Abstract
SHP2, a cytoplasmic protein-tyrosine phosphatase encoded by the PTPN11 gene, plays a critical role in developmental hematopoiesis in the mouse, and gain-of-function mutations of SHP2 are associated with hematopoietic malignancies. However, the role of SHP2 in adult hematopoiesis has not been addressed in previous studies. In addition, the role of SHP2 in human hematopoiesis has not been described. These questions are of considerable importance given the interest in development of SHP2 inhibitors for cancer treatment. We used shRNA-mediated inhibition of SHP2 expression to investigate the function of SHP2 in growth factor (GF) signaling in normal human CD34+ cells. SHP2 knockdown resulted in markedly reduced proliferation and survival of cells cultured with GF, and reduced colony-forming cell growth. Cells expressing gain-of-function SHP2 mutations demonstrated increased dependency on SHP2 expression for survival compared with cells expressing wild-type SHP2. SHP2 knockdown was associated with significantly reduced myeloid and erythroid differentiation with retention of CD34+ progenitors with enhanced proliferative capacity. Inhibition of SHP2 expression initially enhanced and later inhibited STAT5 phosphorylation and reduced expression of the antiapoptotic genes MCL1 and BCLXL. These results indicate an important role for SHP2 in STAT5 activation and GF-mediated proliferation, survival, and differentiation of human progenitor cells.
Introduction
SHP2 is a cytoplasmic protein-tyrosine phosphatase encoded by the PTPN11 gene. SHP2 contains 2 tandem Src homology 2 (SH2) domains (N-SH2 and C-SH2), a protein tyrosine phosphatase (PTP) domain and a C-terminal tail.1 In its inactive state, SHP2 has a low basal PTP activity because of autoinhibition by association of its N-SH2 domain with the PTP domain, which blocks substrate access. SHP2 directly or indirectly associates with activated receptor protein tyrosine kinases or cytokine receptors via its 2 SH2 domains. Binding of SH2 domains to phosphotyrosine sites on other proteins alters the conformation of the N-SH2 domain, preventing its binding to PTP domain and causing catalytic activation. Several studies indicate that SHP2 promotes activation of the Ras and ERK pathway by growth factors (GFs) and cytokines.2,3
SHP2 is widely expressed, with high levels of expression in hematopoietic cells. In murine models, SHP2 has been found to play an essential role in hematopoietic cell development. Homozygous deletion of SHP2 results in embryonic lethality because of severe defects in gastrulation and mesodermal patterning.4 SHP2-deficient embryonic stem (ES) cells exhibit severely decreased differentiation to erythroid and myeloid progenitors in vitro5,6 and fail to contribute to both erythroid and myeloid lineages in chimeric mice derived from SHP2−/− ES cells and wild-type (WT) embryos.7 Reintroduction of WT SHP2 into SHP2−/− ES cells partially rescued these hematopoietic defects. In addition, SHP2 loss-of-function causes a block of lymphocyte development before Pro-T and Pro-B stages.8 Altogether, these data suggest that SHP2 plays a positive role in the development of all blood cell lineages from ES cells, functioning at a very early stage of hematopoietic development. SHP2 haploinsufficiency causes a competitive repopulating defect of HSC in mice.9 Further evidence for the importance of SHP2 in hematopoietic regulation comes from the identification of PTPN11 germline mutations in persons with Noonan syndrome (NS) and LEOPARD syndrome, development disorders associated with abnormal hematopoiesis.10 Somatic gain-of-function PTPN11 mutations are seen in approximately 35% of juvenile myelomonocytic leukemia.11 PTPN11 mutations are also seen in childhood myelodysplastic syndrome, acute lymphoblastic leukemia, and acute myelogenous leukemia.11 These gain-of-function mutations can induce aberrant hyperactivation of the Ras-ERK pathway and GF-independent growth and hypersensitivity to GF stimuli in hematopoietic cells.12-14 Increased SHP2 expression has also been observed in acute leukemia specimens, and a potential role in leukemogenesis has been suggested.15
Despite the essential role for SHP2 in hematopoietic development in mice and association of SHP2 mutations with myeloid malignancies, is not clear whether SHP2 plays a critical role in normal adult hematopoiesis. It is recognized that genes that are essential at the developmental stage may not be equally critical to adult hematopoiesis. In vitro studies using factor-dependent hematopoietic cell lines indicate that SHP2 participates in signal transduction from a variety of hematopoietic GF receptors, including stem cell factor (SCF), IL-3, granulocyte-macrophage colony stimulating factor (GM-CSF), macrophage colony stimulating factor (M-CSF), and erythropoietin.16-22 It was recently reported that SHP2 contributes to ERK activation by granulocyte colony stimulating factor (G-CSF) but not M-CSF. Pharmacologic inhibition of SHP2 specifically inhibited colony-forming units-granulocyte while sparing colony-forming units-macrophage.23 However, these studies do not address the broader role of SHP2 in regulating progenitor cell growth. Moreover, murine hematopoietic cells may differ in their regulation from their human counterparts, and the role of SHP2 in human hematopoietic progenitors has not been described. Investigation of the effect of SHP2 on human hematopoiesis is of considerable significance because there is considerable interest in the development of SHP2 inhibitory compounds for treatment of leukemias and other malignancies, and hematologic toxicities may be a major concern. Here we investigated the function of SHP2 in regulation of GF responsiveness in normal human hematopoietic stem and progenitor cells by inhibiting SHP2 expression in human cord blood (CB) CD34+ cells with stable SHP2 shRNA expression. Our studies demonstrate that SHP2 knockdown in human CD34+ hematopoietic progenitor cells profoundly inhibits their survival, proliferation, and differentiation in response to GF stimuli.
Methods
Plasmid construction
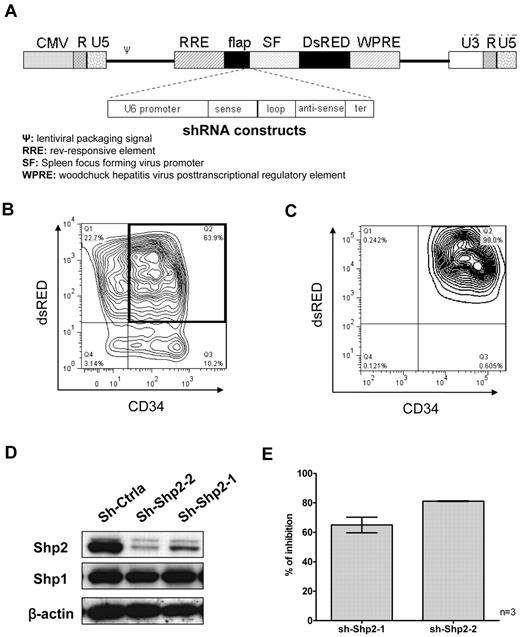
Two 19-nucleotide sequences, sh-Shp2-1 (sense ATATGGCGGTCCAGCATTA) and sh-Shp2–2 (sense ACACTGGTGATTACTATGA), complementary to nucleotides1924 to 1942 and nucleotides 553 to 571 of SHP2 mRNA (NM_002834) were selected for shRNA construction using the Dharmacon siRNA design tool. A nonspecific control shRNA (sh-Ctrla) sequence was purchased from Ambion. The U6-shRNA expression cassette, including the U6 Pol III promoter, the sense and antisense sequence of the shRNA separated by a 9-base loop, and a terminator of 6 thymidines, was constructed by polymerase chain reaction as previously described.24 The resulting shRNA construct was verified by automated DNA sequencing and inserted upstream of the SF promoter of DsRED in the pHIV7-SF-DsRED vector (Figure 1A).
Inhibition of SHP2 expression in human CB CD34+ cells using lentiviral vectors expressing SHP2-specific shRNA. (A) Constructs consisting of U6 promoter driving expression of control and SHP2 specific shRNA were polymerase chain reaction amplified and cloned into an HIV-7 lentiviral vector backbone. Expression of the DsRED selection marker was driven by a spleen focus-forming virus (SF) promoter. (B) Human CB CD34+ cells were transduced with lentiviral vectors carrying control (sh-Ctrla) and SHP2-specific shRNAs (sh-Shp2-1 and sh-Shp2-2), respectively. Representative results for CD34+ cell transduction are shown. (C) The purity of flow cytometry–selected CD34+DsRED+ cells was checked by reanalysis using flow cytometry. Representative results are shown. (D) Inhibition of SHP2 expression was examined using Western blotting. Representative results are shown. (E) Combined data for SHP2 inhibition are shown. Data are mean ± SEM of 3 experiments.
Inhibition of SHP2 expression in human CB CD34+ cells using lentiviral vectors expressing SHP2-specific shRNA. (A) Constructs consisting of U6 promoter driving expression of control and SHP2 specific shRNA were polymerase chain reaction amplified and cloned into an HIV-7 lentiviral vector backbone. Expression of the DsRED selection marker was driven by a spleen focus-forming virus (SF) promoter. (B) Human CB CD34+ cells were transduced with lentiviral vectors carrying control (sh-Ctrla) and SHP2-specific shRNAs (sh-Shp2-1 and sh-Shp2-2), respectively. Representative results for CD34+ cell transduction are shown. (C) The purity of flow cytometry–selected CD34+DsRED+ cells was checked by reanalysis using flow cytometry. Representative results are shown. (D) Inhibition of SHP2 expression was examined using Western blotting. Representative results are shown. (E) Combined data for SHP2 inhibition are shown. Data are mean ± SEM of 3 experiments.
MIG-R1 vectors expressing WT SHP2 and a gain-of-function Shp2 mutant (SHP2-E76K) upstream of an internal ribosome entry site followed by an enhanced green fluorescent protein (GFP) gene were obtained from Dr M. Golam Mohi (Upstate Medical University, Syracuse, NY).25 An shRNA-resistant SHP2 mutant (SHP2-ShR) was generated by introducing 6 point mutations into the sequence in SHP2 targeted by the sh-Shp2-2 shRNA without changing the encoded amino acid sequence. The final mutant sequence was ATACCGGCGACTATTACGA (mutated nucleotides italicized). Wild-type SHP2 and SHP2-ShR cDNA were cloned into the EcoRl site of the MIG-R1 retroviral vector (provided by Dr Warren Pear, University of Pennsylvania, Philadelphia, PA),
Virus vector production
Infectious lentivirus particles were produced by cotransfecting 293T cells with shRNA-expressing lentiviral plasmids together with pCMV-gp, pCMV-rev, and pCMV-VSV-G packaging plasmids using the calcium phosphate coprecipitation method. Supernatants containing infectious virus particles were collected 24 and 48 hours after transfection, filtered, and concentrated using ultracentrifugation. Titration of infectious virus particles was performed on HT1080 cells. Infectious virus titers were typically between 2 to 3 × 108/mL. Retroviral particles carrying MIG-R1–based vectors were produced by transient transfection of 293 cells as previously described.26
Samples and cell preparation
Human umbilical CB samples were obtained under a protocol approved by the Institutional Review Board at City of Hope Cancer Center, in accordance with assurances filed with the Department of Health and Human Services, and meeting all requirements of the Declaration of Helsinki. All donors signed informed consent. Mononuclear cells were isolated using Ficoll-Hypaque density gradient separation. Cells were either used when freshly obtained or were cryopreserved in dimethyl sulfoxide–containing medium in liquid nitrogen tanks (vapor phase). Frozen cells were thawed and incubated in Iscove modified Dulbecco medium (IMDM) supplemented with 20% FBS and DNase I (Sigma-Aldrich) for 3-hour incubation at 37°C before further processing. CD34+ cells were selected using immunomagnetic column separation (Miltenyi Biotec) as previously described.27
Lentivirus transduction of human CD34+ cells
CD34+ cells transduction was performed as previously described.26 Briefly, cells were cultured for 48 hours in serum-free medium (SFM; StemCell Technologies) with 100 ng/mL Flt-3 ligand, 50 ng/mL stem cell factor, 100 ng/mL thrombopoietin, 10 ng/mL IL-6, and 25 ng/mL IL-3 on fibronectin CH-296 peptide (Retronectin; Pan Vera)–coated plates. Cell were then resuspended in virus supernatant (multiplicity of infection [MOI] = 5-10) with the same GF and plated on retronectin-coated plates preexposed to viral supernatants. This procedure was repeated after 24 hours. Forty-eight hours later, cells were labeled with anti-CD34–allophycocyanin antibodies (BD Biosciences), and CD34+DsRED+ cells collected by flow cytometry sorting (MoFlo; Cytomation). CD34+ cells used for in vivo repopulation assays were transduced using a similar protocol. CB CD34+ cells were cultured for 24 hours in serum-free medium with 100 ng/mL Flt-3 ligand, 100 ng/mL SCF, and 100 ng/mL thrombopoietin on fibronectin CH-296 peptide-coated plates. Cell were then resuspended in virus supernatant (MOI = 10) with the same GF and plated on retronectin-coated plates preexposed to viral supernatants. This procedure was repeated after 24 hours. Forty-eight hours later, cells were labeled with anti-CD34–allophycocyanin antibodies and CD34+DsRED+ cells were collected by flow cytometry sorting for transplantation. TF-1 cells cultured in RMPI 1640 supplemented with 10% fetal bovine serum and 2 ng/mL GM-CSF were exposed to retroviral particles carrying MIG-R1, SHP2-WT, SHP2-shR, or SHP2-E76K at MOI = 2, and GFP+ cells were selected 48 hours later by flow cytometry sorting. GFP+ TF-1 cells were expanded in culture and transduced with lentiviral vectors expressing sh-Ctrla or sh-Shp2–2 at MOI of 2. GFP+DsRED+ cells were isolated by flow cytometry sorting 48 hours after transduction.
Evaluation of progenitor growth
CFC assay.
CD34+DsRED+ cells were plated in methylcellulose progenitor culture and assessed for the presence of colony-forming units-granulocyte macrophage, and colony-forming units–erythroid colonies as described previously.28
Cell expansion in liquid culture with high GFs.
CD34+DsRED+ cells were cultured in IMDM with 30% FBS and GFs (3 U/mL erythropoietin, 5 ng/mL SCF, 20 ng/mL granulocyte-macrophage colony stimulating factor [GM-CSF], 20 ng/mL G-CSF, and 5 ng/mL IL-3) as used in colony formation (CFC) culture. Cellular expansion was measured by counting the number of cells generated in culture at different time points as described in “SHP2 knockdown inhibits CD34+ cell proliferation.”
Growth factor dose-response.
CD34+DsRED+ cells were plated in 96-well plates in SFM with graded concentrations of GF for 72 hours. Concentrations used in CFC culture were considered to be 100×, and concentrations from 100× to 0.1× were studied. The number of viable cells was analyzed using an 3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay kit (Promega) as recommended by the manufacturer.
Cell proliferation.
CD34+DsRED+ cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; 20μM, Invitrogen) in IMDM at 37°C for 30 minutes. Cells were incubated overnight to release unbound dye and then cultured in low, physiologic concentrations of GF similar to those present in stroma-conditioned medium (200 pg/mL GM-CSF, 1 ng/mL G-CSF, 200 pg/mL SCF, 50 pg/mL leukemia inhibitory factor, 200 pg/mL macrophage inhibitory protein 1α, and 1 ng/mL IL-6) for 3 days.29 CFSE fluorescence intensity was assessed by flow cytometry (LSRII, BD Biosciences) on day 3 of culture. Proliferation index was analyzed using ModFit LT 3.0 software. The position of the parent generation was set based on the fluorescence of a cell aliquot treated with paraformaldehyde after CFSE labeling and overnight incubation.
Apoptosis.
CD34+DsRED+ cells were cultured overnight in SFM with low concentrations of GF as described for cell proliferation, washed with IMDM, and cultured in SFM with or without GF for 3 days. Apoptosis was assessed on days 1 and 3 by flow cytometry after labeling with annexin V–Cy5 (BD Biosciences PharMingen) and 4,6-diamidino-2-phenylindole (DAPI). Cell apoptosis was also detected during cell expansion in liquid culture with high GFs described in “Cell expansion in liquid culture with high GFs.”
Cell differentiation.
CD34+DsRED+ cells were cultured in conditions as used in cell expansion. Cells were labeled with antibodies (CD33, CD11b, CD14, glycophorin A, CD45, and CD71, eBioscience) and analyzed by flow cytometry at different time points as described in “SHP2 knockdown inhibits myeloid and erythroid differentiation of CD34+ cells.”
Western blotting
CD34+DsRED+ cells were cultured in cell expansion cultures for 7 days. Protein extracts were prepared as previously described.26 Protein were resolved on 4% to 2% SDS-PAGE gels and transferred to nitrocellulose membranes. Membranes were blocked with 10% nonfat milk in phosphate-buffered saline and 0.1% Tween-20 and labeled with primary antibodies [anti-actin (AC-15, Sigma-Aldrich), anti-SHP2 (Santa Cruz Biotechnology), anti-phospho–JAK2 (Tyr 1007/1008) and total JAK (Cell Signaling), anti-phospho–AKT (Ser 473) and total AKT (Cell Signaling), anti-phospho–ERK and total ERK (Santa Cruz Biotechnology), anti-phospho–STAT5 (Tyr 694, BD Biosciences) and total STAT5 (Cell Signaling), anti-phospho–STAT3 (Tyr 705) and total STAT3 (Cell Signaling), anti-Mcl1 (Santa Cruz Biotechnology), anti-BCLXL (BD Biosciences), and anti-Bcl2 (Sigma-Aldrich)], followed by horseradish peroxidase–conjugated secondary anti–mouse and anti–rabbit antibodies (1:8000; Jackson ImmunoResearch Laboratories). Antibody detection was performed using enhanced chemiluminescence (Superfemto kit; Pierce Biotechnology).
In vivo repopulation assays
All animal studies were performed in accordance with the guidelines of the City of Hope Research Animal Care Committee. NOD/SCID/γ chainnull (NSG) mice (The Jackson Laboratory) were housed in micro-insulator cages in a pathogen-free condition and handled in laminar flow hoods. Six- to 8-week-old NSG mice were sublethally irradiated with 300 cGy from a 137Cs source with attenuator on the day of transplantation and placed on Sulfatrim water after irradiation. A total of 5 × 104 to 2 × 105 CD34+DsRED+ cells were transplanted into NSG mice by tail vein injection. Bone marrow cells from both femurs were harvested from each mouse 8 to 14 weeks after transplantation and human CD45+ cells were detected using flow cytometry.
Statistics
Data obtained from multiple experiments were reported as the mean ± SEM. Significance levels were determined by Student t test and analysis of variance. Data from NSG mice engraftment experiment were analyzed using nonparametric Mann-Whitney test.
Results
Effective SHP2 knockdown in CD34+ cells using lentivirus expressed shRNA
We studied the role of SHP2 in human hematopoietic stem and progenitor cell (HSPC) function by inhibiting SHP2 expression in CB CD34+ cells using shRNA directed against SHP2 (Figure 1A). CD34+ cells were transduced with lentivirus vectors coexpressing SHP2-specific shRNA (sh-Shp2-1 or sh-Shp2-2) or control shRNA (sh-Ctrla) with DsRED and CD34+DsRED+ cells were selected using flow cytometry (Figure 1B). The purity of sorted CD34+DsRED+ cells was confirmed by flow cytometry (Figure 1C). Expression of sh-Shp2-2 and sh-Shp-1 resulted in 81.1% ± 0.2% and 65.0% ± 5.3% inhibition of SHP2 expression, respectively, in CD34+ cells compared with control shRNA (Figure 1D-E). In contrast, expression of the homologous SHP1 protein was not changed in SHP2 shRNA-transduced cells, indicating that knockdown was specific for SHP2.
SHP2 knockdown inhibits CD34+ cell proliferation
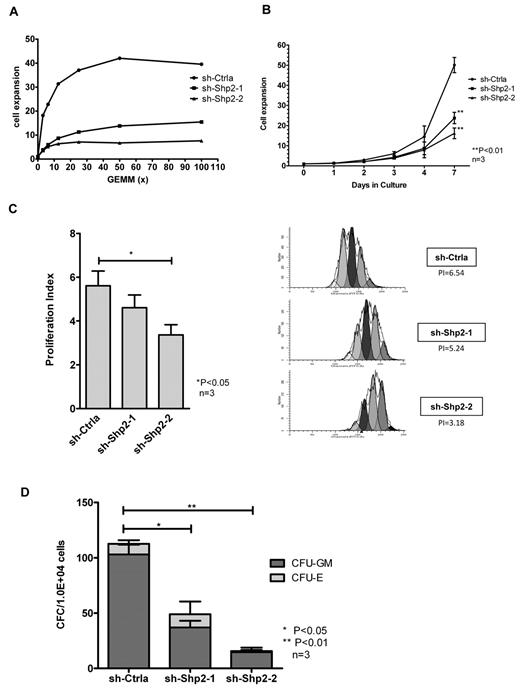
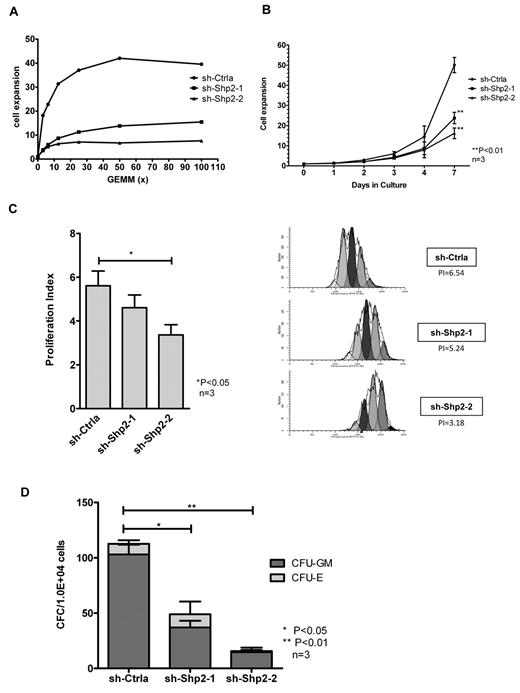
In view of previous reports that SHP2 may regulate hematopoietic GF signaling, we investigated the effect of SHP2 knockdown on CD34+ cell response to GF stimuli. Because primary human HSPCs do not grow very well in the presence of a single GF, we studied the effect of SHP2 inhibition on the dose-response relationship of CD34+ cells to GF stimulation using a cocktail of GFs containing SCF, GM-CSF, G-CSF, IL-3, and erythropoietin. SHP2 has been shown to be involved in the downstream signaling of these GFs in cell line studies. SHP2-knockdown CD34+dsRED+ cells cultured demonstrated markedly reduced dose-dependent proliferation after stimulation with graded concentrations of GFs in an MTS assay compared with control shRNA-expressing cells (Figure 2A). SHP2-knockdown CD34+ cells also demonstrated significantly reduced expansion in cell numbers in high GF-containing cultures compared with controls (50.09 ± 3.78-, 23.75 ± 2.79-, and 16.17 ± 2.65-fold expansion for sh-Ctrla, sh-Shp2-1, and sh-Shp2-2, respectively, at day 7, P < .01; Figure 2B). The effect of SHP2 down-regulation on cell division was analyzed by labeling CD34+DSRED+ cells with CFSE and monitoring cell division based on the reduction of CFSE fluorescence intensity during culture. A proliferation index was calculated using ModFit LT 3.0 software. SHP2-knockdown CD34+ cells showed significantly reduced cell division during culture with proliferation index of 4.65 ± 0.57 and 3.36 ± 0.47 for sh-Shp2-1- and sh-Shp2-2–transduced cells compared with proliferation index of 5.57 ± 0.71 for sh-Ctrla-transduced cells at day 3 (P < .05, Figure 2C). SHP2 knockdown CD34+ cells generated significantly reduced colonies in methylcellulose progenitor culture compared with controls (112.7 ± 11.3, 49.0 ± 9.0, and 15.7 ± 3.2 colonies from 1 × 104 CD34+ cells transduced with sh-Ctrla, sh-Shp2–1, and sh-Shp2–2, respectively; Figure 2D). These results indicated that knockdown of SHP2 resulted in a significantly reduced proliferative response to GF stimuli, proportional to the degree of inhibition of SHP2 expression.
SHP2 knockdown in CD34+ cells results in significantly reduced progenitor proliferation in response to GF stimulation. (A) CD34+DsRED+ cells were cultured in 96-well plates with graded concentrations of a GF combination (SCF, GM-CSF, G-CSF, IL-3, and erythropoietin) including for 72 hours, and the number of cells was analyzed using an MTS assay. Representative results showing inhibition of SHP2 expression significantly reduced cell proliferative response to GF stimulation. (B) CD34+DsRED+ cells were cultured for 7 days in high concentrations of GFs as used in CFC culture and expansion in cell numbers evaluated. Data are mean ± SEM of 3 experiments. **P < .01, SHP2 shRNA-transduced cells compared with sh-Ctrla. (C) CD34+DsRED+ cells were labeled with CFSE and cultured in low, physiologic concentrations of GF for 3 days. Cell division was evaluated on the basis of reduction of CFSE fluorescence intensity. A proliferation index was calculated based on the using ModFit LT 3.0 software. Data are mean ± SEM of 3 experiments. *P < .05. Representative ModFit analysis results from 1 experiment are shown. (D) CD34+DsRED+ cells (1 × 104) were placed in methylcellulose CFC culture, and colonies were counted after 14 days culture. Data are mean ± SEM of 3 experiments. Knockdown of SHP2 significantly reduced colony formation ability of CB CD34+ cells. *P < .05 for sh-Shp2-1, and **P < .01 for sh-Shp2-2 compared with sh-Ctrla.
SHP2 knockdown in CD34+ cells results in significantly reduced progenitor proliferation in response to GF stimulation. (A) CD34+DsRED+ cells were cultured in 96-well plates with graded concentrations of a GF combination (SCF, GM-CSF, G-CSF, IL-3, and erythropoietin) including for 72 hours, and the number of cells was analyzed using an MTS assay. Representative results showing inhibition of SHP2 expression significantly reduced cell proliferative response to GF stimulation. (B) CD34+DsRED+ cells were cultured for 7 days in high concentrations of GFs as used in CFC culture and expansion in cell numbers evaluated. Data are mean ± SEM of 3 experiments. **P < .01, SHP2 shRNA-transduced cells compared with sh-Ctrla. (C) CD34+DsRED+ cells were labeled with CFSE and cultured in low, physiologic concentrations of GF for 3 days. Cell division was evaluated on the basis of reduction of CFSE fluorescence intensity. A proliferation index was calculated based on the using ModFit LT 3.0 software. Data are mean ± SEM of 3 experiments. *P < .05. Representative ModFit analysis results from 1 experiment are shown. (D) CD34+DsRED+ cells (1 × 104) were placed in methylcellulose CFC culture, and colonies were counted after 14 days culture. Data are mean ± SEM of 3 experiments. Knockdown of SHP2 significantly reduced colony formation ability of CB CD34+ cells. *P < .05 for sh-Shp2-1, and **P < .01 for sh-Shp2-2 compared with sh-Ctrla.
SHP2 knockdown inhibits CD34+ cell viability
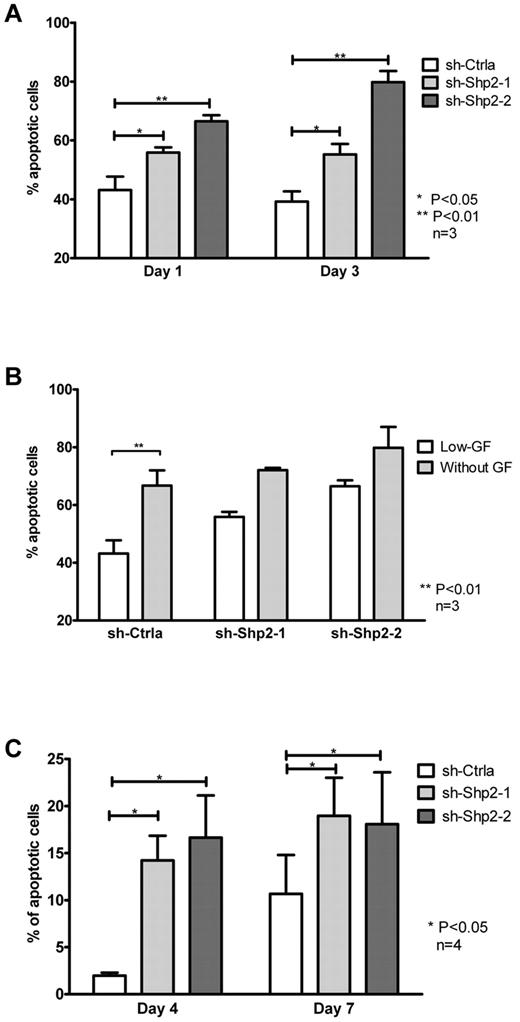
We examined the effect of SHP2 knockdown on survival of CD34+ HSPCs. Apoptosis was detected by flow cytometry after labeling with annexin V and DAPI. SHP2 shRNA-transduced cells exhibited increased apoptosis compared with controls when cultured with low, physiologic concentrations of GF (39.2% ± 3.5%, 55.3% ± 3.6%, and 79.8% ± 3.7% for sh-Ctrla, sh-Shp2-1, and sh-Shp2-2, respectively; Figure 3A). Apoptosis was proportional to the degree of inhibition of SHP2 expression. Although increased apoptosis was observed in all 3 groups on removal of GF from medium, the relative increase of apoptosis on GF deprivation compared with that seen in low GF condition was lower in SHP2 shRNA-transduced cells compared with sh-Ctrla (Figure 3B). These observations suggest that GF-mediated promotion of cell survival is reduced in SHP2-knockdown cells. Increased cell apoptosis was also seen with SHP2-knockdown cells when cultured in high concentrations of GFs compared with control (Figure 3C). In additional experiments, we evaluated cell expansion and apoptosis of SHP2 and control shRNA-transduced CD34+ cells cultured with single GFs (SCF or Flt-3 ligand) or cytokines (IL-3 or GM-CSF) at high concentration (100 ng/mL). SHP2 knockdown resulted in significantly reduced cell expansion in response to IL-3 and GM-CSF compared with control shRNA (supplemental Figure 3A) and decreased survival of cells cultured with IL-3 and SCF (supplemental Figure 3B). These data indicating that signaling complexes involving both GF receptors with tyrosine kinase activities and cytokine signaling without tyrosine kinase activity may be dependent on SHP2 expression.
SHP2 knockdown in CD34+ cells results in significantly reduced progenitor survival. (A) CD34+DsRED+ cells were cultured in SFM with low, physiologic concentrations of GFs for 3 days and analyzed for apoptosis by labeling with annexin V and DAPI. Data are mean ± SEM of 3 experiments. *P < .05, sh-Shp2-1 and **P < .01 for sh-Shp2-2 compared with sh-Ctrla. (B) The effect of GF withdrawal for 24 hours on apoptosis of CD34+DsRED+ cells was evaluated. Data are mean ± SEM of 3 experiments. **P < .01 with or without GF withdrawal. (C) CD34+DsRED+ cells cultured in high concentrations of GFs. SFM were analyzed for apoptosis. Data are mean ± SEM of 4 experiments. *P < .05, sh-Shp2-1 and sh-Shp2-2 compared with sh-Ctrla.
SHP2 knockdown in CD34+ cells results in significantly reduced progenitor survival. (A) CD34+DsRED+ cells were cultured in SFM with low, physiologic concentrations of GFs for 3 days and analyzed for apoptosis by labeling with annexin V and DAPI. Data are mean ± SEM of 3 experiments. *P < .05, sh-Shp2-1 and **P < .01 for sh-Shp2-2 compared with sh-Ctrla. (B) The effect of GF withdrawal for 24 hours on apoptosis of CD34+DsRED+ cells was evaluated. Data are mean ± SEM of 3 experiments. **P < .01 with or without GF withdrawal. (C) CD34+DsRED+ cells cultured in high concentrations of GFs. SFM were analyzed for apoptosis. Data are mean ± SEM of 4 experiments. *P < .05, sh-Shp2-1 and sh-Shp2-2 compared with sh-Ctrla.
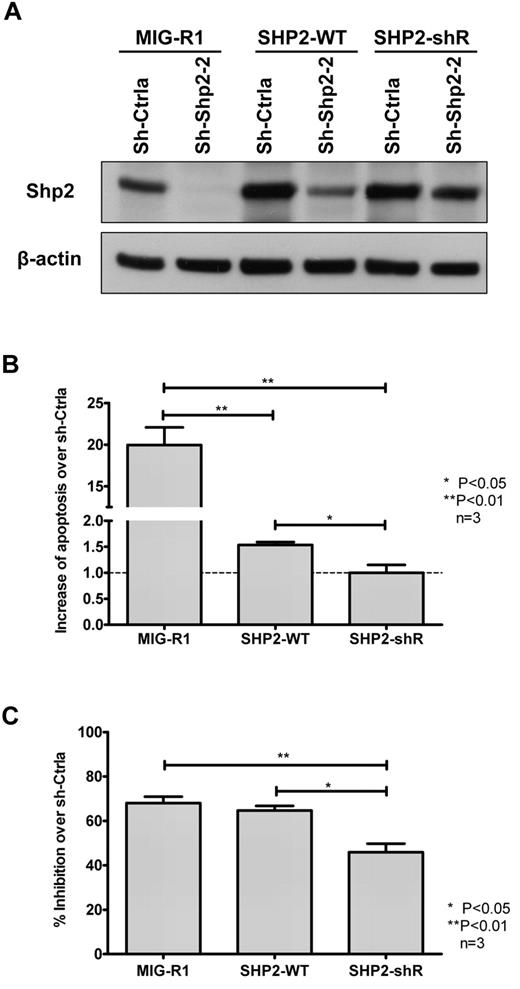
To confirm that the effects of SHP2 knockdown in hematopoietic cells were not related to “off-target” effects, we expressed an SHP2 construct with synonymous mutations in the sh-Shp2-2 targeting sequence in TF-1 cells. TF-1 cells were transduced with vectors expressing GFP (MIG-R1), WT SHP2 cDNA, or mutant SHP2 cDNA (SHP2-ShR) followed by transduction with vectors expressing sh-Ctrla and sh-Shp2–2 shRNA. Western blotting showed that SHP2-ShR maintained SHP2 expression in sh-Shp2-2 shRNA coexpressing cells, whereas expression of SHP2-WT only partially restored SHP2 expression in SHP2-ShR–expressing cells (Figure 4A). Restoration of SHP2 expression by SHP2-ShR completely prevented SHP2 shRNA-mediated apoptosis and partially prevented SHP2 shRNA-mediated inhibition of cell growth (Figure 4B-C). These results indicate that the effects of sh-Shp2-2 are related to inhibition of SHP2 expression rather than “off-target” effects.
Rescue of SHP2 expression in TF-1 cells. (A). TF-1 cells transduced with empty vector (MIG-R1) as well as vectors carrying WT SHP2 cDNA (SHP2-WT) and SHP2 cDNA with noncoding mutations in the sh-Shp2-2 target sequence (SHP2-shR) were transduced with sh-Ctrla– and sh-Shp2-2–expressing vectors, respectively. GFP+DsRED+ cells were sorted by FACS and expression of SHP2 analyzed by Western blotting. SHP2-WT expression resulted in partial restoration of SHP2 expression in sh-Shp2-2 shRNA-expressing cells, whereas SHP2-shR completely rescued expression of SHP2 to normal or even higher levels in sh-Shp2-2 shRNA-expressing cells. (B) Dual-transduced TF-1 cells were cultured with GM-CSF (2 ng/mL). Apoptosis was analyzed by labeling with annexin V and DAPI. Data are mean ± SEM of fold changes of apoptosis from sh-Shp2-2 over sh-Ctrla-expressing cells from 3 experiments. *P < .05 for SHP2-WT compared with SHP2-shR, and P < .01 for MIG-R1 compared with SHP2-WT and SHP2-shR. (C) Dual-transduced TF-1 cells were starved of GM-CSF overnight and then cultured with GM-CSF (0.3 ng/mL). Cell growth in response to the GM-CSF was evaluated by MTS assay. Inhibition of cell growth of sh-Shp2-2–transduced cells relative to the cell growth of the corresponding sh-Ctrla–transduced cells was calculated. Data are mean ± SEM from 3 replicates. *P < .05 for SHP2-WT compared with SHP2-shR, and **P < .01 for MIG-R1 compared with SHP2-shR.
Rescue of SHP2 expression in TF-1 cells. (A). TF-1 cells transduced with empty vector (MIG-R1) as well as vectors carrying WT SHP2 cDNA (SHP2-WT) and SHP2 cDNA with noncoding mutations in the sh-Shp2-2 target sequence (SHP2-shR) were transduced with sh-Ctrla– and sh-Shp2-2–expressing vectors, respectively. GFP+DsRED+ cells were sorted by FACS and expression of SHP2 analyzed by Western blotting. SHP2-WT expression resulted in partial restoration of SHP2 expression in sh-Shp2-2 shRNA-expressing cells, whereas SHP2-shR completely rescued expression of SHP2 to normal or even higher levels in sh-Shp2-2 shRNA-expressing cells. (B) Dual-transduced TF-1 cells were cultured with GM-CSF (2 ng/mL). Apoptosis was analyzed by labeling with annexin V and DAPI. Data are mean ± SEM of fold changes of apoptosis from sh-Shp2-2 over sh-Ctrla-expressing cells from 3 experiments. *P < .05 for SHP2-WT compared with SHP2-shR, and P < .01 for MIG-R1 compared with SHP2-WT and SHP2-shR. (C) Dual-transduced TF-1 cells were starved of GM-CSF overnight and then cultured with GM-CSF (0.3 ng/mL). Cell growth in response to the GM-CSF was evaluated by MTS assay. Inhibition of cell growth of sh-Shp2-2–transduced cells relative to the cell growth of the corresponding sh-Ctrla–transduced cells was calculated. Data are mean ± SEM from 3 replicates. *P < .05 for SHP2-WT compared with SHP2-shR, and **P < .01 for MIG-R1 compared with SHP2-shR.
SHP2 knockdown inhibits myeloid and erythroid differentiation of CD34+ cells
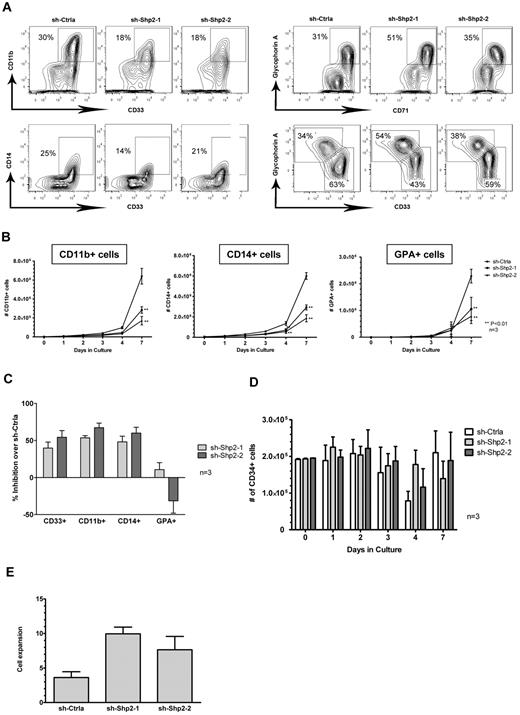
To explore the role of SHP2 in HSPC differentiation, we cultured SHP2 shRNA and control shRNA-transduced CB CD34+ cells with high concentrations of GFs (IL-3 + SCF + G-CSF + GM-CSF + Epo) that promote cell differentiation toward both myeloid and erythroid lineages. Cell differentiation was detected by analyzing expression of lineage markers using multicolor flow cytometry. SHP2 shRNA-transduced CB CD34+ cells generated significantly lower numbers of both myeloid (CD11b+ and CD14+) and erythroid lineage (GPA+) cells compared with sh-Ctrla (Figure 5A-B), with more pronounced inhibition for myeloid lineage cells compared with erythroid lineage cells (Figure 5C). On the other hand, an increased percentage of CD34+ cells was seen after culture of SHP2 shRNA-transduced cells, with retention of CD34+ cell numbers at levels similar to controls (Figure 5D). These results suggest that GF-induced differentiation of CD34+ HSPCs is reduced after knockdown of SHP2 expression. The relative retention of the CD34+ cell population is in contrast to reduced stem cell self-renewal observed after SHP2 knockdown in murine models. Significantly reduced differentiation of SHP2-knockdown cells compared with control shRNA-transduced CD34+ cells was also observed during extended culture in the condition with high concentrations of GFs (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) as well as under GF conditions that selectively induced myeloid differentiation (IL-3 + SCF + G-CSF + GM-CSF; supplemental Figure 1B) or erythroid differentiation (SCF + erythropoietin; supplemental Figure 1C). To exclude the possibility of cellular senescence accounting for retention of CD34+ cells, CD34+ cells from day 7 cultures were selected using flow cytometry and stained for senescence-associated β-galactosidase and cultured with high concentration of GFs for 7 days. SHP2 shRNA and control shRNA-transduced cells were both negative for senescence-associated β-galactosidase (data not shown). Interestingly, SHP2 shRNA-expressing CD34+ cells had higher proliferative potential in culture compared with control shRNA-transduced cells (Figure 5E). These results indicate that the retention of the CD34+ cell population after SHP2 knockdown is not related to cellular senescence, and support the hypothesis that retention of CD34+ cells after SHP2 knockdown may be related to reduced proliferation and differentiation, which could also explain the increased proliferative potential of the retained CD34+ cells.
SHP2 knockdown inhibits CD34+ cell differentiation. (A) CD34+DsRED+ cells were cultured with high concentrations of GFs (IL-3 + SCF + G-CSF + GM-CSF + Epo), and cell differentiation toward both myeloid (CD11b+ and CD14+) and erythroid lineages (GPA+) was analyzed. SHP2 shRNA-transduced CB CD34+ cells generated significantly lower amounts of both myeloid and erythroid lineage cells during the culture compared with sh-Ctrla. Representative flow cytometric analyses from day 4 culture. (B) Total myeloid and erythroid cells generated from CD34+DsRED+ cells (2 × 105) after 7 days of culture. Data are mean ± SEM for 3 experiments. **P < .01 compared with sh-Ctrla. (C) Inhibition of CD34+ cell differentiation to myeloid and erythroid lineage after SHP2 knockdown. Inhibition is calculated relative to control shRNA-expressing cells after 4 days of culture with high concentrations of GFs. Data are mean ± SEM for 3 experiments. (D) Effect of SHP2 knockdown on retention of CD34+ cells during GF culture. The number of CD34+ cells is shown. Data are mean ± SEM of 3 experiments. (E) CD34+DsRED+ cells were cultured with high concentrations of GFs (IL-3 + SCF + G-CSF + GM-CSF + Epo) for 7 days, and CD34+DsRED+ cells were purified from cultured cells by flow cytometry. The selected CD34+DsRED+ cells were cultured with high concentrations of GFs for an additional 7 days, and cell expansion was evaluated based on cell number. Data are mean ± SEM of 3 experiments.
SHP2 knockdown inhibits CD34+ cell differentiation. (A) CD34+DsRED+ cells were cultured with high concentrations of GFs (IL-3 + SCF + G-CSF + GM-CSF + Epo), and cell differentiation toward both myeloid (CD11b+ and CD14+) and erythroid lineages (GPA+) was analyzed. SHP2 shRNA-transduced CB CD34+ cells generated significantly lower amounts of both myeloid and erythroid lineage cells during the culture compared with sh-Ctrla. Representative flow cytometric analyses from day 4 culture. (B) Total myeloid and erythroid cells generated from CD34+DsRED+ cells (2 × 105) after 7 days of culture. Data are mean ± SEM for 3 experiments. **P < .01 compared with sh-Ctrla. (C) Inhibition of CD34+ cell differentiation to myeloid and erythroid lineage after SHP2 knockdown. Inhibition is calculated relative to control shRNA-expressing cells after 4 days of culture with high concentrations of GFs. Data are mean ± SEM for 3 experiments. (D) Effect of SHP2 knockdown on retention of CD34+ cells during GF culture. The number of CD34+ cells is shown. Data are mean ± SEM of 3 experiments. (E) CD34+DsRED+ cells were cultured with high concentrations of GFs (IL-3 + SCF + G-CSF + GM-CSF + Epo) for 7 days, and CD34+DsRED+ cells were purified from cultured cells by flow cytometry. The selected CD34+DsRED+ cells were cultured with high concentrations of GFs for an additional 7 days, and cell expansion was evaluated based on cell number. Data are mean ± SEM of 3 experiments.
SHP2 knockdown inhibits engraftment of CD34+ cells in NSG mice
CB CD34+ cells transduced with Sh-Ctrla and Sh-SHP2–2 were transplanted into sublethally irradiated NSG mice. Bone marrows from mice were evaluated 8 to 14 weeks after transplantation, and human CD45+ cell engraftment detected using flow cytometry. SHP2-knockdown resulted in significantly reduced human cell engraftment in NSG mice compared with the control (P = .012, n = 18 for sh-Shp2-2 and n = 6 for sh-Ctrla; supplemental Figure 2A-B; Table 1). The relatively low engraftment efficiency of control shRNA-transduced cells probably reflects effects of in vitro culture and manipulation required for cell transduction and selection of transduced cells. Despite this limitation, these results clearly indicate that SHP2 deficiency results in significant impairment of human HSC engraftment.
SHP2 knockdown inhibits GF activated ERK, AKT, and Jak/STAT signaling
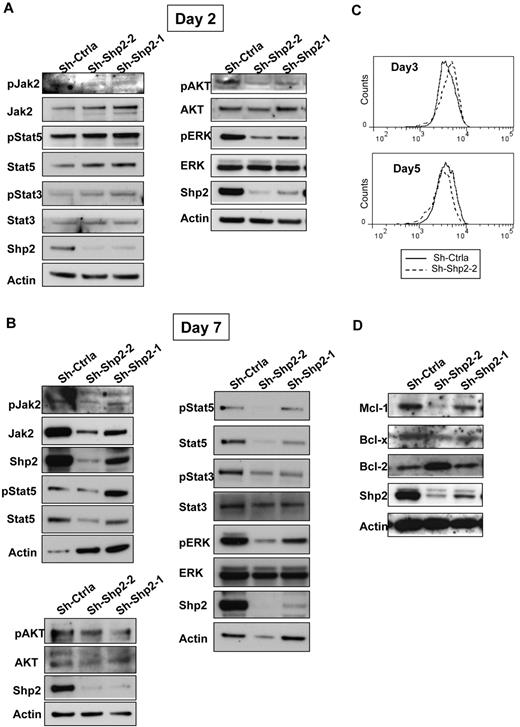
SHP2 can modulate signaling mechanisms involved in hematopoietic cell survival, proliferation, and differentiation, in particular ERK but also JAK/STAT and AKT signaling pathways. To study the effect of SHP2 down-regulation on these cell signaling pathways, we performed Western blotting for phosphorylated JAK2, STAT3, STAT5, AKT, and ERK1/2 on SHP2-knockdown and control CD34+ cells after culture for 2 days (Figure 6A) and 7 days (Figure 6B). SHP2 inhibition was associated with reduction of ERK1/2 and AKT phosphorylation without change in total protein levels at both early and late time points. In contrast, levels of STAT5 phosphorylation and STAT3 phosphorylation were elevated in SHP2 knockdown cells compared with controls at day 2 (Figure 6A) but significantly reduced at day 7 (Figure 6B). Using phospho-flow, we confirmed that phosphorylation of STAT5 (Figure 6C; supplemental Figure 4A) and STAT3 (supplemental Figure 4B) was increased in CD34+ cells at early but not late time points after culture. In addition, SHP2-knockdown resulted in reduction in phosphorylated JAK2 (Y1007/Y1008) at day 2 and in phosphorylated and total JAK2 levels at day 7. Expression of the antiapoptotic genes BCLXL and MCL1, which are known to be regulated by STAT5 and ERK, respectively,30,31 was also decreased in SHP2-knockdown CD34+ cells, with greater inhibition of MCL1 and BCLXL expression in sh-Shp2-2– compared with sh-Shp2-1–expressing cells (Figure 6D). In contrast, BCL2 expression was increased compared with control in sh-Shp2-2–expressing cells. These results suggest a potential role for these signaling mechanisms in the reduced GF-mediated proliferation, differentiation, and survival of SHP2-knockdown cells.
SHP2 knockdown down-regulates STAT5, ERK1/2, and AKT signaling and MCL1 and BCLXL levels. CD34+DsRED+ cells were expanded in culture for 7 days, and protein expression was evaluated at different time points. (A) Western blots showing expression of SHP2, total and phosphorylated ERK, AKT, JAK2, STAT3, and STAT5 at day 2. (B) Western blots showing expression of SHP2, total and phosphorylated ERK, AKT, JAK2, STAT3, and STAT5 at day 7. Results shown are representative of 3 experiments. (C) Western blots showing expression of the antiapoptotic genes BCL2, BCLXL, and MCL1 at day 7. (D) Phosphorylated STAT5 levels measured by flow cytometry in CD34+-expressing cells at day 3 and day 5.
SHP2 knockdown down-regulates STAT5, ERK1/2, and AKT signaling and MCL1 and BCLXL levels. CD34+DsRED+ cells were expanded in culture for 7 days, and protein expression was evaluated at different time points. (A) Western blots showing expression of SHP2, total and phosphorylated ERK, AKT, JAK2, STAT3, and STAT5 at day 2. (B) Western blots showing expression of SHP2, total and phosphorylated ERK, AKT, JAK2, STAT3, and STAT5 at day 7. Results shown are representative of 3 experiments. (C) Western blots showing expression of the antiapoptotic genes BCL2, BCLXL, and MCL1 at day 7. (D) Phosphorylated STAT5 levels measured by flow cytometry in CD34+-expressing cells at day 3 and day 5.
Effect of SHP2 knockdown on cells expressing gain-of-function SHP2 mutants
Because our results suggested that therapeutic SHP2 inhibition could be associated with significant hematopoietic toxicity, it was of interest to examine whether cells expressing gain-of-function mutations in SHP2 demonstrated enhanced sensitivity to SHP2 inhibition. To examine this, we expressed a leukemia-associated SHP2 gain-of-function mutant (E76K) and WT SHP2 in TF-1 hematopoietic cells to evaluate the relative effect of SHP2 knockdown on survival of mutant versus WT SHP2-expressing cells. E76K-expressing cells transduced with sh-Shp2-2–expressing vectors demonstrated increased apoptosis following SHP2 knockdown and similar inhibition of growth compared with WT SHP2-expressing cells (Figure 7). These results suggest that cells expressing gain-of-function SHP2 mutations may have increased dependency on SHP2 expression for survival and may be more sensitive to inhibition of SHP2 expression or function.
Expression of a SHP2 gain-of-function mutant results in increased sensitivity to the knockdown of SHP2. (A) TF-1 cells were transduced with vectors carrying WT SHP2 cDNA (SHP2-WT; coexpressing GFP) and gain-of-function mutant SHP2 cDNA (SHP2-E76K) followed by transduction with sh-Ctrla and sh-Shp2-2 vectors, respectively (coexpressing DsRED). GFP+DsRED+ cells were selected by flow cytometry and cultured in the presence of GM-CSF (2 ng/mL). Apoptosis was analyzed by labeling with annexin V and DAPI, and data from sh-Shp2-2–transduced cells were normalized over the corresponding sh-Ctrla-transduced cells. Data are mean ± SEM of fold changes of apoptosis from 3 experiments. **P < .01, SHP2-WT compared with SHP2-E76K. (B) Dual-transduced TF-1 cells were cultured without GM-CSF overnight and then cultured in the presence of GM-CSF (0.3 ng/mL) for 3 days. Cell growth in response to the GM-CSF was evaluated by MTS assay. Data from sh-Shp2-2–transduced cells were normalized over sh-Ctrla–transduced cells, and the inhibition of cell growth relative to sh-Ctrla was calculated. Data are mean ± SEM from 3 replicates.
Expression of a SHP2 gain-of-function mutant results in increased sensitivity to the knockdown of SHP2. (A) TF-1 cells were transduced with vectors carrying WT SHP2 cDNA (SHP2-WT; coexpressing GFP) and gain-of-function mutant SHP2 cDNA (SHP2-E76K) followed by transduction with sh-Ctrla and sh-Shp2-2 vectors, respectively (coexpressing DsRED). GFP+DsRED+ cells were selected by flow cytometry and cultured in the presence of GM-CSF (2 ng/mL). Apoptosis was analyzed by labeling with annexin V and DAPI, and data from sh-Shp2-2–transduced cells were normalized over the corresponding sh-Ctrla-transduced cells. Data are mean ± SEM of fold changes of apoptosis from 3 experiments. **P < .01, SHP2-WT compared with SHP2-E76K. (B) Dual-transduced TF-1 cells were cultured without GM-CSF overnight and then cultured in the presence of GM-CSF (0.3 ng/mL) for 3 days. Cell growth in response to the GM-CSF was evaluated by MTS assay. Data from sh-Shp2-2–transduced cells were normalized over sh-Ctrla–transduced cells, and the inhibition of cell growth relative to sh-Ctrla was calculated. Data are mean ± SEM from 3 replicates.
Discussion
In this study, we used SHP2-specific shRNAs delivered by lentiviral vectors to knockdown SHP2 expression in CB CD34+ cells to explore the role of SHP2 in normal human HSPC functions. Previous studies have evaluated the role of SHP2 in developmental hematopoiesis in the mouse but have not evaluated its role in adult hematopoiesis or in primary human hematopoietic progenitors. Our results indicate that SHP2 plays an important role in normal human hematopoietic progenitor proliferation, survival, and proliferation through modulation of important GF-activated signaling mechanisms. To our knowledge, the function of SHP2 in primary human hematopoietic stem and progenitor cells has not been previously reported. These results are of importance because they enhance our understanding of critical regulatory signaling mechanisms in adult human hematopoietic progenitors and they have implications for therapeutic strategies targeting SHP2 signaling for the treatment of hematologic malignancies and solid tumors.
Microenvironmental factors play an important role in supporting and regulating normal HSPC survival, proliferation, and differentiation. SHP2 has been found to positively regulate downstream signaling of many GF and cytokine receptors and cell surface molecules, and SHP2 deficiency should impair intracellular signal transduction of human HSPC in response to GF stimuli. Our results demonstrate that SHP2 knockdown in human CD34+ cells resulted in markedly decreased responsiveness to GF stimulation, and this inhibitory effect is correlated with the level of inhibition of SHP2 expression. Knockdown of SHP2 caused significantly reduced cell proliferation and expansion with significantly increased apoptosis, markedly diminished proliferation and reduced generation of differentiated cells during culture in the presence of hematopoietic GF. Our results also indicate that SHP2 is involved in proliferative and antiapoptotic signaling through both GF receptor tyrosine kinases and cytokine receptors without tyrosine kinase activities. Expression of an shRNA-resistant SHP2 mutant completely prevented increase in apoptosis and partially reversed reduced cell growth following shRNA expression, indicating that observed effects of SHP2 shRNA were specifically related to knockdown of SHP2 expression. The partial reversion of growth inhibitory effect of SHP2 shRNA in this setting may be explained by previous observations that overexpression of WT SHP2 can negatively affect GF signaling and cell growth.14,32 Alternatively, our data may indicate that SHP2 regulation of hematopoietic cell growth and survival may occur through different pathways. In murine models, SHP2 deletion is associated with reduced stem cell self-renewal.9 In contrast, we observed relative retention of CD34+ cells in SHP2 knockdown cells with retention of enhanced proliferative potential and without significant cell senescence. Retention of primitive cells after SHP2 knockdown could result from reduced CD34+ cell division and differentiation. On the other hand, SHP2 knockdown human cells demonstrated reduced engraftment in NSG mice, suggesting impaired stem cell capacity. It is unclear whether this reflects altered homing and engraftment capacity, or reduced proliferation and differentiation capacity of SHP2 knockdown cells, which could limit our ability to detect stem cell engraftment.
SHP2 catalytic activity is required for promotion of signaling through the Ras-ERK and phosphatidylinositol 3-kinase pathway. SHP2 may associate with GF receptors directly by docking to phosphorylated tyrosine residues or indirectly via adaptor/scaffolding proteins, such as GAB2, which possess conserved tyrosine sites for engagement of the 2 SHP2 SH2 domains.16,33 Although the key substrates for Ras-ERK activation remain unknown, SHP2-mediated dephosphorylation and inhibition of negative regulators, including RasGAP and sprouty proteins, or dephosphorylation and activation of Src kinase, may play a role. In our studies, SHP2 inhibition in hematopoietic cells was associated with reduced ERK and AKT activation, suggesting a role for SHP2 in GF-mediated activation of ERK and phosphatidylinositol 3-kinase/AKT signaling in normal hematopoietic cells. SHP2 has been reported to both enhance and inhibit signaling in the JAK/STAT pathway, depending on the cell context and extracellular signals.34 SHP2 can negatively regulate STAT family proteins in different cell types through direct dephosphorylation of tyrosine-phosphorylated STATs and/or down-regulation of upstream JAK kinases.35-38 On the other hand, SHP2 can dephosphorylate the Tyr1007 site in JAK2 and prevent subsequent degradation of JAK2,39 and can promote STAT5 activation and up-regulate STAT5 target gene expression.40 Our results indicate that SHP2 knockdown has complex effects on STAT5 signaling in human hematopoietic progenitors. SHP2 knockdown is initially associated with increased STAT5 and STAT3 phosphorylation despite reduced Jak2 phosphorylation, probably related to loss of SHP2-mediated dephosphorylation of STAT535 and STAT341 proteins. Reduced Jak2 phosphorylation may reflect the ability of SHP2 to act immediately downstream of GF receptors to facilitate JAK2 activation.40 On the other hand, chronic knockdown of SHP2 resulted in significantly reduced phosphorylated STAT5 and STAT3, suggesting activation of counter-regulatory mechanisms, such as other PTPs, suppressors of cytokine signaling, and protein inhibitors of activated STATs.42,43 Reduced total JAK2 levels with chronic SHP2 knockdown may reflect enhanced proteasomal degradation of JAK2 because of activation of counter-regulatory proteins, such as suppressors of cytokine signaling 1 and suppressors of cytokine signaling 3.44,45 Flow cytometry–based measurement of P-STAT5 in CD34+-expressing cells confirmed increased STAT5 phosphorylation at day 3 but not at day 5 of culture, indicating that these time-dependent changes did not simply reflect the increased numbers of differentiated cells over time.
We observed that higher degrees of chronic SHP2 knockdown result in increased inhibition of STAT5 activation in hematopoietic cells. Because increased SHP2 knockdown was associated with increased apoptosis and inhibition of proliferation of myeloid and erythroid cells, these observations suggest an important contribution of STAT5 activation in mediating SHP2 effects on hematopoietic progenitor viability and proliferation. We further demonstrated that higher degrees of SHP2-knockdown resulted in reduced levels of MCL1 and BCLXL in hematopoietic cells in association with reduced STAT5 activation. MCL1 is a BCL2 family member that plays a critical role in HSC survival.46 Levels of MCL1 and BCLXL, but not BCL2, in hematopoietic cells are regulated by survival signals from cytokines.47 STAT5 signaling up-regulates MCL1 and BCLXL expression by directly activating transcription30,48 and can also up-regulate MCL1 at the protein level by activating the phosphatidylinositol 3-kinase/AKT pathway.48 These results suggest that SHP2 effects on hematopoietic cell viability may be mediated by inhibition of MCL1 and BCLXL downstream of JAK2 and STAT5. Previous studies have shown that knockdown of STAT5 in CB CD34+ cells results in significantly reduced cell proliferation and colony formation without significant effects on cell differentiation and cell apoptosis.49 The additional effects of SHP2 knockdown on downstream signaling pathways, including JAK2, STAT3, ERK, and AKT, may explain the more extensive effects of SHP2 knockdown on cell growth compared with STAT5 knockdown alone.
There is continued interest in evaluation of SHP2 as a therapeutic target and the development of small molecule SHP2 inhibitors.50 The results of the current studies caution that therapeutic SHP2 inhibition could be associated with significant hematopoietic toxicity. On the other hand, we made the important observation that cells expressing a SHP2 gain-of-function mutant exhibited higher levels of apoptosis after SHP2 knockdown compared with cells expressing WT SHP2. These results indicate that cells dependent on gain-of-function SHP2 mutations may be more sensitive to the proapoptotic effects of SHP2 inhibition. The mechanisms underlying this differential sensitivity are unclear at present. These observations support further study to confirm that malignant cells demonstrate greater dependence on SHP2 and enhanced sensitivity to SHP2-specific inhibitors compared with normal cells. It will also be important to determine whether transient or intermittent inhibition of SHP2 may be sufficient to target tumor cells but less toxic to normal hematopoietic cells compared with constitutive knockdown of SHP2, as in the present study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the City of Hope National Medical Center Analytical Cytometry core and the Animal Resources Center for excellent technical support and StemCyte for their generous gift of CB samples.
This work was supported by the National Institutes of Health (grants R01 HL77847 and R01 CA95684; R.B.).
National Institutes of Health
Authorship
Contribution: L.L. designed and performed research, analyzed data, and wrote the manuscript; H.M. and T.M. performed experiments and reviewed the manuscript; J.-K.Y. and J.R. provided material and reviewed the manuscript; and R.B. designed the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ravi Bhatia, Division of Hematopoietic Stem Cell and Leukemia Research, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: rbhatia@coh.org.