramatically thinned,28,39 highlighting the important contribution the vaginal epithelium plays in preventing viral access to the host.
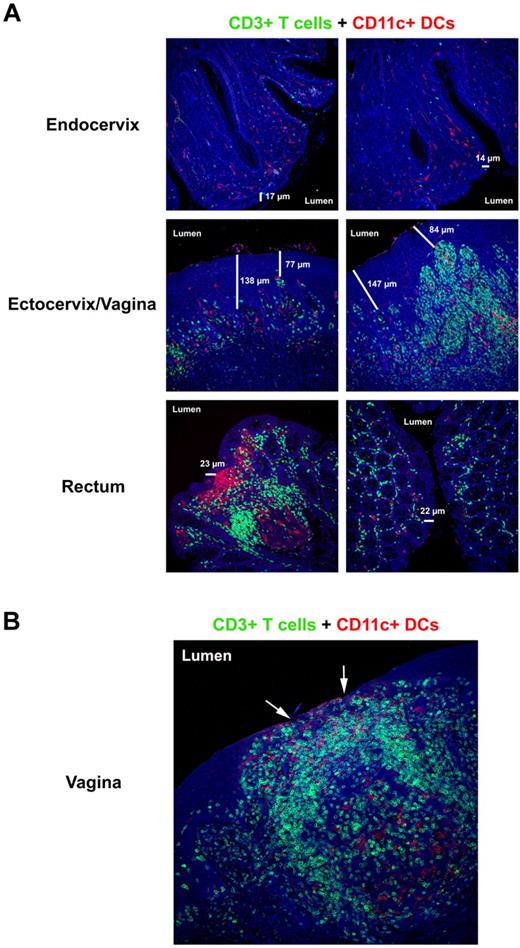
The mucosal barriers and HIV/SIV target cell composition. (A) Immunofluorescent micrographs (200×) of endocervix, ectocervix/vagina, and rectum stained for CD3+ T cells (green) and CD11c+ DCs (red) from a representative SIV-negative RM. The 2 different columns show the diversity and abundance of potential target cells in the same animal along the different mucosal barriers. The thickness of the respective epithelial barriers (in micrometers) is indicated in each image to highlight the heterogeneity that can exist in the thickness of the barrier that separates the host from its environment. (B) Immunofluorescent micrograph (200×) of vagina stained for CD3+ T cells (green) and CD11c+ DCs (red) from a SIV-negative RM with subclinical mucosal inflammation. The arrows point to regions of epithelial thinning where potential target cells are directly juxtaposed to the lumen. Note the densely packed T-cell infiltrate with numerous DCs beneath what remains of the epithelial barrier.
The mucosal barriers and HIV/SIV target cell composition. (A) Immunofluorescent micrographs (200×) of endocervix, ectocervix/vagina, and rectum stained for CD3+ T cells (green) and CD11c+ DCs (red) from a representative SIV-negative RM. The 2 different columns show the diversity and abundance of potential target cells in the same animal along the different mucosal barriers. The thickness of the respective epithelial barriers (in micrometers) is indicated in each image to highlight the heterogeneity that can exist in the thickness of the barrier that separates the host from its environment. (B) Immunofluorescent micrograph (200×) of vagina stained for CD3+ T cells (green) and CD11c+ DCs (red) from a SIV-negative RM with subclinical mucosal inflammation. The arrows point to regions of epithelial thinning where potential target cells are directly juxtaposed to the lumen. Note the densely packed T-cell infiltrate with numerous DCs beneath what remains of the epithelial barrier.
The area of the cervix that represents an abrupt transition between the ectocervix and endocervix is referred to as the transformation zone or the squamocolumnar junction and is the predominant site of human papillomavirus infection.40 The transformation zone is likely an important site for HIV/SIV transmission because of the single layer of epithelium and abundant CD4+ target cells within this region of the FGT.40 Indeed, NHP studies have shown early foci of productively infected cells within transformation zone/squamocolumnar junction,36 highlighting the importance of this anatomical site for viral transmission. In contrast to the vaginal and ectocervical mucosae, the epithelium of the endocervix is separated from the environment by only a single columnar epithelial layer that may present a pathway of least resistance for HIV/SIV to gain entry into the host and access to susceptible target cells.27 However, this relatively thin endocervical epithelial barrier is covered with copious amounts of cervical mucus that can represent a daunting challenge for the virus to penetrate, depending on the stage of the menstrual cycle.41 In fact, mucus itself has been shown to effectively trap SIV in vivo and prevent viral access to underlying epithelium.27
The frequency and density of susceptible potential target cells for HIV/SIV can be vastly different both between and within the tissues in the FGT. These differences can be influenced by both host and external factors leading to local inflammatory responses (Figure 1A). Subclinical (ie, asymptomatic) genital tract infections, such as bacterial vaginosis and trichomoniasis (Trichomonas vaginalis), can increase a woman's susceptibility to HIV infection.42 A recent study of the transmissibility of HIV in different human populations showed that depending on the viral load in the transmitting partner and other factors, infectivity can be as high as 1 transmission per 10 contacts for penile-vaginal contact or even 1 transmission per 3 contacts for penile-anal contact and that sexually transmitted infections are an important cofactor for increased HIV susceptibility.17 Furthermore, an SIV NHP model of genital ulcerative disease demonstrated association of vRNA+ cells in mucosal tissues with visible ulcer/inflammation and a more rapid dissemination to the lymphatic tissues.43 The importance of preexisting inflammation in the FGT in enhancing transmission was also shown in a NHP model in which intravaginal administration of either a TLR9 or TLR7 agonists before SIV exposure enhanced viral transmission or replication.44 These data suggest that clinical or subclinical mucosal inflammatory responses can result in thinning or breach of the intact cervicovaginal mucosae, consequently allowing HIV/SIV virus easier access into the host at a site of plentiful susceptible CD4+ target cells (Figure 1B). Collectively, these studies highlight the dynamic nature of the female genital tract physical barriers and suggest divergent susceptibilities to vaginal transmission depending on the stage of menstruation and local inflammation.
HIV/SIV also faces host innate defenses that represent challenges for the virus to overcome to gain access to and establish infection within the host. Aside from the physical barrier, the mucosal epithelium also contributes innate defenses that have antiviral activity such as the production and secretion of microbicidal defensins, antimicrobial peptides, and secretory inhibitors deposited within the lumen of the FGT.45-48 In one recent study, investigators demonstrated that shortly after SIV vaginal challenge, endocervical epithelial cells express chemokines that presumably result in rapid recruitment of plasmacytoid dendritic cells (DCs) to the basolateral surface of cervical epithelium.35 Further studies will be needed to determine the extent that these responses are mediated by viral particles or other inflammatory mediators contained in the viral inoculum preparations.
Furthermore, chemokine expression by vaginal epithelial cells and inflamed skin epithelial cells have been shown to potently attract Langerhans cell precursors.36,49,50 Collectively, these early recruited cells are important mediators of innate defenses and inflammation, in addition to initiating and linking innate and adaptive immunity. In addition, the mucosally recruited plasmacytoid DCs were shown to produce copious amounts of macrophage-inflammatory protein-1α/β (CCL3/4) and type I interferon (IFN)– α/β,36 which have been shown to have a wide variety of antiviral effects capable of impacting HIV replication through numerous mechanisms, including induction of apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G, latent endoribonuclease through the expression of oligoadenylate synthetase, myxovirus-family proteins, and other IFN-inducible proteins.51,52 Collectively, these innate responses to viral exposure and the first few days of infection within the mucosal lamina propria likely inhibit or limit viral replication, perhaps accounting in part for the small founder populations in SIV infection despite exposure to a large dose of virus.36,53 However, these ostensibly “protective” early host innate responses also lead to mucosal inflammation and CD4+ T-cell recruitment that can ignite local mucosal viral replication if infectious virus persists at the mucosal portal of entry.36
Although there is some argument on the phenotype of the first cell populations that are productively infected in the female genital submucosae, with some groups showing early SIV vRNA+ cells to be predominately CD4+ T cells,9,36 and other groups demonstrating that the SIV vRNA+ cells are in fact DCs, particularly Langerhans cells.54,55 Collectively, these studies suggest that SIV, and by extension HIV, may take advantage of multiple cellular targets to gain an initial foothold in the portal of entry after successfully crossing the mucosal surface. However, once virus gains access to the host, either by infecting resident CD4+ T cells or Langerhans cells, the appearance of viral spread within the submucosae of the female genital tract is not typically demonstrable until 3-4 days after exposure to a high dose of virus (ie, 100% animal infectious dose).27
The fate and location of the virus within these first days after challenge remains largely unknown. However, several groups have demonstrated the presence of both virus (PCR) or SIV vRNA+-infected cells within the genital draining lymph nodes, albeit at very low levels, shortly after intravaginal SIV challenge, suggesting that rapid dissemination to lymphatic tissues can occur after mucosal exposure.27,54,55 However, there is little evidence of active viral replication in draining or systemic lymphatic tissues until 5-6 days after vaginal challenge.27,54 Therefore, although viral particles themselves might rapidly gain access to draining lymphatic tissues after intravaginal SIV inoculation through lymphatic drainage or carried by DCs (likely mucosal Langerhans cells),56 it appears that these early viruses are insufficient to establish systemic replication that may be related to a failure to reach an obligatory threshold of infectious virus necessary to establish productive infection in these lymphatic tissues. It will be important to determine whether the viruses that cross mucosal surfaces replicate in resident CD4+ T cells and/or Langerhans cells at low levels or whether these viruses are sequestered within mucosal DCs or macrophages until the recruitment of inflammatory CD4+ T cells. Given the biologic properties of myeloid derived DCs and macrophages from the female genital mucosae, these cells may play a key role in maintaining HIV in an infectious state until the arrival of increasing levels of activated CD4+ T cells within the submucosae.33,55,57 Regional differences in resident antigen-presenting cell population size and function found in distinct mucosal tissues may thus dramatically influence transmission across these variable mucosal surfaces.57
After HIV/SIV vaginal transmission, local viral replication and amplification within the portal of entry appears to be necessary before dissemination and lymphatic spread.27,36 The eclipse phase observed in humans58 between presumptive exposure and development of either detectable viremia or clinically apparent symptoms of an acute viral syndrome strongly supports NHP models of mucosal transmission having a need for local mucosal viral amplification as a prerequisite to establishing a systemic productive infection in the host.27 Furthermore, epidemiologic data showing increased HIV incidence with sexually transmitted coinfections,59 along with the empirical NHP transmission data, support the notion of a requirement for a “threshold” level of mucosal susceptible CD4+ target cells, clustered in close proximity to cells harboring infectious virus (ie, mucosal DCs, macrophages, or CD4+ T cells) derived from the viral inoculum that crossed the epithelial barrier from the initial exposure to establish local infection. This “threshold” of susceptible CD4+ target cells can result from active recruitment to mucosal tissues through early host inflammatory processes36 or as a result of preexisting inflammation. Furthermore, the requirement for mucosal replication and amplification before lymphatic tissue seeding highlights why preexisting inflammation within the cervicovaginal mucosae, resulting in a greater density of DCs and activated CD4+ T cells within the submucosae before HIV/SIV (Figure 1B), is associated with more efficient establishment of infection than vaginal transmission in the absence of inflammatory changes. Collectively, these data suggest a need for sufficient CD4+ T cells to be present within the mucosal tissues to support first local viral replication before lymphatic tissue dissemination, which highlights a potential vulnerability of the virus that may be exploited by HIV-prevention modalities.
MGT transmission
The exposed portion of the male genital tract (MGT) is separated from the environment by a keratinized stratified squamous epithelial layer. HIV transmission across the MGT is poorly understood; however, male circumcision was recently shown in 3 randomized controlled clinical trials to reduce female-to-male transmission by approximately 60%,60-62 providing evidence that the foreskin can play an important role in this route of HIV transmission. In addition, although SIV has been demonstrated to be transmitted across the intact mucosae of the foreskin and glans of the penis of RMs,31 the early events that lead to transmission across the MGT and productive systemic infection have not been elucidated. All major HIV/SIV target cells (ie, CD4+ T cells, Langerhans cells, DCs, and macrophages) have been identified in the foreskin and glans penis, but most CD4+ T cells typically reside below the basement membrane in these mucosal tissues, whereas CD4+ Langerhans cells exclusively reside in the epithelium,63,64 and ex vivo tissue cultures of human foreskin have shown that the MGT is susceptible to HIV infection with CCR5-using virus.63,65 As with all mucosal tissues, inflammation of the glans penis alone (balanitis) or with the foreskin (balanoposthitis) likely increases an individual's susceptibility to HIV infection.66 This is highlighted by the fact that the presence of a foreskin predisposes a patient to sexually acquired infections (including genital herpes, candidiasis, gonorrhea, syphilis, and human papillomavirus infection) and inflammatory dermatoses,67,68 providing one plausible mechanism for why uncircumcised patients are at greater risk of HIV infection than circumcised men.66
Rectal mucosae
The rectum is a particularly susceptible site for HIV/SIV infection given that the rectal mucosae is (1) separated from the environment by only a single layer of columnar epithelium and (2) contains numerous CD4-bearing target cells (CD4+ T cells, DCs, and macrophages) juxtaposed to the basolateral surface of the intestinal epithelium (Figure 1A). Remarkably, there is a paucity of in vivo data detailing the early host-viral events that transpire after this route of transmission, even though rectal challenge is an extensively used NHP transmission model for vaccine-based studies. In one detailed study in which the authors looked at viral dissemination in the RM model after rectal challenge demonstrated that paracolic lymph nodes are the first lymphatic tissues that are seeded by virus and SIV vRNA+ cells; however, the earliest time point used to assess viral replication was 7 days after challenge, and the analysis focused on secondary lymphatic tissues and not the rectal mucosae or the associated gastrointestinal-associated lymphoid tissue.69
Thus, the initial target cells, local amplification within the rectal mucosae, the exact mechanism of viral dissemination to rectal draining lymph nodes, and whether alternative pathways of viral dissemination occur after rectal transmission remain to be determined. In humans, in addition to the potential epithelial abrasions that occur with receptive anal intercourse, the anorectal localization of other sexually transmitted infections that can induce inflammatory tissue responses likely contributes to the greater incidence of HIV seen in both men and women who practice receptive anal intercourse.70 This finding highlights the important role preexisting inflammation plays in HIV mucosal transmission, regardless of the mucosal site. In contrast, for cervicovaginal transmission, the cervical mucus has been shown to be capable of impeding the mobility of HIV virions,41 potentially limiting transmission. Although the large bowel, including the rectum, produces copious amounts of mucus that is continuously deposited into the lumen of the large bowel,71,72 the potential impact of this mucus on anorectal transmission is understudied and could prove useful in vaccine or microbicide studies designed to inhibit anal transmission.
Summary
There are several anatomic and physiologic host barriers to infection that in aggregate can explain the viral bottleneck seen in HIV transmission. For mucosal infections, mucus and an intact epithelial barrier most likely represent the greatest obstacle to infection (Figure 2). HIV and SIV appear to be capable of flexibly exploiting multiple mechanisms to transit different epithelial barriers and gain access to susceptible target cells and the opportunity to establish a systemic infection. Even after mucosal barriers are breached, the infecting virus must still contact susceptible target cells (directly, of through indirect means such as via a DC or other antigen presenting cells), complete the multiple steps of the viral replication cycle and generate progeny at a basic reproductive ratio large enough to overcome innate immune responses. SGA analysis of transmitted/founder viruses has confirmed that transmitted viruses are predominantly CCR5-using viruses. Ironically, local expansion at mucosal sites in cervicovaginal transmission, which appears to be required for establishment of systemic infection, is facilitated by recruitment of activated CD4+ T cells intended to quench virus that otherwise might be contained locally. On systemic dissemination, viral amplification is massive and extensive death of CD4+ T cells occurs, particularly at mucosal sites. Although the low multiplicity of infection after sexual exposure provides a unique opportunity to exploit a vulnerability of the virus, the rapid expansion/diversification and seeding of long-term reservoirs that follow suggests that interventions that act early and at initial mucosal sites of infection are likely to be most effective.
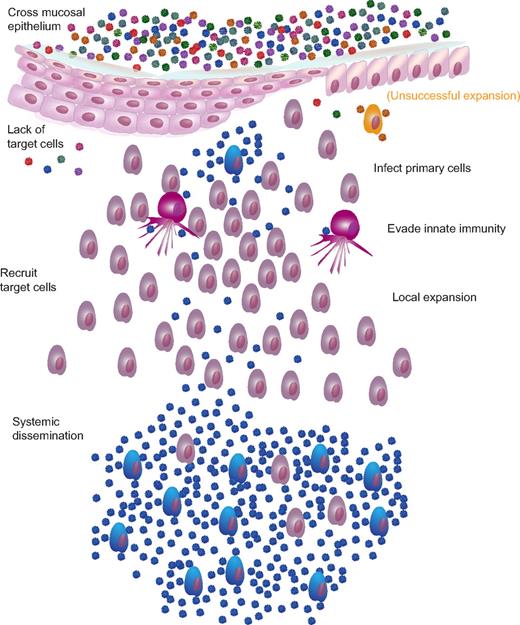
Mucosal transmission and single variant systemic dissemination. There are multiple barriers to infection and, most likely, multiple mechanisms by which the virus overcomes these barriers. Barriers include mucus, pH, intact epithelium, target cell availability, innate cells, and inhibitory chemokines/cytokines. After successfully traversing the mucosal epithelium, cell-free virus interacts with CD4 and coreceptor to initiate primary infection of susceptible target cells, either with or without previous transport and facilitated transfer of virions by Langerhans cells or DCs. During the next 2-5 days, the virus replicates and is amplified locally, initially fueled by preexisting targets but paradoxically expands rapidly with the recruitment of activated target cells chemotactically recruited to the site to quench the budding infection. For cervicovaginal transmission, this local expansion of virus and infected cells appears to be a prerequisite for dissemination to local lymph nodes and then systemically. Although the exact proportion of virions “lost” at each barrier is unknown, presumably, virions can be successful at crossing the epithelial barrier only to be ineffective at finding target cells (red and green virions) or unable to expand locally (orange infected cell) at a sufficiently large reproductive ratio before target cell recruitment and dissemination as to be unable to establish a systemic, disseminated productive infection.
Mucosal transmission and single variant systemic dissemination. There are multiple barriers to infection and, most likely, multiple mechanisms by which the virus overcomes these barriers. Barriers include mucus, pH, intact epithelium, target cell availability, innate cells, and inhibitory chemokines/cytokines. After successfully traversing the mucosal epithelium, cell-free virus interacts with CD4 and coreceptor to initiate primary infection of susceptible target cells, either with or without previous transport and facilitated transfer of virions by Langerhans cells or DCs. During the next 2-5 days, the virus replicates and is amplified locally, initially fueled by preexisting targets but paradoxically expands rapidly with the recruitment of activated target cells chemotactically recruited to the site to quench the budding infection. For cervicovaginal transmission, this local expansion of virus and infected cells appears to be a prerequisite for dissemination to local lymph nodes and then systemically. Although the exact proportion of virions “lost” at each barrier is unknown, presumably, virions can be successful at crossing the epithelial barrier only to be ineffective at finding target cells (red and green virions) or unable to expand locally (orange infected cell) at a sufficiently large reproductive ratio before target cell recruitment and dissemination as to be unable to establish a systemic, disseminated productive infection.
Acknowledgments
The authors thank Jeff Lifson for his critical reading of the manuscript.
This work was supported with federal funds from the National Cancer Institute, National Institutes of Health under contract HHSN266200400088C. This work represents the opinions of the authors and not necessarily the National Institutes of Health or the Department of Health and Human Services.
National Institutes of Health
Authorship
Contribution: B.F.K. and J.D.E. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brandon F. Keele, AIDS and Cancer Virus Program, SAIC-Frederick, NCI-Frederick, 1050 Boyles Street, Frederick, MD 21702; e-mail: keelebf@mail.nih.gov.