Abstract
Hypoxia is emerging as an important characteristic of the hematopoietic stem cell (HSC) niche, but the molecular mechanisms contributing to quiescence, self-renewal, and survival remain elusive. Vascular endothelial growth factor A (VEGFA) is a key regulator of angiogenesis and hematopoiesis. Its expression is commonly regulated by hypoxia-inducible factors (HIF) that are functionally induced in low-oxygen conditions and that activate transcription by binding to hypoxia-response elements (HRE). Vegfa is indispensable for HSC survival, mediated by a cell-intrinsic, autocrine mechanism. We hypothesized that a hypoxic HSC microenvironment is required for maintenance or up-regulation of Vegfa expression in HSCs and therefore crucial for HSC survival. We have tested this hypothesis in the mouse model Vegfaδ/δ, where the HRE in the Vegfa promoter is mutated, preventing HIF binding. Vegfa expression was reduced in highly purified HSCs from Vegfaδ/δ mice, showing that HSCs reside in hypoxic areas. Loss of hypoxia-regulated Vegfa expression increases the numbers of phenotypically defined hematopoietic stem and progenitor cells. However, HSC function was clearly impaired when assessed in competitive transplantation assays. Our data provide further evidence that HSCs reside in a hypoxic microenvironment and demonstrate a novel way in which the hypoxic niche affects HSC fate, via the hypoxia-VEGFA axis.
Introduction
The process of blood formation originates with the hematopoietic stem cell (HSC) which is responsible for the life-long supply of mature blood cells through the unique ability to combine self-renewal and differentiation. During the later stages of mammal development, HSCs are present in the fetal liver (FL) from where they seed the bone marrow (BM), which remains the main site of hematopoiesis throughout adult life. The HSC niche is a term used to describe the location and regulatory microenvironment of HSCs. In the BM, cellular HSC niche components include bone-forming osteoblasts1,2 and perivascular cells,3,4 that through production of various secreted factors as well as through direct cell-cell interactions can affect HSC behavior. As for location it is thought that HSCs are present at a higher frequency at the endosteum close to the bone surface, because ex vivo labeled HSCs tend to localize to the endosteal areas of the BM.5,6 However, within these areas HSCs are likely to associate with both osteoblastic and sinusoidal endothelial cells.7 The FL HSC niche is much less defined than that in the BM, but a candidate FL niche component is a CD3+ Ter119− population that can provide HSC support.8
Apart from HSC regulatory cues such as cell-cell contact and production of soluble factors, it is possible that variations in oxygen availability can affect HSCs, and it has been hypothesized that hypoxia is a characteristic of the HSC niche.9 Oxygen levels are generally lower in BM compared with peripheral blood (PB).10 Two studies have demonstrated that fractions of BM that were not well perfused by injected Hoechst dye, an indirect measure of oxygen availability, were also the fractions that showed the highest HSC activity in transplantation assays.11,12 Similarly, by assuming that low levels of reactive-oxygen species (ROS) corresponds to low levels of oxygen, it was shown that BM cell populations with low ROS activity have higher HSC potential compared with populations where ROS activity is high.13 Moreover, the HSC enriched endosteal areas of BM are positively stained for the hypoxic marker Pimonidazol,14 as are long-label-retaining cells with a sinusoidal location.15 In addition, several studies have shown that both human and murine hematopoietic stem and progenitor cell (HSPC) proliferation and differentiation can be affected by hypoxic treatment ex vivo.9,16-22 Cells exposed to hypoxia generally respond by induction of Hypoxia-Inducible Factors (HIF) HIF-1 and/or HIF-2.23-25 HIFs are transcription factors that consist of 2 subunits: a HIF-α subunit (HIF1A, EPAS1, or HIF3A) that associates with HIF-β (also known as ARNT1). The activity of HIFs is posttranslationally regulated in such a way that the α-subunit is degraded in normoxia because of the action of oxygen-sensing prolyl-hydroxylase-domain proteins. This leads to binding by the E3-ubiqutin-ligase complex von Hippel Lindau (VHL), subsequently leading to proteasomal degradation of the subunit.26-28 In situations of hypoxia this process is no longer active, allowing HIF-α and HIF-β subunits to form heterodimers that can bind to hypoxia-response elements (HRE) in promoters and activate transcription of target genes.24 HIF-1α was recently shown to be an important regulator of HSCs in the hypoxic niche. Loss of HIF-1α activity in HSCs was associated with increased cycling and exhaustion of stem cell activity after secondary transplantation, while stabilization of HIF-1α by deletion of Vhl led to a marked reduction in HSC cycling.29 Consistent with these findings, we have shown that in vitro hypoxic culture as well as chemical or retroviral stabilization of HIF-1α leads to reduced cycling of HSPCs.30 Apart from regulating HSC quiescence, oxygen availability and HIF activity may affect hematopoiesis in other ways yet unknown.
Vascular endothelial growth factor A (VEGFA) is a well-known angiogenic factor that is induced by hypoxia.31,32 Complete absence or haploinsufficiency of Vegfa during development causes embryonic lethality because of a failure to establish vascular angiogenesis as well as hematopoiesis.33,34 Vegfa is also absolutely required for adult hematopoiesis. By conditional deletion of the Vegfa gene in the hematopoietic system, Gerber et al could show that Vegfa is necessary for maintenance of BM activity. Furthermore Vegfa-deficient HSCs fail to engraft lethally irradiated mice despite the presence of VEGFA in the microenvironment of the recipients, demonstrating a cell-intrinsic effect of VEGFA on HSCs. This effect was shown to be regulated by an internal, autocrine loop because antibodies blocking VEGF-receptor activity on the cell surface did not lead to any effect on in vitro colony formation, whereas intracellular inhibitors of VEGF-signal transduction caused apoptosis.35 In addition to the HSC intrinsic effect, VEGFA may also regulate HSC function via extrinsic mechanisms. Establishment of HSC osteoblastic niches via endochondral ossification was shown to depend on VEGFA availability.36 Furthermore, engraftment of transplanted HSCs is dependent on proper expression of a VEGF-receptor, Kdr, in the irradiated BM.37
We hypothesized that a potential role of the hypoxic HSC niche is to up-regulate Vegfa expression in HSCs, thereby contributing to HSC survival. To test this hypothesis we have investigated the hematopoietic phenotype in a model where hypoxia-regulated Vegfa expression is abrogated. In Vegfaδ/δ mice, the HRE in the Vegfa promoter has been deleted at both alleles, thereby inhibiting HIF-binding and subsequent activation of Vegfa expression after hypoxia.38 Vegfa expression is reduced in highly purified HSCs from BM of Vegfaδ/δ mice but not in mature cells. This observation provides further support for the hypothesis that HSCs reside in hypoxic locations of BM. We show that ablation of the hypoxic regulation of Vegfa expression within the hematopoietic system affects hematopoietic differentiation and increases the number of phenotypically identified HSCs, but at the same time leads to a functional defect of HSCs identified by competitive transplantation experiments.
Methods
Mice
Vegfatm2Pec mice38 (referred to as Vegfaδ) were kindly provided by P. Carmeliet, Leuven University, Belgium. Ethical permission to perform animal experiments was obtained from the Board for Research Animal Ethics, Lund. Vegfaδ/+ on a mixed 129 SvS6 × Swiss Tac background (CD45.2) were intercrossed with C57Bl/6J (CD45.2) wild-type (WT) mice. F1Vegfaδ/+ mice from this crossing were mated to obtain mice and fetal livers with the Vegfaδ/δ genotype. WT littermates were used as controls in all experiments. The colony was maintained on a C57Bl/6J × 129 SvS6 × Swiss Tac mixed background. On this background, the mice had a mixed genotype at the H2-locus (H2b/H2q). For BM transfer experiments, mice were therefore bred to obtain a homozygous H2b/H2b genotype, compatible with C57Bl/6 and B6.SJL used as recipients and source of competitor cells. Vegfaδ/δ mice display a partial prenatal lethality and adult Vegfaδ/δ mice that survive develop an amyotrophic lateral sclerosis-like disease around 6 months. In our study we analyzed mice before the onset of disease, to avoid any unspecific effects on the hematopoietic system.
Flow cytometry
For a full list of antibody clones, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For analysis of chimerism in transplanted mice, PB and BM were stained with CD45.1-PerCp and CD45.2-FITC. To detect B cells, T cells, and myeloid cells, samples were stained with B220-PE, B220-APC, CD3-PE, and Mac-1-APC. For analysis of the lineage−, Sca-1+, c-kit+ (LSK) compartment in the BM, samples were first stained for lineage-specific markers by unconjugated antibodies against B220, CD3, CD4, CD8, Ter119, Mac-1, and Gr-1 followed by Tricolor-conjugated goat anti–rat antibodies (Invitrogen) and Sca-1-PE, c-kit-APC and CD34-FITC. For purification of LSK CD34+/− cells, samples were enriched for c-kit expression before staining using MidiMACS columns (Miltenyi). For analysis of ROS content, pre-stained cells were incubated in 10μM 2′,7′-Dichlorofluorescin diacetate (DCFDA; Sigma-Aldrich) for 20 minutes at 37°C. For analysis of the LSK compartment in FL, samples were first depleted of Ter119+ cells and thereafter stained for the lineage-specific markers B220, CD5, CD8, Ter119, and Gr-1 followed by Tricolor-conjugated goat anti–rat and Sca-1-FITC, c-kit-APC, and CD150-APC. Cells were analyzed or sorted using a FACS CantoII, FACS Calibur, FACS Diva or FACS Aria (BD Bioscience) and FlowJo Version 9.1 software (TreeStar). Total blood counts were measured by automated hematocytometry on a Sysmex system (Sysmex GmbH).
Competitive-repopulation assay
Recipient mice, 8-12 weeks old, (C57Bl/6 × B6.SJL; CD45.1/2) were lethally irradiated with 900 cGy 20-24 hours before transplantation. 2 × 105 BM cells from Vegfaδ/δ or WT (CD45.2) mice were mixed with 2 × 105 competitor BM cells from B6.SJL (CD45.1) mice and injected into the tail vein of irradiated recipients. Peripheral-blood chimerism was measured by FACS analysis of CD45.1 and CD45.2 cell surface expression at 4, 8, 12, and 15 weeks post transplantation. At 15 weeks, chimerism in the BM LSK compartment was analyzed. For quantification of competitive repopulating units (CRU), groups of recipient mice were transplanted with 5 × 103, 2 × 104, 5 × 104, and 2 × 105 BM cells from Vegfaδ/δ or WT mice together with 2 × 105 competitor cells. Positive engraftment was defined as > 1% lymphoid and myeloid engraftment at 22 weeks post transplantation. CRU content was analyzed using L-calc software (StemCell Technologies).
Statistics
Statistical significance was determined by calculating 2-tailed P values using t test or Mann-Whitney test.
Results
Vegfa expression is reduced in HSCs from Vegfaδ/δ mice
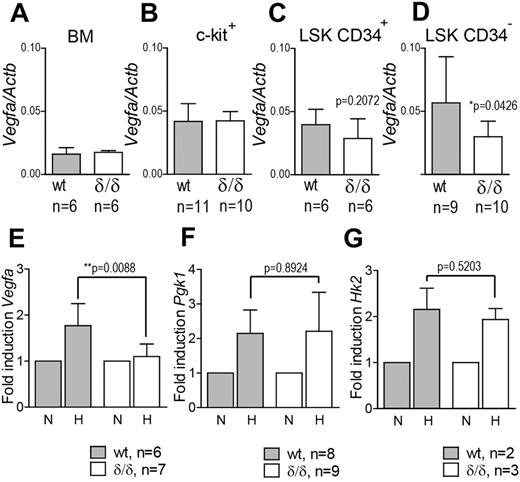
To investigate whether hypoxia-induced Vegfa expression was indeed perturbed in the hematopoietic system of Vegfaδ/δ mice, we incubated c-kit+ cells from WT or Vegfaδ/δ mice in normoxic or hypoxic conditions and thereafter measured Vegfa expression by quantitative real-time PCR (Q-RT-PCR). Vegfa expression was increased in WT cells exposed to hypoxia compared with normoxic expression levels. At the same time, Vegfa expression remained largely unchanged in Vegfaδ/δ cells on hypoxic exposure (Figure 1E). We also measured expression of Pgk1 and Hk2, 2 glycolysis-associated genes previously reported to be induced by hypoxia.39 Expression of these 2 genes was increased in both genotypes after hypoxic culture, demonstrating the specificity of the lacking Vegfa induction in Vegfaδ/δ mice (Figure 1F-G). We next measured Vegfa expression in different BM cell populations. In total BM cells, c-kit+, and LSK CD34+ cells, there were no differences in Vegfa expression between WT and Vegfaδ/δ, arguing for the fact that these subsets have similar basal expression but lack hypoxia/HIF-induced up-regulation of Vegfa (Figure 1A-C). However, in LSK CD34− cells, a population highly enriched for long-term repopulating HSCs, Vegfa expression was significantly lower in Vegfaδ/δ samples compared with WT (Figure 1D). These results indirectly indicate that HSCs are localized in hypoxic areas where Vegfa expression is induced, while more mature cells are not.
Vegfa expression is reduced in LSK CD34− cells from Vegfaδ/δ BM but not in more differentiated BM cells.Vegfa expression was measured by Q-RT-PCR in (A) unfractionated BM, (B) c-kit enriched cells, (C) LSK CD34+ cells (P = .2072, t test), and (D) LSK CD34− cells (*P = .0426, t test) from Vegfaδ/δ or WT mice. Vegfa expression was normalized to Actb expression. Bars represent mean ± SD. mRNA levels of (E) Vegfa (*P = .0088, t test), (F) Pgk1 (P = .8924, t test), and (G) Hk2 (P = .5203, t test) were measured in c-kit enriched cells from Vegfaδ/δ or WT mice previously incubated in normoxic or hypoxic conditions for 12 hours. Data in panels E,F, and G are presented as fold increase in gene expression compared with normoxia. Bars represent mean ± SD. Results shown are data combined from 3 independent experiments. N = normoxia, H = hypoxia.
Vegfa expression is reduced in LSK CD34− cells from Vegfaδ/δ BM but not in more differentiated BM cells.Vegfa expression was measured by Q-RT-PCR in (A) unfractionated BM, (B) c-kit enriched cells, (C) LSK CD34+ cells (P = .2072, t test), and (D) LSK CD34− cells (*P = .0426, t test) from Vegfaδ/δ or WT mice. Vegfa expression was normalized to Actb expression. Bars represent mean ± SD. mRNA levels of (E) Vegfa (*P = .0088, t test), (F) Pgk1 (P = .8924, t test), and (G) Hk2 (P = .5203, t test) were measured in c-kit enriched cells from Vegfaδ/δ or WT mice previously incubated in normoxic or hypoxic conditions for 12 hours. Data in panels E,F, and G are presented as fold increase in gene expression compared with normoxia. Bars represent mean ± SD. Results shown are data combined from 3 independent experiments. N = normoxia, H = hypoxia.
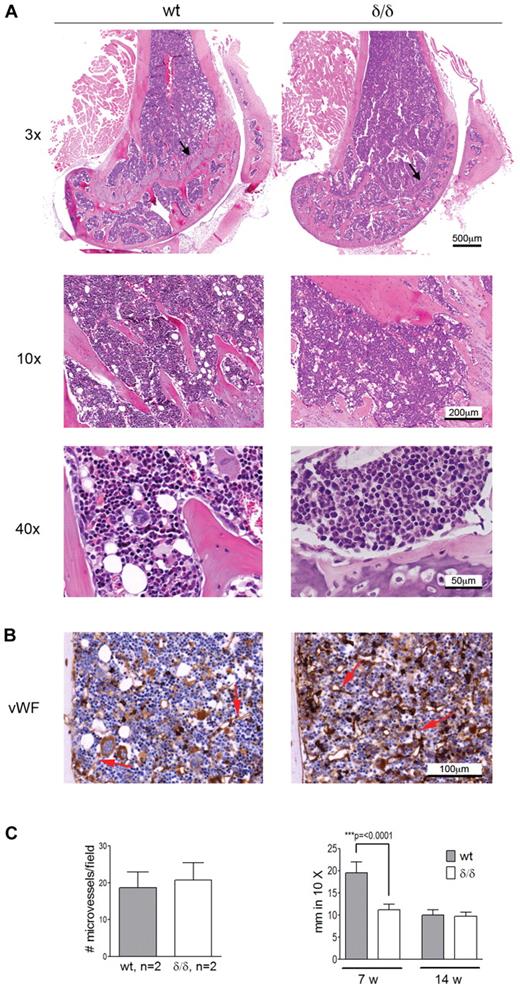
Endochondral ossification is impaired in Vegfaδ/δ mice while BM morphology and microvessel density are not affected
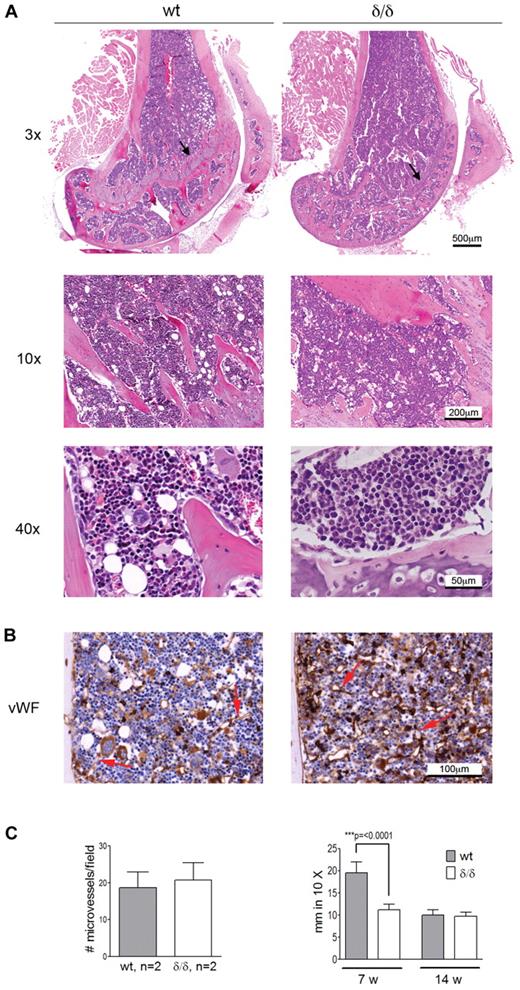
Apart from hematopoiesis, VEGFA is involved in processes like angiogenesis and endochondral ossification. Because both bone-associated and vascular-associated HSC niches have been proposed, we wanted to investigate if the bone or BM vascular architecture was affected by the Vegfaδ/δ mutation because such changes could cause secondary effects on hematopoiesis by altering the HSC niche. BM sections from femur of Vegfaδ/δ mice appeared normal with comparable BM cellularity and distribution of bone trabeculae compared with WT sections. However, the epiphyseal growth plate in the femur of young, but not adult, Vegfaδ/δ mice was reduced compared with WT, suggesting a defect in endochondral bone formation (Figure 2A). Sections of bone were stained with antibodies against VWF to visualize distribution of microvessels. No differences in microvessel density could be observed between the 2 genotypes (Figure 2B-C).
BM morphology and microvessel density of Vegfaδ/δ mice are normal while the endochondral ossification is decreased. (A) Representative histology images of mouse BM sections were captured from 1 WT mouse and 2 Vegfaδ/δ mice in the trabecular area of distal femur samples at original magnification ×3, ×10, and ×40. Histologic specimens were stained with hematoxylin and eosin. Arrows indicate the epiphyseal growth plate. (B) BM sections were immunohistochemically stained with VWF to detect microvessels. Original magnification ×20. Red arrows indicate microvessels. (C) The average number of microvessels was counted in 3 different images per mouse captured from 2 mice per group. Bars represent mean ± SD. The thickness of the epiphyseal growth plate in femur was measured manually in 10× magnifications. Bars represent mean ± SD of 7-11 measurements from representative images, P < .0001, t test. All images were captured using a Zeiss slide scanner containing a Sony DFW-X710 camera and MIRAX software.
BM morphology and microvessel density of Vegfaδ/δ mice are normal while the endochondral ossification is decreased. (A) Representative histology images of mouse BM sections were captured from 1 WT mouse and 2 Vegfaδ/δ mice in the trabecular area of distal femur samples at original magnification ×3, ×10, and ×40. Histologic specimens were stained with hematoxylin and eosin. Arrows indicate the epiphyseal growth plate. (B) BM sections were immunohistochemically stained with VWF to detect microvessels. Original magnification ×20. Red arrows indicate microvessels. (C) The average number of microvessels was counted in 3 different images per mouse captured from 2 mice per group. Bars represent mean ± SD. The thickness of the epiphyseal growth plate in femur was measured manually in 10× magnifications. Bars represent mean ± SD of 7-11 measurements from representative images, P < .0001, t test. All images were captured using a Zeiss slide scanner containing a Sony DFW-X710 camera and MIRAX software.
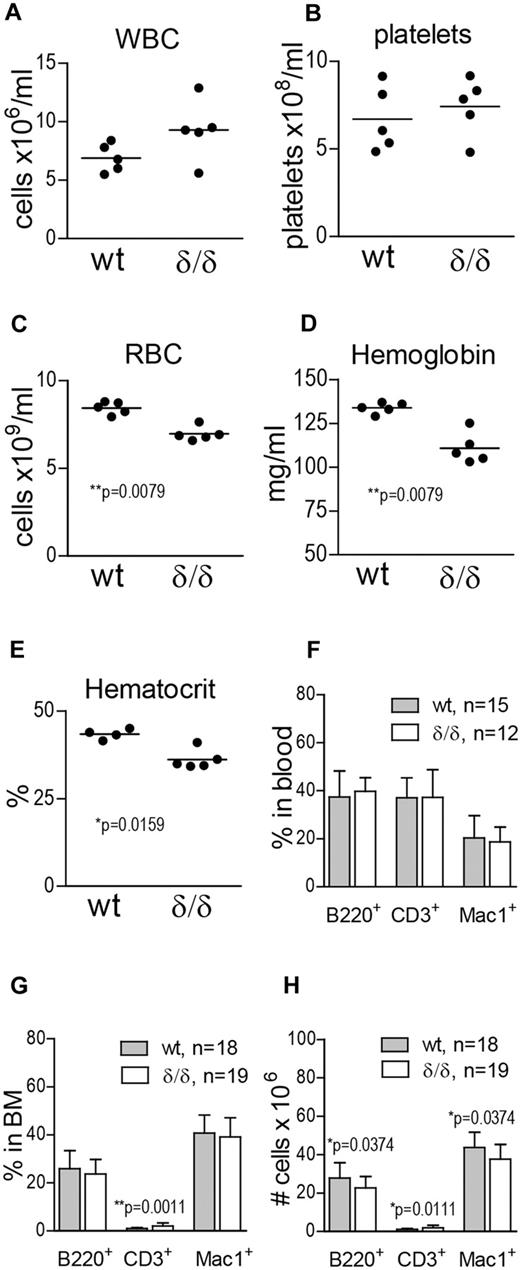
Vegfaδ/δ mice have anemia and altered numbers of differentiated cells in the BM
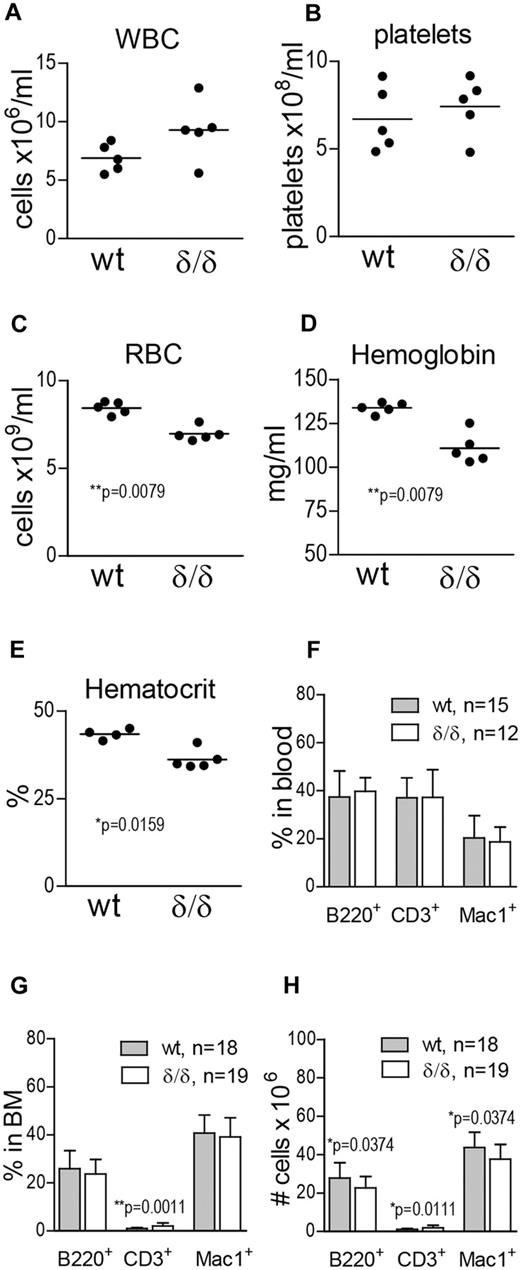
To investigate the effect of ablated hypoxia-regulated Vegfa expression on hematopoietic output, we measured WBCs, platelets, RBCs, hemoglobin, and hematocrit in the PB of WT and Vegfaδ/δ mice. WBC and platelet counts were not significantly different between the groups (Figure 3A-B). However, RBC, hemoglobin, and hematocrit values were reduced in the PB of Vegfaδ/δ mice compared with WT littermates, suggesting a deficiency in erythropoiesis (Figure 3C-E). When erythroid blast forming unit (BFU-E) formation in response to recombinant erythropoietin was assessed in vitro, we could not detect any deficiency in Vegfaδ/δ cells (supplemental Figure 1D). To investigate whether erythropoietic development was blocked at a specific stage, we performed FACS analysis using markers defining maturing erythrocytes. We found that proerythroblasts and basophilic erythroblasts were slightly increased in the BM of Vegfaδ/δ mice, while the later stages (late baso- and chromatophilic erythroblasts and orthochromatic normoblasts) tended to be decreased (supplemental Figure 1A-C). To investigate differentiation capacity toward lymphoid and myeloid lineages, FACS analysis for B cells (B220+), T cells (CD3+), and monocytes (Mac-1+) was performed. We observed a slight increase in T cells and a decrease in B-cell and monocyte numbers in the BM of Vegfaδ/δ mice compared with WT (Figure 3G-H), while there were no differences in lineage distribution in the PB (Figure 3F).
Vegfaδ/δ mice display mild anemia and altered numbers of differentiated cells in the BM. (A) WBC count, (B) platelet count, (C) RBC count, (D) hemoglobin, and (E) hematocrit levels were measured on blood samples from Vegfaδ/δ or WT mice. Results are shown from 5 individual mice per group. P values are from Mann-Whitney test. (F) Percentages of B220+, CD3+, and Mac-1+ cells in PB were determined by FACS analysis. Bars represent mean ± SD. Results shown are data combined from 5 independent experiments. (G) BM lineage distribution. Bars represent mean ± SD, **P = .0011, Mann-Whitney test. (H) Total numbers of B220+, CD3+, and Mac-1+ cells in 2 × femur and tibia. Bars represent mean ± SD, P values are from Mann-Whitney test. Results shown in panels G and H are data combined from 6 independent experiments.
Vegfaδ/δ mice display mild anemia and altered numbers of differentiated cells in the BM. (A) WBC count, (B) platelet count, (C) RBC count, (D) hemoglobin, and (E) hematocrit levels were measured on blood samples from Vegfaδ/δ or WT mice. Results are shown from 5 individual mice per group. P values are from Mann-Whitney test. (F) Percentages of B220+, CD3+, and Mac-1+ cells in PB were determined by FACS analysis. Bars represent mean ± SD. Results shown are data combined from 5 independent experiments. (G) BM lineage distribution. Bars represent mean ± SD, **P = .0011, Mann-Whitney test. (H) Total numbers of B220+, CD3+, and Mac-1+ cells in 2 × femur and tibia. Bars represent mean ± SD, P values are from Mann-Whitney test. Results shown in panels G and H are data combined from 6 independent experiments.
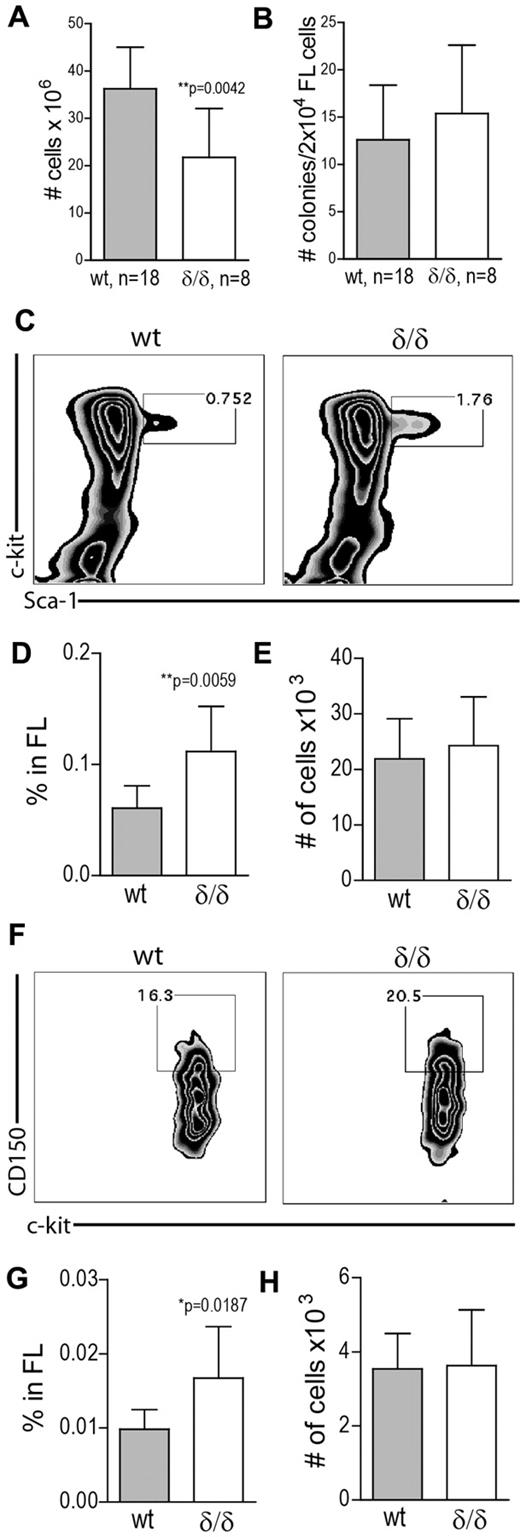
Ablated hypoxia-regulated Vegfa expression increases adult HSPC frequency
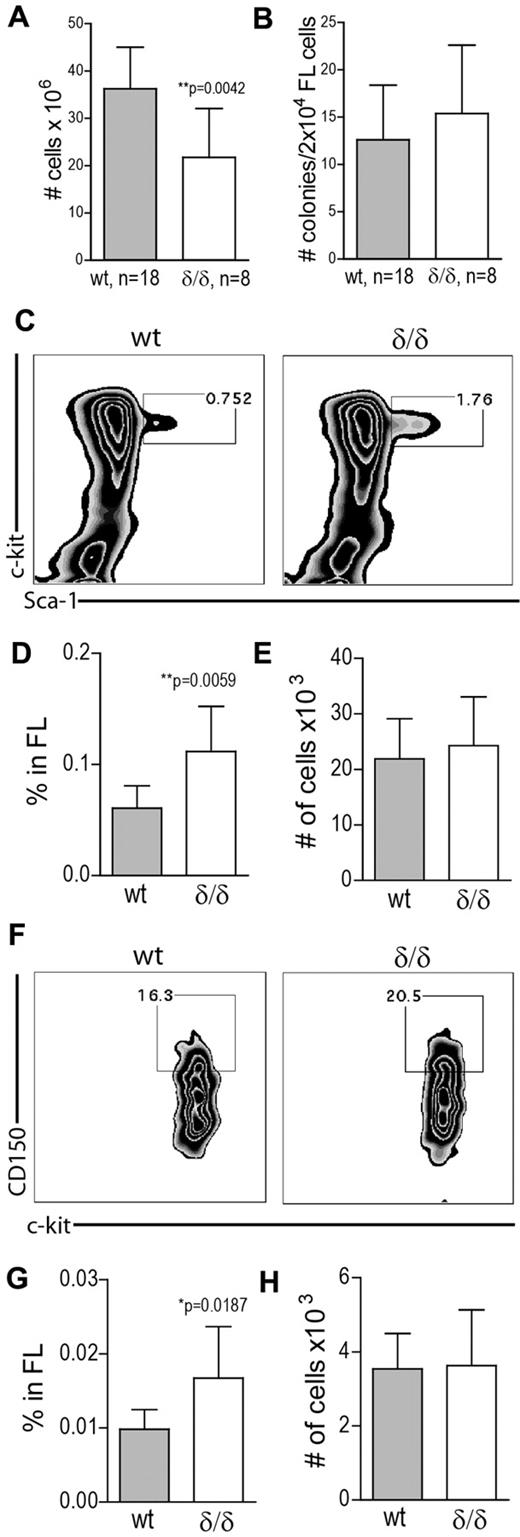
To determine whether hypoxia-related Vegfa expression is needed to maintain HSPC integrity, we measured the frequency of different cell populations in the BM of WT and Vegfaδ/δ mice. Total BM cellularity was slightly but significantly decreased in Vegfaδ/δ mice compared with WT (Figure 4A). Hematopoietic colony forming unit (CFU) activity in vitro was similar between cells from WT and Vegfaδ/δ BM (Figure 4B). Recombinant VEGFA was added to the cultures with the intent to rescue a possible defect in CFU-formation, but the addition of the protein does not seem to have any effect on colony formation by itself. CFU assays were also performed in hypoxic conditions (1% O2). Although it has previously been reported that hypoxia can increase colony formation,19,20 we did not detect any major changes compared with normoxia (Figure 4B). FACS analysis showed that the frequency and total numbers of LSK cells in BM of Vegfaδ/δ mice were not different between the genotypes (Figure 4C-E). The frequency of cells in the LSK CD34− compartment was slightly increased in contrary to our hypothesis, but total numbers were not (Figure 4F-H).
HSPC frequency is increased in BM with defect hypoxia-regulated Vegfa expression. (A) BM cellularity was determined by counting mononuclear cells from femur, tibia, and iliac crest from Vegfaδ/δ or WT mice. Data are presented as ratio of WT BM cellularity and results shown are combined data from 8 independent experiments. Bars represent mean ± SD, **P = .0065, t test. (B) Total CFU frequency was measured by plating 2 × 104 BM cells/mL in methylcellulose, with or without mVEGFA, followed by incubation in normoxia or hypoxia for 7 days. Results shown are data combined from 4 independent experiments. (C) The frequency of LSK cells in the BM was measured by FACS analysis. Representative FACS plots of lineage− gated cells. (D) Percentage LSK cells in BM. (E) Total number of LSK cells per 2 × femur and tibia. Results shown in D-E are data combined from 5 independent experiments. Bars represent mean ± SD, n = 15-17 mice per genotype. P values are from t test. (F) The frequency of LSK CD34− cells in BM was measured by FACS analysis. Representative FACS plots of LSK gated cells. (G) Percentage LSK CD34− cells in BM. (H) Total number of LSK CD34− cells per 2 × femur and tibia. Results shown in G-H are data combined from 4 independent experiments. Bars represent mean ± SD, n = 13 mice per genotype. P values are from t test.
HSPC frequency is increased in BM with defect hypoxia-regulated Vegfa expression. (A) BM cellularity was determined by counting mononuclear cells from femur, tibia, and iliac crest from Vegfaδ/δ or WT mice. Data are presented as ratio of WT BM cellularity and results shown are combined data from 8 independent experiments. Bars represent mean ± SD, **P = .0065, t test. (B) Total CFU frequency was measured by plating 2 × 104 BM cells/mL in methylcellulose, with or without mVEGFA, followed by incubation in normoxia or hypoxia for 7 days. Results shown are data combined from 4 independent experiments. (C) The frequency of LSK cells in the BM was measured by FACS analysis. Representative FACS plots of lineage− gated cells. (D) Percentage LSK cells in BM. (E) Total number of LSK cells per 2 × femur and tibia. Results shown in D-E are data combined from 5 independent experiments. Bars represent mean ± SD, n = 15-17 mice per genotype. P values are from t test. (F) The frequency of LSK CD34− cells in BM was measured by FACS analysis. Representative FACS plots of LSK gated cells. (G) Percentage LSK CD34− cells in BM. (H) Total number of LSK CD34− cells per 2 × femur and tibia. Results shown in G-H are data combined from 4 independent experiments. Bars represent mean ± SD, n = 13 mice per genotype. P values are from t test.
Ablated hypoxia-regulated Vegfa expression causes late gestational lethality and increases HSPC frequency in the FL
The Vegfaδ/δ mutation is partly embryonically lethal for still unknown reasons. We therefore wanted to investigate the time point at which embryos die in utero to evaluate the possibility that the lethality is associated with the time point of FL hematopoiesis or BM seeding. Prenatal lethality was previously reported to be approximately 60% in mice on 129 SvS6 × Swiss Tac background.38 This number was increased in our hands, where an additional element of the C57Bl/6 strain has been added, and reached 76%. In addition, we observed that back-crossing further than one generation to C57Bl/6 led to a complete absence of homozygous pups born from δ/+ × δ/+ matings (100% lethality). By comparing genotype outcomes from mice surviving past birth to embryos killed at day 15.5 of embryogenesis, we could observe that the frequency of the δ/δ genotype was higher, although not reaching mendelian ratios, at day 15.5 compared with postbirth time points (18% and 6% respectively; Table 1), suggesting substantial lethality during the late gestational period. To investigate whether hypoxia-regulated Vegfa expression is needed to maintain FL hematopoiesis, we measured the frequencies of HSPCs in FLs of WT and Vegfaδ/δ mice. Consistent with the BM findings, cellularity was significantly lower in Vegfaδ/δ FLs compared with WT (Figure 5A). CFU activity was similar between the 2 genotypes (Figure 5B). By FACS analysis, we detected an increase in the frequency of LSK cells, but not absolute numbers, in Vegfaδ/δ FLs compared with WT (Figure 5C-E). The LSK CD150+ frequency, but not absolute numbers, was increased in Vegfaδ/δ FLs (Figure 5F-H).
HSPC frequency is increased in FL cells lacking hypoxic Vegfa induction. (A) FL cellularity of Vegfaδ/δ or WT mice. Results shown are combined data from 5 independent experiments. Bars represent mean ± SD, **P = .0042 Mann-Whitney test. (B) Total CFU frequency was measured by plating 2 × 104 FL cells/mL in methylcellulose followed by incubation in normoxia for 7 days. Results shown are data combined from 4 independent experiments. (C) The frequency of LSK cells in FL was measured by FACS analysis. Representative FACS plots of lineage− gated cells. (D) Percentage LSK cells in FL. **P = .0059, t test. (E) Total number of LSK cells per FL. (F) The frequency of LSK CD150+ cells in FL was measured by FACS analysis. Representative FACS plots of LSK gated cells are shown. (G) Percentage LSK CD150+ cells in FL. *P = .0187, t test. (H) Total number of LSK CD150+ cells per FL. Results shown in panels C-H are data combined from 4 independent experiments. Bars represent mean ± SD, n = 8-9 mice per genotype.
HSPC frequency is increased in FL cells lacking hypoxic Vegfa induction. (A) FL cellularity of Vegfaδ/δ or WT mice. Results shown are combined data from 5 independent experiments. Bars represent mean ± SD, **P = .0042 Mann-Whitney test. (B) Total CFU frequency was measured by plating 2 × 104 FL cells/mL in methylcellulose followed by incubation in normoxia for 7 days. Results shown are data combined from 4 independent experiments. (C) The frequency of LSK cells in FL was measured by FACS analysis. Representative FACS plots of lineage− gated cells. (D) Percentage LSK cells in FL. **P = .0059, t test. (E) Total number of LSK cells per FL. (F) The frequency of LSK CD150+ cells in FL was measured by FACS analysis. Representative FACS plots of LSK gated cells are shown. (G) Percentage LSK CD150+ cells in FL. *P = .0187, t test. (H) Total number of LSK CD150+ cells per FL. Results shown in panels C-H are data combined from 4 independent experiments. Bars represent mean ± SD, n = 8-9 mice per genotype.
HSCs lacking hypoxia-regulated Vegfa expression have impaired competitive repopulation capacity
To evaluate HSC function, we performed competitive-repopulation assays, where 2 × 105 BM cells from WT or Vegfaδ/δ mice were transferred to lethally irradiated recipient mice together with an equal dose of BM competitor cells from CD45 congenic mice. Both short-term and long-term reconstitution was significantly reduced in PB of recipients of Vegfaδ/δ BM compared with WT, indicating HSC impairment (Figure 6A-C). Engrafted cells from WT and Vegfaδ/δ mice showed comparable contribution to myeloid and lymphoid lineages (Figure 6D). After 15 weeks we evaluated chimerism in the BM LSK compartment of recipient mice. The levels of CD45.2-expressing LSK cells were significantly decreased in recipients of Vegfaδ/δ BM, confirming that HSCs lacking hypoxic Vegfa expression have defect engraftment ability (Figure 6E). Furthermore, a CRU assay where limiting doses of BM cells were transplanted to groups of recipients, showed that CRU frequency was approximately 3-fold lower in BM from Vegfaδ/δ mice compared with WT (1/1.1 × 105 and 1/3.7 × 104, respectively; Figure 6F).
HSCs lacking hypoxia-induced Vegfa expression have impaired competitive reconstitution potential. Test cells (2 × 105; CD45.2) and competitor cells (2 × 105; CD45.1) were transplanted to lethally irradiated recipients. Results are shown from 1 representative experiment of 2 performed where 3 different donors per genotype were injected into 4-5 recipients each. Test cell contribution to PB at (A) 4 weeks, *P = .0107, t test, (B) 8 weeks, **P = .0014, t test, and (C) 15 weeks after transplantation, ***P < .0001, t test. (D) Lineage distribution of engrafted cells in PB at 15 weeks after transplantation. Bars represent mean ± SD. (E) Test cell contribution to the BM LSK compartment at 15 weeks after transplantation. **P = .0023, Mann-Whitney test. (F) CRU frequency was determined by limiting-dilution transplantations. Percentages of recipients with negative engraftment of test cells are plotted against the cell dose given. n = 5 recipient mice per cell dose and n = 3 donors per genotype. Poisson distribution gives that 1 CRU is present at the derived cell dose where the frequency of negatively engrafted recipients is 37%.
HSCs lacking hypoxia-induced Vegfa expression have impaired competitive reconstitution potential. Test cells (2 × 105; CD45.2) and competitor cells (2 × 105; CD45.1) were transplanted to lethally irradiated recipients. Results are shown from 1 representative experiment of 2 performed where 3 different donors per genotype were injected into 4-5 recipients each. Test cell contribution to PB at (A) 4 weeks, *P = .0107, t test, (B) 8 weeks, **P = .0014, t test, and (C) 15 weeks after transplantation, ***P < .0001, t test. (D) Lineage distribution of engrafted cells in PB at 15 weeks after transplantation. Bars represent mean ± SD. (E) Test cell contribution to the BM LSK compartment at 15 weeks after transplantation. **P = .0023, Mann-Whitney test. (F) CRU frequency was determined by limiting-dilution transplantations. Percentages of recipients with negative engraftment of test cells are plotted against the cell dose given. n = 5 recipient mice per cell dose and n = 3 donors per genotype. Poisson distribution gives that 1 CRU is present at the derived cell dose where the frequency of negatively engrafted recipients is 37%.
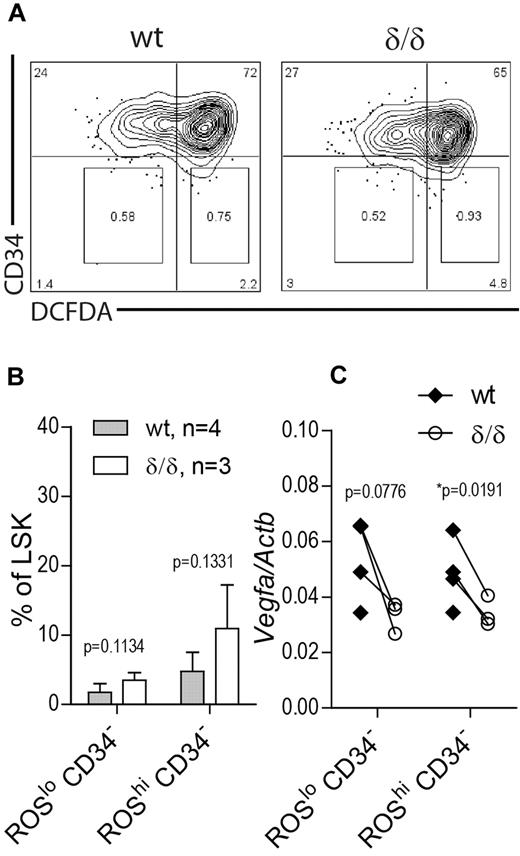
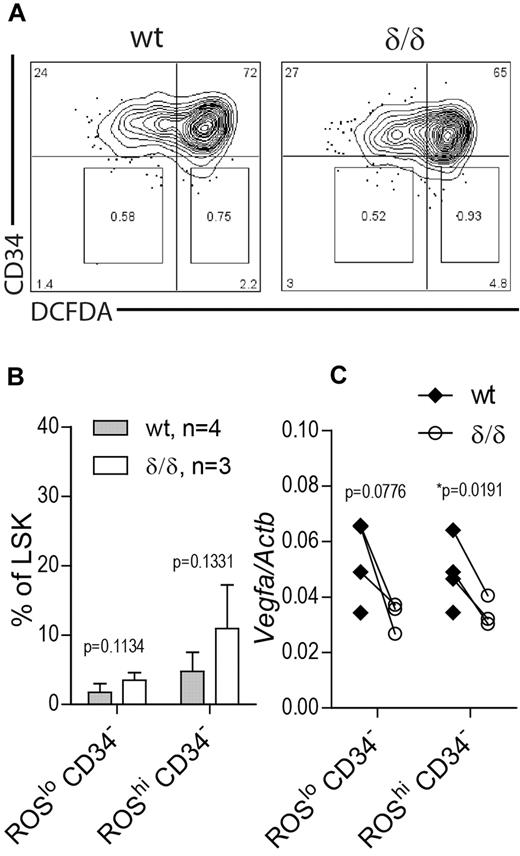
Increased HSC frequency in Vegfaδ/δ mice is not because of selective expansion of a subpopulation of normoxic HSCs
The unexpected finding of increased HSC frequency as defined by surface markers in BM of Vegfaδ/δ mice may reflect a situation of stressed hematopoiesis because RBC output is lower and HSC function is clearly impaired. Therefore we wanted to investigate whether this increase is because of expansion of a subpopulation of HSCs that reside in normoxia and thereby are able to escape the drastic effect of a nonfunctional hypoxic Vegfa induction. To this end we measured Vegfa levels in LSK CD34− cells that were further subdivided based on ROS content, measured by DCFDA staining. Because ROS are formed as a consequence of oxygen dependent reactions, ROS levels are assumed to correlate with oxygen availability. We used this approach to divide the LSK CD34− compartment into hypoxic (ROSlo) and normoxic (ROShi) populations (Figure 7A). Distinct ROSlo and ROShi populations were detected in both Vegfaδ/δ and WT mice. There was a trend toward an increase in the ROShi CD34− population as well as the ROSlo CD34− population in Vegfaδ/δ mice compared with WT but the changes were not statistically significant (Figure 7A-B). When we measured Vegfa gene expression in the sorted subpopulations, we again detected lower Vegfa expression in both ROSlo CD34− and ROShi CD34−Vegfaδ/δ samples compared with WT, suggesting relatively hypoxic locations for both populations (Figure 7C).
Vegfa expression in ROSlo and ROShi HSC populations. BM cells from Vegfaδ/δ or WT mice were stained with markers to detect LSK CD34 and DCFDA to detect ROS. (A) Representative FACS profiles of LSK gated cells showing gates used for sorting of CD34− ROSlo and CD34− ROShi populations. (B) Percentage LSK CD34− ROSlo and LSK CD34− ROShi cells within LSK gated cells. Bars represent mean ± SD. P values are from t test. (C) Vegfa expression in sorted subpopulations. Each dot represents an individual mouse and connecting lines are between littermate pairs. P values are from paired t test. Results shown are data combined from 2 independent experiments.
Vegfa expression in ROSlo and ROShi HSC populations. BM cells from Vegfaδ/δ or WT mice were stained with markers to detect LSK CD34 and DCFDA to detect ROS. (A) Representative FACS profiles of LSK gated cells showing gates used for sorting of CD34− ROSlo and CD34− ROShi populations. (B) Percentage LSK CD34− ROSlo and LSK CD34− ROShi cells within LSK gated cells. Bars represent mean ± SD. P values are from t test. (C) Vegfa expression in sorted subpopulations. Each dot represents an individual mouse and connecting lines are between littermate pairs. P values are from paired t test. Results shown are data combined from 2 independent experiments.
Discussion
Several pieces of evidence for the existence of BM HSC niches low in oxygen have been presented, but the importance of such areas to HSC fate has only begun to be elucidated. It is clear that hypoxia, indirectly measured by low ROS-activity,13 low perfusion as measured by uptake of injected Hoechst dye,11,12 or low metabolic activity,40 correlates with increased HSC activity. Indeed, these findings fit with the idea that HSCs, to last for a complete life-time, need to be preserved from stress imposed by potentially mutagenic molecules that are bi-products of oxygen consumption. Recent data demonstrate an important role for HIF-1α in HSC function. HSCs lacking expression of Hif1a are able to engraft lethally irradiated recipients but fail to sustain hematopoiesis for extended periods or after serial transplantation, indicating exhaustion from extensive proliferation.29 In agreement with this, we have shown that in vitro hypoxic treatment or constitutive activation of HIF-1α activity in HSCs reduces proliferation and leads to up-regulation of cell cycle-inhibitory genes.30 Thus, one important function of the hypoxic niche seems to be keeping HSCs in relative quiescence. However, because HIF-1α is such a master regulator of the hypoxic response with hundreds of known target genes, it is tempting to believe that there are other ways in which hypoxia affects HSC fate. Although being one of the typical hypoxia-regulated genes as shown in several tissues, the importance of the hypoxia-VEGFA axis in HSC biology has never been examined. Because HSCs likely reside in a hypoxic microenvironment, and because Vegfa is a highly hypoxia-dependent gene crucial for HSC survival, we hypothesized that abrogated hypoxia-induced expression in our model would affect HSC numbers and/or function.
Q-RT-PCR experiments showed that Vegfa expression in WT cells was lowest in unfractionated BM but increased in the more primitive c-kit+, LSK CD34+, and LSK CD34− populations. In purified HSCs from Vegfaδ/δ mice, where hypoxia is not able to affect Vegfa expression, Vegfa was reduced compared with WT control cells. The reduction was specifically seen in the LSK CD34− population and thus demonstrates that long-term HSCs reside in hypoxic areas of BM while more mature cells are not. Whether these areas are preferentially endosteal, vascular or other, cannot be concluded from our data.
Differentiation toward myeloid, B-cell and T-cell lineages as measured by FACS was normal in PB of Vegfaδ/δ mice, while in BM a slightly distorted distribution was observed. Vegfaδ/δ mice are slightly anemic with lower levels of RBCs, hemoglobin, and hematocrit in the PB compared with WT. FACS analysis of progenitors at different stages of erythropoiesis showed slightly lower levels of late developing erythroblasts with a corresponding increase in the earlier progenitor populations: proerythroblasts and basophilic erythroblasts. Furthermore, the more primitive BFU-E, as measured by colony formation in response to erythropoietin in vitro, was normal. It is therefore possible that the decreased levels of peripheral RBCs in Vegfaδ/δ mice is because of a general survival defect of late erythrocytes that is dependent on hypoxia-induced Vegfa. Vegfaδ/δ mice have lower numbers of BM cells compared with WT littermates. However, the BM did not appear hypocellular in histologic sections and therefore the decreased BM count is probably rather a secondary effect of the smaller overall size of Vegfaδ/δ mice compared with their WT littermates than a direct effect on hematopoiesis. The frequency of phenotypically defined long-term HSCs, LSK CD34− cells, are increased in BM of Vegfaδ/δ mice, in contrary to our hypothesis that ablated hypoxic Vegfa up-regulation would decrease HSC numbers. However, phenotypically defined HSCs do not necessarily correspond to functionally defined HSCs. This unanticipated phenomenon has been observed in other transgenic mice lacking genes important in HSC function like Gfi1 and Cxcr4.41,42 One way to interpret the data are that the increased frequency of HSCs in Vegfaδ/δ mice is a way to compensate for less functional ones. This is in agreement with the results that we obtained from the HSC functional assays, where HSCs from Vegfaδ/δ mice were shown to have a lower capacity to competitively reconstitute irradiated hosts compared with WT HSCs. The effect was seen as early as 4 weeks but was more pronounced at 15 weeks post transplantation, showing that short-term and more importantly long-term reconstitution was affected. In addition, Vegfaδ/δ contribution to the LSK compartment at 15 weeks was reduced while WT chimerism was high. Although equal doses of test and competitor cells were transplanted, WT chimerism was higher than the expected 50%. However, WT test cells in our experiment are likely to compete better than the competitor cells because of their 129Sv background; It has been demonstrated that 129Sv mice have increased HSC activity compared with B6 strains.43 The engraftment defect of Vegfaδ/δ HSCs clearly demonstrate that although steady-state hematopoiesis can be maintained, HSCs that are not able to up-regulate Vegfa in response to hypoxia are less fit and perform poorly when challenged. Because it has previously been demonstrated that the role VEGFA plays in HSCs is mainly to mediate survival, we suggest that a hypoxia-driven Vegfa boost is needed for survival when HSCs seed their new niches after transplantation, while baseline levels of VEGFA together with other factors present in the BM is enough to maintain HSCs in unchallenged mice. However, it cannot be ruled out that processes like homing and lodging are responsible for the observed results. Because normal levels of VEGFA are present in BM of WT recipient mice, our results confirm that the need for VEGFA is indeed HSC intrinsic as previously suggested.35
Based on ROS staining, the discrepancy between phenotypic and functional HSCs in Vegfaδ/δ mice could not be explained by the existence of 2 separate populations within the LSK CD34− compartment where normoxic HSCs selectively expand to compensate for lower survival of hypoxic HSCs. Rather, both ROSlo and ROShi LSK CD34− cells tend to be increased and both these populations have somewhat decreased Vegfa expression. It is possible that oxygen/ROS levels in the LSK compartment is already relatively low compared with more mature cells in the BM and this could be one reason why we could not detect differences in Vegfa expression between ROS subpopulations. Low ROS levels selects for primitive cells when lineage− CD45+ cells are used as starting material, indicating that these cells are hypoxic but whether ROS content correlates with oxygen concentration in populations further enriched for HSCs is not clear.13 Alternatively, ROS might not be a good indicator of oxygen concentration in general. Evidence exists that hypoxia can induce ROS and that ROS is even required for hypoxic signaling.44 We believe that the increased frequency of HSCs in Vegfaδ/δ mice is a sign of a stressed situation in the hematopoietic system. Despite the lack of hypoxic Vegfa induction, these HSCs survive if unchallenged, but have a severe disadvantage when competing against WT HSCs.
In young Vegfaδ/δ mice we observed that the epiphyseal growth plate of femur, where endochondral ossification takes place, was diminished. This discovery fits well with a previously described role of VEGFA in the process where chondrocytes in hyaline cartilage proliferates and provides the scaffold for bone formation.45 This process has also been connected to hypoxia and HIF-activity.46,47 The volume of bone, and consequently the amount of available endosteal surfaces constituting HSC niches, is well known to affect HSC numbers. Therefore, the ossification phenotype discovered here could possibly influence the HSC phenotype. However, we could not reveal any obvious loss of trabecular bone. Because our analysis showed an increase in HSC numbers rather than a decrease, it is not likely that the reduced ossification have affected HSCs. Still, the defect in endochondral bone formation could be a reason why Vegfaδ/δ mice are growth arrested because the length of bones should be reduced when bone growth is impaired.
Whether hypoxic HSC niches also exist in the FL remains unknown. FL hematopoiesis peaks around embryonic day 15 in the mouse, and thereafter HSCs start to migrate from the FL to the BM cavities, where hematopoiesis continues during young and adult life. As in adult BM, a decrease in cellularity was observed in FL from Vegfaδ/δ mice compared with WT. Correspondingly, FL from Vegfaδ/δ mice contained a higher frequency of both LSK and LSK CD150+ cells. Again, it is possible that this increase in HSPCs is a way to compensate so that efficient hematopoietic output can be maintained in the FL despite inferior HSC survival. Oosthuyse et al reported that the Vegfaδ/δ mutation causes an approximately 60% prenatal lethality and we show here that this lethality increases to 76% when C57Bl/6 is added to the strain background and 100% when the background approaches pure C57Bl/6. However, neither the cause, nor the time point for this lethality was previously examined although suggested to be caused by insufficient vascular growth. The observation that an increase in the C57Bl/6 contribution increases lethality, suggests that expression patterns of Vegfa differs between mouse strains and that a critical threshold level is needed for survival. Indeed, inter-strain differences in base-line Vegfa expression levels have been documented.48 We show here that a major part of embryonic lethality takes place during a time window from embryonic day 15.5 until birth. Taking into account the competitive-repopulation assay results of adult HSCs, where Vegfaδ/δ HSCs have reduced repopulation capacity compared with WT, it is interesting to speculate that Vegfaδ/δ FL HSCs would have a similar defect. The process of HSC reconstitution after transplantation, where HSCs need to successfully migrate to and subsequently survive in BM niches, is likely similar to what is taking place when FL HSCs populate BM niches. Our data raise the possibility that hypoxic regulation of Vegfa is involved in seeding of HSCs from FL to BM, although further investigations are needed to prove this.
Takubo et al reported that Vegfa levels in HSCs from Hif1a conditional knock-out mice were not statistically significantly decreased,29 excluding Vegfa as an important mediator of the phenotype in Hif1a deficient HSCs. However, redundancy by other HIF variants compensating for the loss of Hif1a, as well as biologic effects of undetectable changes in Vegfa concentrations, cannot be disqualified. Deletion of the HRE in the Vegfa promoter in Vegfaδ/δ mice should efficiently abrogate binding of all HIF-variants to the Vegfa promoter. We confirmed this in our system by measuring Vegfa mRNA levels in hematopoietic cells after hypoxic exposure in vitro. While hypoxic up-regulation of Vegfa was perturbed in Vegfaδ/δ mice, baseline expression was clearly detected. Other documented regulators of Vegfa expression include Estrogen49 and Ppargc1a50 . Vegfa is needed for several developmental processes other than hematopoiesis. It is therefore possible that the presence of the Vegfaδ/δ mutation during development selects for individuals where Vegfa expression unrelated to hypoxia is high, in this way blunting the effect of the mutation in mice that survive past birth. This selection might be a reason for the relative weak phenotype observed in unchallenged mice.
Taken together, although HSPC numbers and differentiation capacity are affected in developing and adult Vegfaδ/δ mice, unchallenged hematopoiesis is maintained up to 16 weeks of age. However, in a situation of stress, such as recovery after HSC transplantation, hypoxia/HIF-driven Vegfa up-regulation is clearly needed. In conclusion, we demonstrate here a novel role of the hypoxic HSC niche. Together with previously described roles of hypoxia, like balancing quiescence and protecting HSCs against ROS, we have shown that one important function of the hypoxic HSC niche is to provide HSCs intrinsically with the survival factor VEGFA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Håkan Axelsson, Stefan Karlsson, Jonas Larsson, and Thomas Blom for critical discussions and all the members of the Division of Molecular Medicine and Gene Therapy for help and discussion. They also thank Zhi Ma for FACS cell sorting, Elise Nilsson for excellent immunohistochemical staining, and the BMC animal facility staff for taking care of mice.
This work was supported by the Swedish Research Council (#K2007-64X-20 412-01-3, Linneaus center of excellence grant), the ALF grant from Skåne University hospital, and the Ake Wiberg stiftelse to J.C., and Longterm structural Methusalem funding (Flemish Government), Federal Government IUAP Program (P06/30) to P.C.
Authorship
Contribution: M.R. designed and performed experiments, analyzed data and wrote the manuscript; A.O. and K.R. performed experiments; E.D. and G.L. assisted in designing and performing immunohistochemical experiments; P.C. generated the Vegfaδ/δ mice; and J.C. directed research, designed experiments and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jörg Cammenga, Molecular Medicine and Gene Therapy, BMC A12, 22184 Lund, Sweden; e-mail: jorg.cammenga@med.lu.se.