Abstract
This review presents a cohesive approach to treating patients with Gaucher disease. The spectrum of the clinical presentation of the disease is broad, yet heretofore there was only one disease-specific treatment. In the past 2 years, a global shortage of this product has resulted in reassessment of the “one enzyme–one disease–one therapy” mantra. It has also showcased the multiple levels that engage the patient, the treating physician, and the third-party insurer in providing adequate treatment to all symptomatic patients. The key points summarizing the way I manage my patients include accurate enzymatic diagnosis with mutation analysis (for some prognostication and better carrier detection in the family), a detailed follow-up every 6-12 months (with an option to see consultants and attention to comorbidities), and initiation of enzyme replacement therapy according to symptoms or deterioration in clinically significant features or both. I do not treat patients with very mild disease, but I consider presymptomatic therapy for patients at risk, including young women with poor obstetric history. I prefer the minimal-effective dose rather than the maximally tolerated dose, and when the difference between high-dose and lower-dose regimens is (merely statistically significant but) clinically meaningless, minimizing the burden on society by advocating less-expensive treatments is ethically justified.
Introduction
The purpose of this review is to present a cohesive approach to treating patients with Gaucher disease (GD). As can be appreciated, the spectrum of the clinical presentation of the disease is broad, yet heretofore there was only one disease-specific treatment. In the past 2 years, a global shortage of this product has resulted in reassessment of the “one enzyme–one disease–one therapy” mantra. It has also showcased the multiple levels that engage the patient, the treating physician, and the third-party insurer, in providing adequate treatment for all symptomatic patients with GD. As a treating physician dedicated to GD even before the era of enzyme replacement therapy (ERT), my mandate and inspiration has always been to provide the best therapy with the least malfeasance.
Description of GD
GD is one of the most common glycolipid storage disorders, caused by an inherited deficiency of the lysosomal enzyme β-glucocerebrosidase, leading to accumulation of the substrate glucocerebroside in the cells of the macrophage-monocyte system.1 Accordingly, key disease features are related to splenomegaly with hypersplenism, hepatomegaly, and bone involvement. Although a single-gene disorder, phenotypic expression is extremely variable, ranging from totally asymptomatic (only detectable by DNA analysis or enzyme deficiency) to a lethal newborn form with hydrops fetalis and ichthyosis. Many manifestations, some not-uncommon and others very rare, cannot be explained by storage per se; examples include immunologic abnormalities, increased prevalence of certain malignancies with relative paucity of others, neurologic comorbidities (peripheral neuropathy and Parkinsonism), calcification of cardiac valves, and pulmonary hypertension. Yet, most patients will present or develop signs/symptoms that are commonly managed by hematologists, for example, anemia, thrombocytopenia, and splenomegaly. In the past 2 decades, GD has become a model for other lysosomal storage disorders (LSDs), particularly because of the introduction of safe and effective (albeit extremely expensive) ERT,2 as well as substrate reduction therapy (SRT),3 and other modalities.
Since the glucocerebrosidase gene was cloned,4,5 > 300 mutations have been identified,6 partly explaining the great phenotypic heterogeneity. Because many are private mutations and others may be either single or combined mutations in a complex allele, whole-gene sequencing is recommended for accurate genotyping. I underscore this point because, although genotype-phenotype correlations are imperfect, accurate genotyping for individual patients is important to predict probable prognoses.
Classic subtypes of GD
GD has been traditionally classified into 3 clinical forms, based on the absence (type I) or presence (types II and III) of neurologic involvement. Type I, also known as chronic, nonneuronopathic, is by far the most common form in the Western hemisphere, whereas in Asian and Arab countries type III is probably predominant.7,8 GD is pan-ethnic, but like other autosomal-recessive disorders, there are some ethnic predilections: importantly, a high prevalence of type I GD, especially the N370S and 84GG mutations, among Ashkenazi Jews (carrier frequency, 1:17; disease prevalence, 1:850).1 There are also 2 unique associations with neuronopathic forms: type IIIb, characterized by predominance of visceral features over neurologic signs, initially identified in northern Sweden; and type IIIc, the very rare cardiac variant, among Palestinian Arabs (Table 1).9 The prevalence of GD in the general population is probably > 1:40 000.10
Key characteristics of the 3 clinical forms (including subclassifications) are summarized in Table 1. With the identification of new phenotypes and appreciation that even patients with type I may evince some (late-onset) neurologic manifestations, there is growing support to view GD as a continuum of phenotypes11 ; however, subcategorization into archetypical forms, despite its arbitrary nature, which may not fit all patients, is still useful when talking about management options and in particular as a basis for genetic counseling. In addition, it should be clarified that the neurologic features in type I GD are different from those reported in the neuronopathic forms (type II and III), consisting mainly of peripheral neuropathy, Parkinsonism, and neurologic changes secondary to bone complications.12
Diagnosis
Enzyme activity: a requisite for disease identification
Because most patients with significant visceral involvement (types I and III) will benefit from ERT, and earlier ERT may prevent development of irreversible complications such as avascular necrosis (AVN) of large joints and height retardation in children, accurate and early diagnosis is critical. Detection of low enzymatic activity of β-glucocerebrosidase in peripheral blood cells compared with (same-day) normal controls is still the “gold standard” for diagnosis of GD. It is perturbing that, despite availability of this test for nearly 4 decades and considered to be “textbook knowledge,”1,13 many patients still experience a “diagnostic odyssey” before achieving the correct diagnosis14 ; moreover, we still see misuse of BM aspiration for diagnosis.15 BM examination is the wrong diagnostic test not only because it may be traumatic for patients, but also primarily because it is nonspecific (pseudo-Gaucher cells are detected in many hematologic disorders) and therefore may result in a false-positive diagnosis.
In addition, we are aware of false-positive diagnoses because of PCR-based mutation analysis: carriers of GD may be seen as having GD because 2 mutations are identified, but actually they are on the same (complex) allele.16 A further source of false-positive diagnoses is inherent in dry (whole) blood-spot methods.17 Although this method may be suitable for large-scale screening (of many LSDs), it cannot be relied on for definitive diagnoses. Unfortunately, the true status of persons misdiagnosed on the basis of any of the above-mentioned reasoning is usually discovered only after lack of response to ERT.
Enzymatic assay is indicated in patients with any of the clinical features in Table 1 and in patients diagnosed by BM aspiration or mutation analysis; it is also recommended for prenatal testing when both parents are carriers and the question of early termination of an affected fetus is being considered. Unfortunately, there are only a few laboratories that do perform reliably the enzymatic diagnosis on chorionic villous samples or amniocytes, and most of the genetic institutes today prefer full gene sequencing.
When is a BM aspirate appropriate?
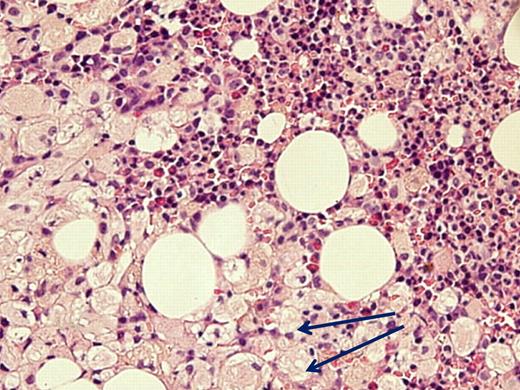
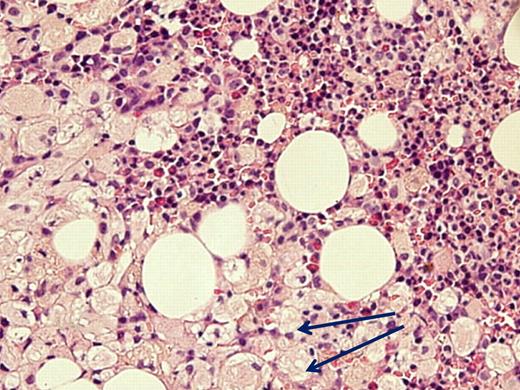
Despite advocating elimination of BM aspiration for the sole purpose of GD diagnosis, there are valid indications for this examination in GD, all related to associated diseases. (1) When the clinical presentation is atypical and differential diagnosis includes entities that can be identified/excluded by BM smear such as idiopathic thrombocytopenic purpura or acute leukemia. There are invariably patients who have 2 independently sorting disorders at presentation, such as GD and a hematologic malignancy (eg, one of our patients had a monoclonal IgA peak on routine work-up for GD; another with severe pancytopenia had acute myelogenous leukemia; Figure 1) and for whom BM examination will pinpoint the diagnosis/diagnoses. (2) When there is an unexplained deterioration in blood counts despite ERT in a symptomatic patient, or acute deterioration in a heretofore stable untreated patient, BM examination may show/exclude hematologic pathology. (3) When there is no response to ERT.
Acute myelogenous leukemia in a patient with otherwise asymptomatic GD. The Gaucher cells are indicated by arrows. Courtesy of Drs Ginette Shibi, Iris Barshack, and Hannah Maayan, Sheba Medical Center, Tel-Hashomer, Israel.
Acute myelogenous leukemia in a patient with otherwise asymptomatic GD. The Gaucher cells are indicated by arrows. Courtesy of Drs Ginette Shibi, Iris Barshack, and Hannah Maayan, Sheba Medical Center, Tel-Hashomer, Israel.
Note that for most the above-mentioned situations, particularly for unexplained cytopenias or when dealing with associated diseases wherein the large number of Gaucher cells may interfere with identifying the pathology, a biopsy should be performed in addition to aspiration to get a clearer histologic picture. In fact, not uncommonly, there will be a dry tap that would inevitably lead to a biopsy of the BM in any case.
Routine follow-up of patients
There is no substitute for a good intake interview, and this is also true in patients with GD. There are associated conditions that are truly independently sorting, whereas others, such as gallstones,18,19 multiple myeloma,20-22 or early-onset Parkinsonism,23,24 are more prevalent in GD. Similarly, it is recommended to specifically query about Gaucher-like signs and symptoms, as well as of associated disorders, in first-degree relatives.25 In patients with rare genotypes and severe presentation at an early age, supranuclear horizontal gaze palsy (pathognomonic of neurologic GD involvement) may not be overt; therefore, a high index of suspicion should be maintained for type III GD.
Among routine blood tests (complete blood count and biochemistry) taken every 6-12 months, I want to mention the following: fasting glucose levels for adults (because of the presence of type 2 diabetes mellitus among some ERT-treated patients26 ), immunoglobulins (because of increased incidence of multiple myeloma and other gammopathies), and liver enzymes (because abnormal findings may implicate unrelated liver disease). Iron, folate, vitamin D, and vitamin B12 are important parameters not only to exclude other causes of anemia, but also because deficiencies may lead to symptoms mimicking those of GD. Many mistake high ferritin levels seen in GD as pathologic, yet in most patients it is a benign biomarker associated with disease severity and decreases with ERT.27
Biomarkers
Biomarkers add quality assurance to biochemical diagnoses but, more importantly, quantify dynamic changes in the clinical course over time. I see no added value in previously used biomarkers such as tartrate-resistant acid phosphatase or angiotensin-converting enzyme or hexosaminidase. Currently, the most reliable biomarker in GD is chitotriosidase28 ; CCL-18 is a substitute option for persons who genetically lack chitotriosidase.29 Reduction in chitotriosidase activity reflects improvement; therefore, chitotriosidase levels are among the evaluable therapeutic goals of treatment.30 Unchanged chitotriosidase activity or regression to higher levels may indicate need for reassessment of status (eg, misdiagnosis, comorbidity) or reevaluation of ERT dose or modality or both. For untreated, mildly affected patients, static biomarker activity is expected; increased chitotriosidase activity should galvanize the physician to closely monitor disease-specific parameters and, if those too have deteriorated, consider treatment; nonetheless, there is no justification in starting ERT merely because of high chitotriosidase levels in an otherwise asymptomatic patient. The use of absolute level of chitotriosidase activity as a sole index of disease burden is also hampered by the variation in genetic polymorphisms in the chitotriosidase gene.29
Imaging modalities
My experience with ultrasound scanning for routine follow-up of organ volumes has been rewarding despite reliance on a single radiologist31 ; for specific indications and for clinical trial assessments, magnetic resonance imaging is preferable.
Although (secondary) pulmonary hypertension (PH) is an expression of severe GD, primary-like PH may be a complication of ERT.32,33 I request annual echocardiography in adults to gauge tricuspid insufficiency but have discontinued it in the pediatric population because I have never diagnosed PH in children.34
Bone density at the lumbar spine and femoral neck is commonly performed in adults biannually (regardless of ERT status), and, recently, because pediatric normal standards have become available, I have added this examination for younger patients in the hope of being able to track/predict skeletal complications. Paradoxically, high density at the femoral neck may indicate osteonecrosis. Individual adult patients with low bone density, ERT-treated and untreated, benefit from bisphosphonates as an adjunct.35,36
Bone scans have not proven helpful except in ascertaining concomitant disorders.
I give patients the opportunity to consult with specialists experienced in GD as part of routine follow-up, particularly orthopedists and obstetricians, which I view as integral to good medical care.37,38 Patients' expectations about improvement in disease-specific parameters with therapeutic interventions are an important aspect of routine visits. Patients may or may not have realistic ideas of what disease-specific therapy may accomplish. It is for this reason that treating physicians should be familiar with the therapeutic goals that currently serve as benchmarks for ERT.25,39 Most patients (regardless of dosing) are expected to achieve satisfactory improvement in hematologic parameters and biomarkers within 2-3 years with subsequent plateauing on near normalization. Inevitably, there are some poor responders who do not derive optimal benefit (most commonly, remain thrombocytopenic). I would recommend patients be cautioned that these goals are approximates at best, and overall improved quality of life is the ultimate goal rather than specific numbers of platelets or multiples of normal of splenic volumes. Lung and skeletal involvement can be viewed as inured to immediate response.
Management
Surgical procedures: splenectomy
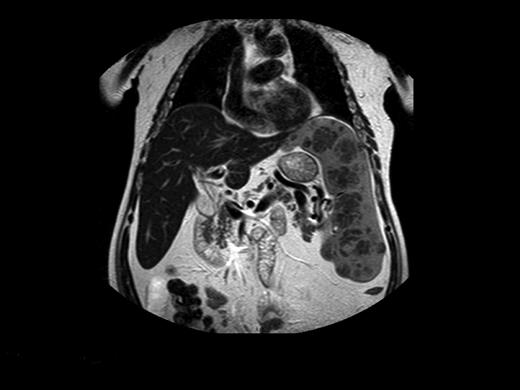
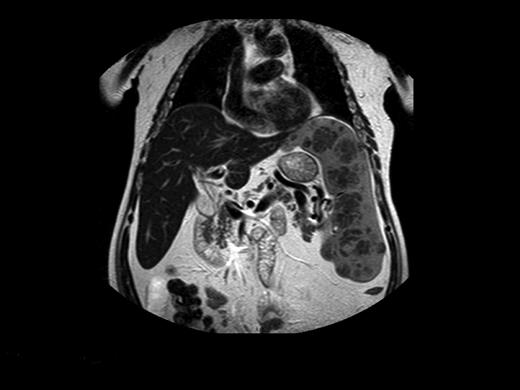
Before commercially available ERT in the 1990s, management of patients with GD was largely symptomatic. Beyond blood transfusions, iron supplementation, and treatment of infections, the important therapeutic modality was surgery. Total or partial splenectomy was the mainstay for massive splenomegaly or severe pancytopenia, but it is less needed since the advent of ERT. However, even with ERT, there are patients with massive splenomegaly and devastating parenchymal damage, including focal lesions, fibrosis, or ischemia, which may limit the organ's ability to respond to ERT (Figure 2).40 We have performed total splenectomies in very few such patients who failed to improve despite years of ERT (including high-dose regimens), specifically, unchanged thrombocytopenia or splenic volume. Dramatic improvement after splenectomy has several features: immediate normalization of platelet counts; decreased fatigue and bleeding tendencies; amelioration of the mechanical component that induced early satiety, abdominal discomfort, and possibly exertional dyspnea; and, for young women, freeing the abdominal cavity for an expanding uterus during pregnancies. I therefore consider the option of splenectomy for patients with huge splenomegaly who are unresponsive to therapy. ERT should be continued after splenectomy to protect against skeletal damage and hepatic complications, although this cannot be guaranteed. Splenectomy today (even for huge organs) may be performed laparoscopically, with improved postoperative recovery,41 a procedure that has been performed successfully in 3 of our patients with massive splenomegaly.
Magnetic resonance image of a 72-year-old man who did not respond to long-term high-dose ERT and improved after splenectomy. Courtesy of Dr George Blinder, Mar-Mor, Jerusalem, Israel.
Magnetic resonance image of a 72-year-old man who did not respond to long-term high-dose ERT and improved after splenectomy. Courtesy of Dr George Blinder, Mar-Mor, Jerusalem, Israel.
Surgical procedures: orthopedic surgeries
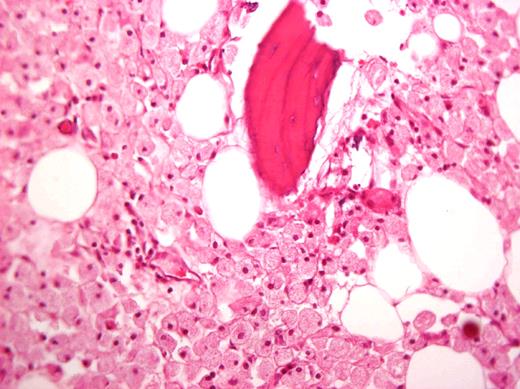
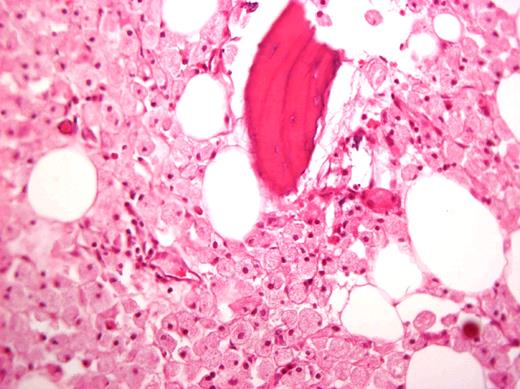
In the past, large joint replacement was not offered in GD because of concern of introducing prostheses into a compromised milieu. This bias has been proven to be wrong, and patients with GD should be candidates for arthroplasty on the basis of criteria of pain and poor function just like others.38-43 Moreover, orthopedic surgery is still a mainstay of GD management for patients who have developed irreversible joint damage before ERT and patients with skeletal complications despite ERT.43 I recommend a team approach before surgery particularly for patients with bleeding tendencies. Interestingly, Gaucher cells in the femoral head are often seen despite long-term ERT (Figure 3).44,45
Histologic specimen from a femoral head removed during arthroplasty of a patient after 15 years of ERT. Courtesy of Dr Ehud Lebel, Shaare Zedek Medical Center, Jerusalem, Israel.
Histologic specimen from a femoral head removed during arthroplasty of a patient after 15 years of ERT. Courtesy of Dr Ehud Lebel, Shaare Zedek Medical Center, Jerusalem, Israel.
Surgical procedures: obstetric considerations
Cesarean sections, when indicated for obstetric reasons, may be associated with increased (mainly hemorrhagic) risks.46 An antenatal birth plan, taking into account all eventualities, especially hemorrhage, and method of pain relief, is ultimately the best preparation for birth.47
A related issue is fertility, which is unimpaired in GD (female and male), and large families are not to be discouraged if this is the patient's inclination. I have some patients who have successfully undergone in vitro fertilization.
Another important issue is genetic counseling. In GD, as in other rare diseases, I strongly recommend meeting a recognized expert who can provide the best possible medical information, in addition to meeting with a genetic counselor.48,49 Carrier screening for GD is controversial because type 1 GD is often asymptomatic and effective treatment exists. However, both premarital and prenatal screenings are offered to Ashkenazi Jews worldwide and have been practiced in Israel since 1995. My general approach is to encourage continuation of pregnancy when the affected fetus is homozygous for the N370S mutation and to support pregnancy termination when the genotype is predictive of a neuronopathic type. I currently offer the option of preimplantation genetic diagnosis as a tenable approach for couples (carriers and patients) in whom a lethal or severe genotype is probable. Here, too, I have had good success.50
ERT: alglucerase
The introduction of the first enzymatic therapy in 1991, the placenta-derived macrophage-targeted glucocerebrosidase, alglucerase (Ceredase; Genzyme Corp) led to a veritable revolution in GD management and subsequently introduced the option of ERT for other LSDs.1,2 Unquestionably, it has been a personal triumph for Roscoe Brady, who championed the concept of ERT and persevered with unfailing enthusiasm, persistence, and dedication.51
The results of the seminal trial, albeit relatively small (12 patients) and short term (9 months), highlight what is achievable with ERT and what is still expected of ERT today: reduction in hepatosplenomegaly, improvement in hypersplenism, decreased biomarkers, and amelioration of bone pain, together with a reliable safety profile.1,2,27,51-54 The original dosage used was 60 U/kg of body weight (BW) every other week (the high-dose regime”), which is still the most frequently used in clinical trials and accordingly highly promoted by the manufacturers.
The key disadvantages of ERT were also noted soon after its introduction: potentially life-long dependency on intravenous treatment, side effects of repeat infusions, for some premedications for allergic reactions, and, finally, the nontrivial aspect of exorbitant cost. In an attempt to combat cost, Ernest Beutler introduced the low-dose regimen, originally administered at the high frequency of 3 times weekly,55 but which was no less effective at the low-frequency regimen of every other week.56 Today, the schedule of 15 U/kg every other week for adult patients and 30 U/kg every other week for children has proven to be safe and effective even in patients with severe disease and will probably result in near-normalization of disease-specific hematologic parameters and organ volumes in a timeframe not greatly different from that of patients on higher doses.57 The issue of maintenance regimens remains unresolved.
ERT: imiglucerase
Imiglucerase, the Chinese hamster ovary cell–derived recombinant ERT (Cerezyme; Genzyme Corp) was approved by the Food and Drug Administration (FDA) in 1994 and shortly thereafter replaced alglucerase, based, again, on a short-term high-dose comparative trial (imiglucerase vs alglucerase) that showed comparable safety and efficacy58 and on our center's low-dose (thrice a week vs every other week) schedule, with similar safety and efficacy results.56 Interestingly, no regulatory authority requested a safety switch-over trial from alglucerase to imiglucerase.
Until recently, imiglucerase enjoyed a monopoly with > 5000 patients receiving imiglucerase; key demographic and clinical data for segments of this cohort have been collected and analyzed by the Genzyme-sponsored international Gaucher registry (ICGG).39,48,59-64
In addition to theoretically better long-term safety (recombinant vs tissue extracted), the efficacy of imiglucerase has been comparable to that of alglucerase. Ancillary long-term observations have also been underscored: individual heterogeneity in magnitude of response, plateauing of response of all parameters to ERT (all doses) after 2-5 years, and greater resistance of bones and lungs to ERT.
The safety profile of imiglucerase has withstood the test of time; the incidence of side effects (based on pharmacovigilance reports of > 16 years; ICGG data and personal experience) has been low, and mostly mild and transient in nature,2,56-65 allowing clinicians to implement home therapy66 and encouraging clinicians to use ERT during pregnancy to improve outcome.53,67 Antiglucocerebrosidase antibodies, mostly nonneutralizing, were reported in 15% of treated patients, whereas allergic reactions developed in 6.6% of treated patients, not necessarily those with antibodies. What is missing in the literature is the number of patients receiving premedication, specifically those receiving intravenous hydrocortisone before infusion, and whether this induced AVN.
Unfortunately, because of its inability to penetrate the blood-brain barrier, even in megadoses, ERT does not affect neurologic features of type III GD.68-70 We have reported our ethical considerations in opposing ERT for infants with type II disease.71 The impotence of ERT in the face of neurologic and cognitive decline in young adults with type III GD is an imperative for new modalities.
The 2009-2010 crisis in imiglucerase supply
Hypothetical concerns about the lack of alternative disease-specific therapies for GD were spotlighted in June 2009, when the Genzyme Corporation announced a viral contamination at its manufacturing site.72 The dramatic reduction in global supply to 20% led to ERT unavailability or significant dose reductions in many patients worldwide. It also led to prelicense availability of 2 ERTs by early access programs at the request of regulatory authorities.73 This shortage certainly stimulated interest in the 2 new ERTs being developed at that time, and the finding that these alternatives were safe and effective was certainly timely.
The unexpected imiglucerase shortage engendered an opportunity to revisit the question of ERT withdrawal for short (but well-monitored) periods, which was controversial.74-76 Current reports from 3 different countries suggest that, although there were no clinically significant short-term complications, most of the patients on drug-holiday had deterioration in platelet counts and biomarkers, with or without reappearance of fatigue, bruising,77,78 or bone pains.79 Hence, I no longer consider drug vacations as a maintenance option.74,78 Whether there will be any long-term effect of ERT withdrawal requires longer follow-up.80
New ERTs: velaglucerase alfa
In February 2010, Shire Human Genetics Therapies received FDA approval for its gene-activated human glucocerebrosidase, velaglucerase alfa (VPRIV), and subsequently approval by the European Medicines Agency in August 2010, as well as in other countries (including Canada, Brazil, and Israel). Velaglucerase alfa, has potential advantages because it is produced in a human cell line with the wild-type human sequence (imiglucerase has a single amino acid substitution at position 495).81 Three phase 3 clinical trials have been successfully completed: a 2-dose trial in treatment-naive patients, a switch-over trial (from imiglucerase), and a head-to-head high-dose comparison with imiglucerase.
Velaglucerase alfa was originally tested in a 9-month phase 1/2 open-label trial in our single center and is on-going as an extension study82 after dose reduction from 60 U/kg BW to 30 U/kg BW fortnightly after 15-18 months. Currently, patients in the extension have achieved 7 years of long-term follow-up.
Today, > 1000 patients worldwide are treated with velaglucerase alfa, including those in compassionate/early access protocols, and it appears that velaglucerase alfa is associated with fewer hypersensitivity reactions and fewer antibodies than the other enzymes.
New ERTs: taliglucerase alfa
Protalix Biotherapeutics produced a plant cell–expressed enzyme, taliglucerase alfa. The main advantages of taliglucerase alfa relate to its manufacturing platform: a high-yield plant cell system that is easily up-scalable in disposable bioreactors, and, because nonmammalian tissues are used, it may provide long-term safety and cost efficacy.83,84 Because of these features, the FDA waived phase 2 after a successful phase 1 trial in healthy volunteers. The phase 3 two-dose clinical trial achieved good safety and efficacy profiles, affording prelicense use in several countries by early-access protocols.73 In addition, there are currently 2 on-going clinical trials that use this enzyme in patients previously treated with imiglucerase (a switch-over study) as well as a 2-dose study in children naive to ERT.
SRT: miglustat
The imino sugar N-butyl deoxynojirimycin, miglustat, an inhibitor of glucocerebroside synthase, the first committed step in glycolipid biosynthesis, was a harbinger of oral substrate inhibitors for GD, as first suggested by Radin in 1976.85 Although clinical trials showed significant effects on key disease parameters,3,86,87 the problematic safety profile led to a relatively narrow label indication when miglustat (Zavesca; Actelion) was approved by the European Medicines Agency for patients with mild-to-moderate GD who are unsuitable for ERT (2002) and by the FDA for patients in whom ERT is not a therapeutic option (2003). Nevertheless, with no other modalities capable of affecting neurologic features, this SRT has the potential to cross the blood-brain barrier and is viewed as a prototype for therapeutic management of neuronopathic forms. Moreover, the drug is oral, obviating many of the inconveniences of intravenous ERT. Unfortunately, the clinical trial with miglustat in type III GD failed to achieve neurologic benefits.88 because of its inferior efficacy in patients with type I GD (compared with ERT) combined with a higher prevalence of side effects,89 I do not prescribe it to my patients.
SRT: eliglustat
Another SRT has recently begun phase 3 clinical trials. Eliglustat (Genzyme Corp) is a ceramide analog of the substrate (unlike the glucose moiety as in miglustat) with a better safety profile and higher potency than miglustat. The 2-year results of the phase 2 trial have shown dramatic improvement in key clinical parameters in 20 of 24 patients with type I GD.90 Although this oral SRT will probably achieve market approval (pending satisfactory safety data), it will require long-term experience (longer than for the new enzymes) because of its complex cytochrome P450 metabolism that complicates the use of some medications91 and because of potential nontrivial cardiotoxicity. Importantly, eliglustat does not penetrate the blood-brain barrier and hence has no added value for type III GD.
Pharmacologic chaperone therapy
Pharmacologic chaperone (PC) therapy is a new strategy to increase residual activity by stabilizing misfolded mutant proteins, preventing endoplasmic-reticulum–associated degradation in proteosomes and allowing trafficking to lysosomes.92,93 This approach is especially applicable in GD because only a modest increase in residual glucocerebrosidase should be sufficient to ameliorate the phenotype. Moreover, these small molecules should be able to cross the blood-brain barrier.
The first PC in clinical trial used isofagomine tartrate (Amicus Therapeutics),93 but phase 2 trials failed to meet endpoints, and further development was abrogated.
A second PC is ambroxol hydrochloride (ExSAR Corporation),94 originally developed as a mucolytic agent 30 years ago (Mucosolvan; Boehringer-Ingelheim), and available over the counter in many countries. Ambroxol has also been used for treatment or prophylaxis or both of neonatal respiratory distress syndrome.95 We have administered Ambroxol off-label to 12 mildly affected patients with type I GD in 2009 with only the 2 thinnest patients having positive results, suggesting the need for higher doses. Hence, formal clinical trials, using higher doses, are necessary before ambroxol can be considered for its potential to benefit type III GD. However, PCs may be the best option moving forward, and I encourage creative thinking in identifying new compounds.
I see a real advantage to oral therapy and in the case of SRTs and PCs can envision these in combination with ERT or developed as maintenance options. It is to be hoped that among these medications there will be at least one that will ameliorate neuronopathic features.
Future modalities
I have not discussed gene therapy96 ; although theoretically promising, there is no ethical justification to experiment on patients who have alternative, safe, and effective treatment. However, stem cell therapy with a low-risk conditioning regimen97 may have a role in GD and other metabolic disorders, but, again, there is the challenge of affecting neurologic features.
Unresolved and controversial issues
Traditionally, one concludes discussions of the current state of knowledge of GD by mentioning unresolved issues. This rubric includes those disease features that challenge our understanding of GD pathophysiology and its management, such as accurate prognostication, the differential effect of therapies on bones and lungs, the effect of putatively little storage material on brain, associated conditions, the relationship to energy expenditure and glucose/insulin resistance, the long-term effects of exogenous enzyme, and more.
However, unresolved issues in GD also include the complicated interplay among the diverging experiences with the disease and its therapies that eventually devolve down to the fundamentals of whether to initiate treatment in asymptomatic patients, the dosage, and the commitment to life-time therapy. Although many think that all patients should be treated, I am convinced that a wait-and-see attitude has merit in very mildly affected patients; whereas most begin with high doses, I recommend starting with 15 U/kg fortnightly; whereas many think that high-dose life-long ERT is absolutely benign, I disagree.98
Worrisome are reports of association of mild GD with cancers,99,100 cardiovascular and cerebrovascular events, and decreased life expectancy.101 Because many of the larger series are culled from the ICGG database, one cannot disregard these concerns. Although we also noticed the increased incidence of monoclonal gammopathies and multiple myeloma in GD,20,21 we have not seen an increased incidence of other malignancies; indeed, we query the low incidence of breast, ovarian, and prostate cancers relative to the general Ashkenazi Israeli population.20,98,102 Similarly, although not formally studied, we have not seen any unusual trends for increased vascular events or for decreased life expectancy among our patients.
Summary: how I treat
The following summarize critical points in optimal management of patients with GD.
First and foremost is accurate enzymatic diagnosis combined with whole-gene sequencing mutation analysis and very detailed medical and family history.
Genetic counseling should always be available.
Routine biannual follow-up for all treated patients, children, and untreated adult patients with unstable parameters; annual evaluations for adults with stable disease.
Initiation of disease-specific therapy (currently ERT) should not be determined solely by absolute numbers, but rather by symptoms or deterioration over time in clinically significant GD features. For patients with a “bad” genotype or a family history of older siblings with severe manifestations (eg, AVN), presymptomatic ERT should be considered.
Presentation of symptoms in toddlerhood is always concerning, and the index of suspicion (when there is no N370S mutation) for neuronopathic disease should be high.
However, for patients with mild mutations, the probability of a lifetime free of signs/symptoms is realistic, and disease-specific therapy would not be warranted.
A caveat to the above is that some young women may benefit from ERT through childbearing years, even if their disease is milder, to maximize good outcome of pregnancies and deliveries; this does not apply to asymptomatic patients with uncomplicated obstetric histories.67
Preparation for surgery (including dental procedures) and birth should include platelet function tests in addition to the routine assessment of blood counts and coagulation factors.
Dosage of ERT remains controversial in the sense that no overriding authority has evidence-based data to resolve the issue. I believe it is better to administer the minimally effective dose rather than the maximally tolerated dose.
There indeed are poor responders, and increasing doses will probably make little difference for these patients. Changing from one ERT to another may be indicated (if, for example, there is a hypersensitivity or neutralizing antibodies to the first ERT) and, possibly, in the future, changing modalities would be reasonable.
In discussing the availability of new ERTs and emergence of small molecules in clinical trials, I maintain an open-minded attitude and am nonjudgmental once the patient/parents reach a decision. Happily, today, there are choices, and, although differences are rather less significant than similarities, the basis for the ultimate choice is usually difficult to divine a priori. Being prepared with all pertinent data is the best way to discuss therapeutic options.
The “personalized” approach to the patient (and his or her family) is the cornerstone of my clinic's perspective of GD, although our clinic has > 750 patients.
The context of cost of therapy for GD is a heavy component in national health budgets, and many would question whether this is equitable.103,104 It behooves us therefore to remind ourselves, the patients, and the pharmaceutical companies, that, although it is a privilege to receive excellent treatment, it is also our joint responsibility to minimize the burden on society wherever possible. I believe this is an attainable goal if we appreciate that at this point most ERTs, for instance, are approximately equally safe and effective, and if cost is drastically less for one, this has importance to the person as well as to society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
I thank Deborah Elstein, my PhD partner since 1993, for her helpful comments and insights that have contributed to the way I manage our patients and the team of physicians working with us at the Gaucher Clinic: Aya Abrahamov, Irith Hadas-Halpern, Gheona Altarescu, Mici Phillips, Ehud Lebel, Menachem Itzchaki, Sorina Granovsky-Grisaru, Rosa Ruchlemer, Josef Alberton, Marc Klutstein, Bettina Birmanns, and Alexander Ioskovitch.
Authorship
Contribution: A.Z. was the single author of this paper.
Conflict-of-interest disclosure: A.Z. receives consultancy fees from Shire Human Genetic Therapies, receives consultancy fees and has options in Protalix Biotherapeutics and sits on their Scientific Advisory Board, receives support from Genzyme Therapeutics for participation in the ICGG registry, and receives honoraria from Actelion Pharmaceuticals.
Correspondence: Ari Zimran, Gaucher Clinic, Shaare Zedek Medical Center, PO Box 3235, Jerusalem 91031, Israel; e-mail: azimran@gmail.com.