Abstract
GATA3 has been identified as a master regulator of T helper cells, as well as being important for early thymic progenitors and T-cell commitment. However, Gata3 expression initiates already at the hematopoietic stem cell (HSC) level, implicating a potential role also in the regulation of HSCs. Herein we used a conditional Gata3 knockout strategy in which Gata3 expression was completely deleted from the earliest stage of embryonic hematopoietic development after emergence of HSCs from hemogenic endothelium. Through a detailed analysis of HSCs at the phenotypic and functional level, we demonstrate that steady-state levels of HSCs are normal in Gata3fl/flVav-Cretg/+ mice. Moreover, through long-term primary and secondary transplantation experiments, we also unequivocally demonstrate that Gata3 has a redundant role in post-transplantation HSC self-renewal.
Introduction
GATA1, GATA2 and GATA3 are expressed at distinct stages in the hematopoietic hierarchy. GATA1 is required for erythropoiesis, megakaryocytes, and eosinophils,1-3 GATA2 for primitive hematopoietic stem/progenitor cells,4,5 and GATA3 for multiple stages of T-cell development.6-8 However, Gata3 is expressed already in hematopoietic stem cells (HSCs),9 multipotent progenitors,10 and early thymic progenitors.11 Although the role of GATA3 has been carefully investigated in T-lymphopoiesis, its role in HSCs has been much less explored.12 Recent studies showing that Gata3 null fetal liver cells can normally generate short-lived myeloid cells 10 weeks after transplantation8 are compatible with GATA3 having little or no role in HSC regulation. However, the HSC phenotype was not directly analyzed in either the fetal liver or the bone marrow (BM) of transplanted mice, nor was the defining stem cell property to self-renew after transplantation into secondary recipients.
Methods
Mice
Wild type (WT) transplantation recipients and competitor cells were on a pure C57BL/6 (CD45.1) background. Gata3fl/fl, Vav-iCre, and R26R-eYFP mice have been described.13-15 Animal experiments were approved by the local ethics committee at the University of Oxford and the United Kingdom Home Office.
Fluorescence-activated cell sorter analysis
BM cells were stained according to previously described protocols.16 For specification of antibodies, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Gene expression analysis
Global gene expression analysis was performed using Mouse Genome 430 2.0 Arrays. Gata3 deletion efficiency was evaluated with dynamic array Biomark Fluidigm analysis (supplemental Methods). Gata3 was amplified and analyzed for gene expression using a dynamic array (Biomark Fluidigm) as previously described.17 A total of 100 cells were sorted/well (2-4 wells/genotype). Data were analyzed using BioMark Real-Time PCR Analysis Software Version 2.0 (Fluidigm), and the ΔCt method was applied.18 Hprt TaqMan Gene Expression Assay ID Mm01337569_m1 was used.
Statistical analysis
Statistical significances were determined with 2-tailed Mann-Whitney test.
Results and discussion
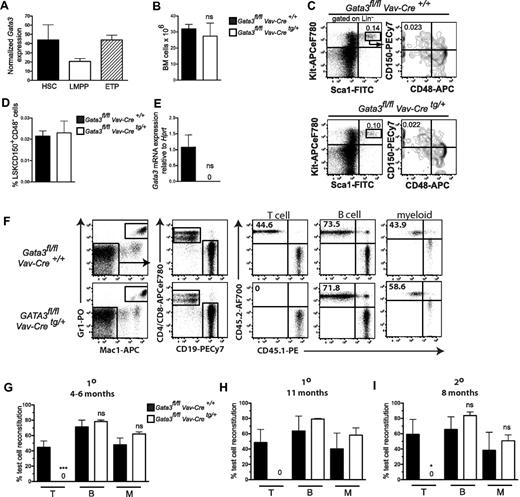
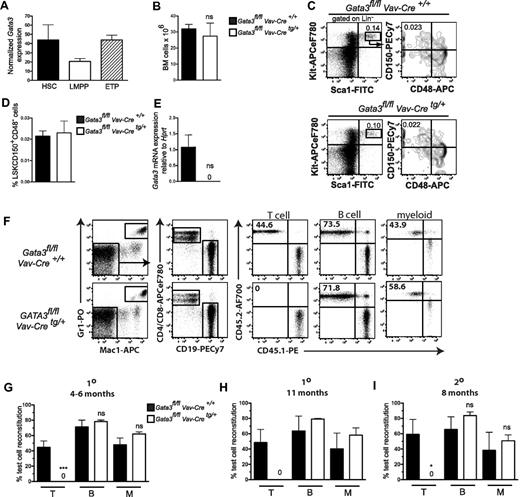
We not only confirmed that Gata3 mRNA is expressed in highly purified LSKFLT3−CD48−CD150+ HSCs,16 but that it is subsequently down-regulated in lymphoid-primed multipotent progenitors19,20 in which thymic-seeding progenitors are thought to reside, before being up-regulated again in LIN−CD25−KIThiFLT3+ early thymic progenitors11 (Figure 1A). GATA3fl/fl mice13 were crossed with Vav-iCre mice,14 ensuring Gata3 deletion shortly after the emergence of definitive HSCs from the hemogenic endothelium.21 Vav-iCre mice crossed with R26R-eYFPfl/fl mice15 demonstrated that Vav-iCre ensures recombination in virtually all (> 99%) BM LSK cells (supplemental Figure 1). BM cellularities of control (Gata3fl/flVav-Cre+/+) and Gata3-deficient (Gata3fl/flVav-Cretg/+) littermates were indistinguishable (Figure 1B), as were the frequencies of LSKCD150+CD48− HSCs (Figure 1C-D), in which complete Gata3 deletion was confirmed (Figure 1E). These findings suggest that Gata3 null HSCs expand normally after their emergence in the embryo and remain unaffected in postnatal BM.
Phenotypic and functional characterization of HSCs in Gata3fl/fl Vav-Cretg/+ mice. (A) Gata3 mRNA expression in 1-week-old WT LSKFLT3−CD150+CD48− HSCs, LSKFLT3hiIL7R− lymphoid-primed multipotent progenitors, and Lin−CD25−KIT+FLT3+ early thymic progenitors. Data are expressed as mean (SEM) normalized Robust Multi-Array Averages (RMA) expression (“Gene expression analysis”). n = 3 experiments. (B) BM cellularity in 1-week-old Gata3fl/flVav-Cre+/+ and Gata3fl/flVav-Cretg/+ mice. Data are mean (SD); n = 3 or 4 mice/genotype. ns indicates not significant. (C) Fluorescence-activated cell sorter profiles from representative mouse showing gating strategy for LSKCD150+CD48− cells in 1-week-old mice. Numbers indicate percentage of total BM cells within the indicated gate. n = 3 or 4 mice/genotype. (D) Bar graphs represent mean (SD) frequency of LSKCD150+CD48− cells. n = 3 or 4 mice/genotype. ns indicates not significant. (E) Bar graphs show mean (SEM) Gata3 mRNA expression levels (relative to Hprt) in LSKCD150+CD48− HSCs isolated from 1- to 2-week-old Gata3fl/fl Vav-Cre+/+ or Gata3fl/fl Vav-Cretg/+ mice. (F-I) A total of 0.5 to 2 million BM cells from 1- to 2-week-old Gata3fl/fl Vav-Cre+/+ or Gata3fl/fl Vav-Cretg/+ mice (CD45.2) were transplanted into lethally irradiated (900 cGy) WT (CD45.1) recipients together with a similar dose of competitor WT (CD45.1) adult BM cells. (F) Representative fluorescence-activated cell sorter analysis of peripheral blood 4 months after transplantation. Numbers indicate percentage of indicated gate within reconstituted T (CD4+/CD8+), B (CD19+), and myeloid (Gr1+Mac1+) cells. n = 3 to 6 mice/group. (G-H) Mean (SEM) percentage of test cell (CD45.2+) reconstitution within the T, B, and myeloid blood cell lineages, respectively, 4 to 6 (G) or 11 (H) months after transplantation. (G) n = 6 to 9 mice/group. (H) n = 2 or 3 mice/group. (I) Six months after primary transplantation, one-half femur equivalent from each primary recipient was transplanted into one secondary WT (CD45.1) recipient. Secondary recipients were analyzed 8 months later. Data show mean (SEM) percentage of test cell reconstitution within T, B, and myeloid reconstituted cells, respectively. N = 3 to 6 mice/group. 1° indicates primary reconstitution; and 2°, secondary reconstitution. *P < .05. ***P < .001. ns indicates not significant.
Phenotypic and functional characterization of HSCs in Gata3fl/fl Vav-Cretg/+ mice. (A) Gata3 mRNA expression in 1-week-old WT LSKFLT3−CD150+CD48− HSCs, LSKFLT3hiIL7R− lymphoid-primed multipotent progenitors, and Lin−CD25−KIT+FLT3+ early thymic progenitors. Data are expressed as mean (SEM) normalized Robust Multi-Array Averages (RMA) expression (“Gene expression analysis”). n = 3 experiments. (B) BM cellularity in 1-week-old Gata3fl/flVav-Cre+/+ and Gata3fl/flVav-Cretg/+ mice. Data are mean (SD); n = 3 or 4 mice/genotype. ns indicates not significant. (C) Fluorescence-activated cell sorter profiles from representative mouse showing gating strategy for LSKCD150+CD48− cells in 1-week-old mice. Numbers indicate percentage of total BM cells within the indicated gate. n = 3 or 4 mice/genotype. (D) Bar graphs represent mean (SD) frequency of LSKCD150+CD48− cells. n = 3 or 4 mice/genotype. ns indicates not significant. (E) Bar graphs show mean (SEM) Gata3 mRNA expression levels (relative to Hprt) in LSKCD150+CD48− HSCs isolated from 1- to 2-week-old Gata3fl/fl Vav-Cre+/+ or Gata3fl/fl Vav-Cretg/+ mice. (F-I) A total of 0.5 to 2 million BM cells from 1- to 2-week-old Gata3fl/fl Vav-Cre+/+ or Gata3fl/fl Vav-Cretg/+ mice (CD45.2) were transplanted into lethally irradiated (900 cGy) WT (CD45.1) recipients together with a similar dose of competitor WT (CD45.1) adult BM cells. (F) Representative fluorescence-activated cell sorter analysis of peripheral blood 4 months after transplantation. Numbers indicate percentage of indicated gate within reconstituted T (CD4+/CD8+), B (CD19+), and myeloid (Gr1+Mac1+) cells. n = 3 to 6 mice/group. (G-H) Mean (SEM) percentage of test cell (CD45.2+) reconstitution within the T, B, and myeloid blood cell lineages, respectively, 4 to 6 (G) or 11 (H) months after transplantation. (G) n = 6 to 9 mice/group. (H) n = 2 or 3 mice/group. (I) Six months after primary transplantation, one-half femur equivalent from each primary recipient was transplanted into one secondary WT (CD45.1) recipient. Secondary recipients were analyzed 8 months later. Data show mean (SEM) percentage of test cell reconstitution within T, B, and myeloid reconstituted cells, respectively. N = 3 to 6 mice/group. 1° indicates primary reconstitution; and 2°, secondary reconstitution. *P < .05. ***P < .001. ns indicates not significant.
BM cells from Gata3fl/flVav-Cre+/+ or Gata3fl/flVav-Cretg/+ mice were transplanted in competition with WT BM cells into lethally irradiated recipients. As evidence of the complete recombination induced by Vav-Cre, Gata3 null BM cells failed to contribute to T-cell reconstitution, whereas B-cell (CD19+) and myeloid (Gr1+Mac1+) reconstitution was unaffected 4 to 6 months (Figure 1F-G) and 11 months (Figure 1H) after transplantation. The myeloid and B-cell lineages remained reconstituted to a similar degree in Gata3-deleted as nondeleted BM cells, as late as 8 months after transplantation into secondary recipients (Figure 1I), demonstrating a dispensable role for GATA3 in HSC self-renewal.
Vav-Cre-induced recombination in the present studies ensured highly efficient hematopoietic recombination14 while allowing viable Gata3 null embryos to develop despite Gata3 deletion occurring already shortly after emergence of HSCs in the early embryo. This approach, combined with state-of-the-art HSC phenotypical analysis, and long-term HSC reconstitution assessment, including secondary transplantations, unequivocally establishes that GATA3 is dispensable for HSC regulation at multiple levels, including steady-state maintenance and self-renewal, as well as the expansion that takes place during development and after transplantation.22
GATA2 and GATA3 can partially rescue the Gata1-deficient erythroid phenotype.23,24 As GATA2 has been demonstrated to play a key role in HSC regulation,5 it remains plausible that a role of GATA3 in HSC regulation might be redundant to that of GATA2, and that this could be revealed in Gata2-deficient HSCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Denise Jelfs, Caroline Barnwell, Jordan Tanner, Dominik Woznica, and Fred Dickinson for expert animal support, Helen Ferry for help with cell sorting, Adam Mead for assistance in fluidigm gene expression analysis, Luca Melchiori for assistance in fluorescence-activated cell sorter analysis, Dr Jinfang Zhu for the GATA3fl/fl mice, Dr Kioussis for the Vav-iCre mice, and Dr Shankar Srinivas for the R26R-eYFP mice.
This work was supported by the EuroSyStem Integrated project (grants EU-FP7 STEMEXPAND and EU-FP7) and the Medical Research Council, United Kingdom (program grant; S.E.W.J.). S.D. was supported by the Fundação para a Ciência e a Tecnologia, Portugal.
Authorship
Contribution: S.E.W.J. and N.B.-V. designed and conceptualized the overall research, analyzed the data, and wrote the manuscript; N.B.-V. performed the transplantations and phenotypic fluorescence-activated cell sorter analysis; S.D. performed gene expression analysis; S.D., P.S.W., and T.B.-J. performed FACS analysis of stem/progenitors and peripheral blood; and S.L. performed affymetrix analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sten Eirik W. Jacobsen, Haematopoietic Stem Cell Laboratory, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, University of Oxford, Headley Way, OX3 9DS Oxford, United Kingdom; e-mail: sten.jacobsen@imm.ox.ac.uk.