Abstract
Myeloproliferative neoplasms (MPNs) are associated with recurrent activating mutations of signaling proteins such as Janus kinase 2 (JAK2). However, the actual downstream signaling events and how these alter myeloid homeostasis are poorly understood. We developed an assay to measure basal levels of phosphorylated signaling intermediates by flow cytometry during myeloid differentiation in MPN patients. Our study provides the first systematic demonstration of specific signaling events and their comparison with disease phenotype and JAK2 mutation status. We demonstrate increased basal signaling in MPN patients, which occurs in both early and later stages of myeloid differentiation. In addition, the pattern of signaling is not correlated with JAK2 mutation status and signaling intensity is poorly correlated with mutant JAK2 allele burden. In contrast, signaling differences are detected between different MPN disease phenotypes. Finally, we demonstrate that signaling can be inhibited by a JAK2-selective small molecule, but that this inhibition is not JAK2 V617F specific, because MPN patients with mutant JAK2, wild-type JAK2, and control patients were inhibited to a similar degree. Our data suggest that, in addition to JAK2 mutations, other factors contribute significantly to the MPN phenotype, results that are relevant to both the pathogenesis and therapy of MPN.
Introduction
The human chronic myeloproliferative neoplasms (MPNs) include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). These diseases arise in long-term HSCs and are characterized by overproduction of one or more myeloid lineages.1,2 In addition, although these disorders are associated with full terminal differentiation, they have a variable propensity to progress either to a myelofibrosis (MF) phase or to acute myeloid leukemia (AML). These characteristics allowed us to use MPNs as a model system with which to examine the consequences of oncogene activation on normal myeloid ontogeny before this is further distorted by the acquisition of mutations that block differentiation. MPNs also provide us with important in vivo model systems with which to probe the multistep progression to AML. Until recently, little was known of the molecular pathogenesis of MPNs; however, the discovery of acquired mutations in signaling pathways has irrevocably changed this. Significant data already existed implicating the activation of intracellular signaling downstream of cytokine receptors in MPNs, but the identification of the Janus kinase 2 (JAK2) V617F mutation in almost all PV patients and in approximately half of ET and PMF patients3-6 has provided a molecular basis for this finding. Subsequently, activating mutations have also been described in exon 12 of JAK2 and in exon 10 of MPL,7,8 demonstrating the critical importance of altered intracellular signaling in the generation of the MPN phenotype.
Despite their importance, the nature of signaling abnormalities in primary cells from MPN patients is poorly understood. Most work has been described in cell lines or in animal model systems, in which JAK2 and MPL mutations activate the STAT5, STAT3, PI-3K/AKT, and RAS-MAPK-ERK pathways.4,8,9 However, these model systems may not adequately reflect the cellular context or the molecular complexity of human MPNs. In addition, where patient material has been used, technical aspects, such as the use of intervening in vitro culture conditions that do not reflect the proper in vivo milieu or the analysis of highly heterogeneous populations of cells using Western blotting or immunohistochemistry of fixed trephine sections, may limit the interpretation of these experiments.10,11 A more detailed and physiologic characterization of the signaling abnormalities in human MPNs and an understanding of how these abnormalities determine the cellular characteristics and disease phenotype are therefore critical tools required to further decipher MPN pathogenesis.
Perhaps the most intriguing question in the pathogenesis of MPNs is how the same mutation may be associated with distinct disease phenotypes, such as JAK2 V617F with PV, ET, and PMF. Mechanisms explaining this genotype-phenotype paradox have been speculated to include germline disease modifiers, potential collaborating mutations, and the gene dosage of mutant JAK2.1,2,12 Studies in BM transplantation and transgenic mouse models have suggested that gene dosage of JAK2 V617F determines the disease phenotype, with higher expression associated with a PV phenotype and lower expression with an ET phenotype.13,14 Evidence in patient material may corroborate this, with colonies homozygous for JAK2 V617F and a higher V617F mutant allele burden in granulocytes present in PV and PMF patients compared with ET patients.15,16 In addition, determining the extent to which JAK2 or other signaling mutations drive the MPN phenotype is of potential clinical importance because this may predict the utility of specific inhibition of these signaling molecules.
We developed a phospho-specific flow cytometry assay to reliably measure basal signaling activity in the BM of MPN patients in primitive (CD34+) and differentiating myeloid ontogeny (CD34−). The assay demonstrated increased basal signaling downstream of activated JAK2 in MPN patients compared with controls in both cell types. In addition, although some signaling differences were noted between MPN disease phenotypes, the pattern of signaling was not correlated with the JAK2 mutation status or mutant allele burden. Finally, we demonstrate modest inhibition of signaling with a JAK2-selective inhibitor in MPN patients. However, this signaling was not specific to patients with mutant JAK2 and inhibited MPN patients with wild-type JAK2 and controls to similar levels.
Methods
Patient samples
Patients were recruited from the specialist MPN clinic at Addenbrooke's Hospital in Cambridge (United Kingdom). Institutional ethics committee approval was obtained in writing. Patient diagnosis was defined according to the World Health Organization criteria for PV, ET, and PMF. Cases of MF secondary to either preceding PV or ET were diagnosed according to accepted criteria17 and were classified together with PMF as MF (Table 1). Mononuclear fractions were immediately separated after Ficoll density centrifugation (Histopaque 1077; Sigma-Aldrich) according to the manufacturer's guidelines. CD34+ cells were enriched from mononuclear fractions by immunomagnetic beads (CD34+ progenitor isolation kit; Miltenyi Biotec). The flow-through from the magnetic column was collected, counted, and is referred to as the CD34− population. All CD34+ fractions were > 85% positive as determined by flow cytometry (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All cells were frozen in 95% FCS and 5% DMSO in liquid nitrogen. Subsequent analyses were performed in batches on immediately thawed material with no intervening culture period unless otherwise stated.
Flow cytometry analysis, cell sorting, and mutational analysis
Staining and sorting for Lin−/CD34+/ CD38− HSCs and individual myeloid progenitor compartments was performed as described previously.12 Cells were double sorted on a MoFlo Cell Sorter (Beckman Coulter). All antibodies were purchased from Invitrogen. V617F genotyping and allele quantitation was performed by pyrosequencing on a PyroMarkQ96 ID (QIAGEN) as described previously.18
Western blotting
HEL cells were maintained in RPMI medium with 10% FCS (Sigma-Aldrich). Extracts from 5 × 106 cells were made in RIPA buffer (50mM Tris, pH 8, 150mM NaCl, 1% NP-40, 0.5% DOC, 0.1% SDS, and protease inhibitors). Protein concentrations were quantitated and equal amounts of protein were loaded on a 10% SDS-PAGE gel. ImageJ software was used to calculate the intensity of each band to calculate the percentage inhibition. Western blotting was performed using standard methods with the following antibodies: phospho-STAT5 (611818; BD Biosciences), phospho-STAT3 (9145; NEB), phospho-ERK (9101; Cell Signaling Technology), and anti-actin (Sigma-Aldrich).
Intracellular phosphoprotein analysis using flow cytometry
Antibodies used for phosphoprotein detection were p-STAT5 Alexa Fluor 488 (612598), p-ERK1/2 Alexa Fluor 488 (612592, both from BD Biosciences); p-STAT3 Alexa Fluor 488 (4323) and p-AKT Alexa Fluor 488 (2336, both from Cell Signaling Technology). All individual antibodies were from the same batch and were used at the dilutions recommended in the manufacturer's data sheets. The isotype control for the ERK and STAT5 antibodies was mouse IgG1 Alexa Fluor 488 (BD Biosciences) and for STAT3 and AKT was rabbit IgG Alexa Fluor 488 (Cell Signaling Technology). Isotype controls were used at the same concentrations as test antibodies. The same operator performed all analyses using the same reagents, protocol, instrument, and baseline settings. Thawed CD34+ and CD34− cells were suspended in PBS without Ca2+/Mg2+ plus 2% BSA, and an equal volume of prewarmed BD Cytofix Buffer (BD Biosciences) was added for 10 minutes at 37°C. Cells were washed and permeabilized by resuspension in ice-cold 90% methanol for 30 minutes. After washing in PBS with 2% BSA, cells were resuspended in 1 mL of blocking buffer (PBS without Ca2+/Mg2+ with 4% BSA and 10 μg/mL of human IgG; Sigma-Aldrich) for 30 minutes on ice. Cells were then aliquoted into FACS tubes after washing and primary antibodies or isotype controls were added at the recommended dilutions. Incubation was performed at room temperature for 30-45 minutes in the dark.
Samples were washed with PBS and analyzed immediately on a FACSCalibur flow cytometer (BD Biosciences). Where patient cell number would allow, the analyses were repeated (for 15 of 30, 50% of CD34+ MPN and control patients and for 21 of 37, 57% of CD34− MPN and controls) and an average of the 2 values used. Chronic myeloid leukemia (CML) control samples were analyzed only once.
Stimulation and inhibition of signaling
For erythropoietin (EPO) stimulation, cells were resuspended in RPMI medium plus 10% FCS and incubated in a 37°C incubator for 30 minutes with recombinant human EPO (R&D Systems) at a concentration of 1 IU/mL. Sodium pervanadate was freshly prepared by mixing sodium orthovanadate (Sigma-Aldrich) and hydrogen peroxide as described previously.19 Cells were stimulated with sodium pervanadate for 10 minutes at 37°C to a final concentration of 20μM.
As a positive control for P-ERK activation, phorbol-12 myristate 13-acetate (PMA; Sigma-Aldrich) was added to a final concentration of 4nM for 10 minutes at 37°C.
EPO-R and glycophorin A (GPA) staining was performed in PBS plus 2% BSA. The antibodies used were PE-EPOR (FAB307P, clone 38409; R&D Systems) and PE-GPA (MHGLA04; Invitrogen). The respective isotype controls used for gating were PE-mIgG2b (IC0041P; R&D Systems) and PE-mIgG1 (MG104; Invitrogen). The -fold stimulation was calculated by dividing the median fluorescence intensity (MFI) of treated sample by the MFI of basal phosphorylation (untreated).
The JAK2 inhibitor TG101209 (Symansis) was dissolved in DMSO and used at a concentration of 0.6μM.20 Imatinib methane sulfonate (LC Laboratories) was used at a concentration of 5μM.21 Cells were treated for 4 hours with either TG101209 or imatinib. In untreated cells, DMSO was added. The following formula was used to calculate the percent inhibition: percent inhibition = (1 − [inhibitor MFI − isotype control MFI]/[untreated MFI − isotype control MFI]) × 100.
Data collection and analysis
Data were collected using CellQuest PRO Version 4.8 software (BD Biosciences) and analyzed using FlowJo Version 7.0 software (TreeStar).
Statistics and graphical representation
Graphs and the indicated statistical analyses were generated using Prism Version 5.0 software (GraphPad). Analysis of entire signaling patterns (for all 4 signaling intermediates) was undertaken using a fully saturated, 3-way factorial ANOVA model (with the 3 factors being disease type (normal, ET, PV, or PMF), protein and cell-type (CD34+ or CD34−). Further targeted pairwise comparisons to evaluate the significance between individual diseases were performed by the 2-sided Student t test. Results are expressed as the means ± SEM.
Results
Patients and controls
To limit the inherent heterogeneity present in MPN patients, analysis was performed predominantly on fractionated BM taken from MPN patients, the majority of whom were untreated and at diagnosis. BM from 6 controls and 6 CML samples were also included in the analyses for comparison (Table 1 and supplemental Table 1).
Measurement of basal signaling pathways by phospho-flow
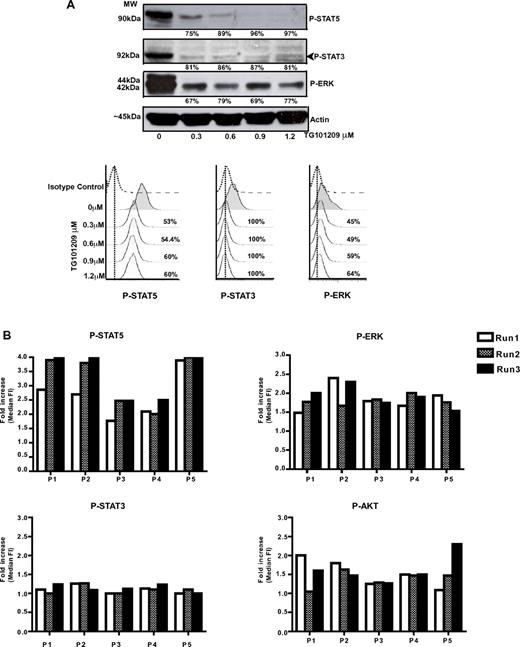
To allow us to consistently interrogate the small numbers of cells obtained from the CD34+ compartment of patient samples; we used intracellular protein staining with phospho-specific antibodies followed by detection using flow cytometry with phospho-flow.22 In CML patients, the phospho-flow method has been shown to be reproducible in measuring CrkL phosphorylation in < 104 CD34+ stem cells,23 where it was found to be of comparable sensitivity to Western blotting. We first validated this technique against standard Western blotting in the JAK2 V617F–positive human cell line HEL. Cells were treated with different concentrations of the JAK2-selective inhibitor TG101209 for 4 hours and then analyzed in parallel for inhibition of signaling pathways by Western blotting and phospho-flow (Figure 1A). To allow comparative analysis of different doses of the inhibitor, the results of phospho-flow were expressed as the percent inhibition and calculated as described in “Methods.” As shown in Figure 1A, inhibition of phospho-STAT5, phospho-STAT3, and phospho-ERK was detected by both methods. The phospho-flow method was found to be as sensitive as Western blotting, could be reproducibly performed in limiting cell numbers, and demonstrated a wider dynamic range (eg, P-ERK).
Comparison of flow cytometric quantification of specifically phosphorylated signaling intermediates (phospho-flow) to Western blotting. (A) HEL cells were treated with different concentrations of the JAK2-selective agent TG101209 for 4 hours. Aliquots were taken from each concentration and divided in half, with one half analyzed by Western blotting (top panel) and the other half by phospho-flow analysis (bottom panel). The percent inhibition by each method is shown. Inhibition of phospho-STAT5, phospho-STAT3, and phospho-ERK was demonstrated using both methods. (B) To ensure that the phospho-flow assay was robust and reproducible in patient samples, we performed repeat experiments, assessing 4 signaling pathways (phospho-ERK, phospho-STAT5, phospho-STAT3, and phospho-AKT) in each experiment for 5 patients. For each patient, experiments were performed in triplicate, each at a different time point, from frozen aliquots of cells. A high degree of correlation can be seen between runs for each individual patient.
Comparison of flow cytometric quantification of specifically phosphorylated signaling intermediates (phospho-flow) to Western blotting. (A) HEL cells were treated with different concentrations of the JAK2-selective agent TG101209 for 4 hours. Aliquots were taken from each concentration and divided in half, with one half analyzed by Western blotting (top panel) and the other half by phospho-flow analysis (bottom panel). The percent inhibition by each method is shown. Inhibition of phospho-STAT5, phospho-STAT3, and phospho-ERK was demonstrated using both methods. (B) To ensure that the phospho-flow assay was robust and reproducible in patient samples, we performed repeat experiments, assessing 4 signaling pathways (phospho-ERK, phospho-STAT5, phospho-STAT3, and phospho-AKT) in each experiment for 5 patients. For each patient, experiments were performed in triplicate, each at a different time point, from frozen aliquots of cells. A high degree of correlation can be seen between runs for each individual patient.
We next documented the reproducibility of our phospho-flow assay in replicate testing, performed on 5 patient samples that were thawed and analyzed on 3 separate occasions. We assessed 4 signaling pathways (phospho-STAT5, phospho-STAT3, phospho-AKT, and phospho–ERK1/2), all suspected to be downstream of mutant JAK2 and MPL. The data were quantified as the -fold increase of signaling (over isotype control), and were calculated by dividing the MFI of the phospho-specific antibody by that of the isotype control. In general, the -fold increase was relatively modest and between 1.0-2.5 times that of the isotype control, although signaling via STAT-5 was sometimes higher. We found a high degree of correlation between different runs for an individual patient, particularly in the middle range of the assay (Figure 1B).
Altered signaling is detected in both the stem/progenitor and later compartments in human MPNs
Being assured of the robustness of our assay, we systematically assessed intracellular signaling in both the early CD34+ and later CD34− compartments of MPN patients. Our cohort included patients diagnosed with PV, ET, MF, and control BM, in which all samples were analyzed following prior separation of the CD34+ and CD34− populations by immunomagnetic beads. This strategy allowed us to optimize reproducibility by batch-analyzing multiple samples as well as optimizing the number of events documented for the rare CD34+ population. We first compared signaling in CD34+ cells from 24 MPN patients against signaling in the same compartments from the BM of 4 controls and from 4 untreated CML patients at diagnosis as negative and positive controls, respectively (Figure 2 and supplemental Table 1). To correct for patient- and compartment-specific variations in antibody staining, the -fold increase of the specific phospho-antibody relative to the isotype control antibody was calculated and is expressed as a ratio for each patient, as described in “Measurement of basal signaling pathways by phospho-flow.”
Phospho-flow demonstrates the induction of multiple signaling pathways in CD34+ cells from patients with MPN. (A) Representative histogram profiles of phospho-ERK (top row), phospho-STAT5 (middle row), and phospho-STAT3 staining (bottom row) in a control and an MF patient (left and middle panels, respectively) are shown. Note that the median florescence intensity (MFI) of the isotype control (green) is different between each of the 2 patient and control samples. Therefore, for each patient sample, a relative value, the -fold increase, was calculated by dividing the MFI of the test antibody (red) by the MFI of the isotype control (green). The bar charts on the right show the results from all patient samples represented as the -fold increase ± SEM. No significant difference in signaling was noted between the 3 different MPNs. However, significant activation of basal signaling was seen for ERK pathway in ET and MF patients compared with controls. (B) A signaling heat-map table is shown for all control and MPN samples analyzed. For each individual in our cohort, the results of the signaling analysis are shown graphically. The patient and control numbers correspond with those shown in the histograms in panel A. Where the patient is JAK2 V617F positive, we have also documented the allele burden of the CD34+/CD38− compartment. A degree of signaling heterogeneity is demonstrated, even between patients within the same disease/control phenotype.
Phospho-flow demonstrates the induction of multiple signaling pathways in CD34+ cells from patients with MPN. (A) Representative histogram profiles of phospho-ERK (top row), phospho-STAT5 (middle row), and phospho-STAT3 staining (bottom row) in a control and an MF patient (left and middle panels, respectively) are shown. Note that the median florescence intensity (MFI) of the isotype control (green) is different between each of the 2 patient and control samples. Therefore, for each patient sample, a relative value, the -fold increase, was calculated by dividing the MFI of the test antibody (red) by the MFI of the isotype control (green). The bar charts on the right show the results from all patient samples represented as the -fold increase ± SEM. No significant difference in signaling was noted between the 3 different MPNs. However, significant activation of basal signaling was seen for ERK pathway in ET and MF patients compared with controls. (B) A signaling heat-map table is shown for all control and MPN samples analyzed. For each individual in our cohort, the results of the signaling analysis are shown graphically. The patient and control numbers correspond with those shown in the histograms in panel A. Where the patient is JAK2 V617F positive, we have also documented the allele burden of the CD34+/CD38− compartment. A degree of signaling heterogeneity is demonstrated, even between patients within the same disease/control phenotype.
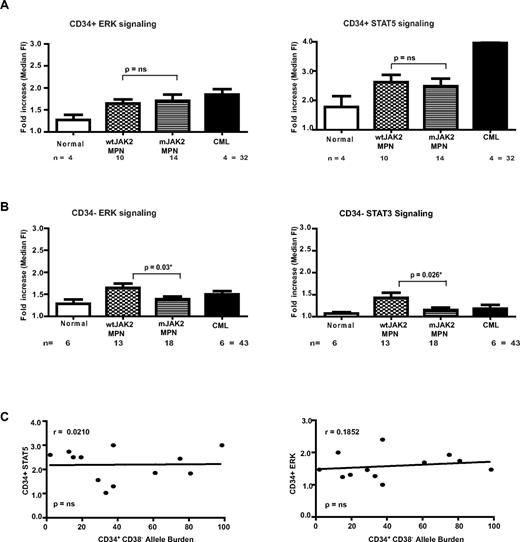
Compared with control BM samples, there was unequivocal increased activation of signaling pathways within the CD34+ stem and progenitor compartment. Representative FACS profiles from our patient cohort are shown in Figure 2A. The histograms demonstrate activation of the STAT5 and ERK pathways in the CD34+ compartment of MPN patients over controls, with this difference reaching statistical significance for ERK signaling in ET and MF patients (P = .03 and P = .04, respectively; Figure 2A). No difference from controls was detected for AKT, in which the control cells also appeared to have a moderate baseline increase over isotype control (Figure 2B and supplemental Figure 2A). As might be expected from the phenotype of MPNs, the -fold increase of signaling over the isotype control in comparison with controls was relatively modest (a 1- to 2.5-fold increase for P-ERK, P-STAT3, and P-AKT), although P-STAT5 signaling was higher. This is illustrated in the heat map in Figure 2B, in which the JAK2 mutation status and the allele burden for each patient are also shown (Figure 2B).
A similar analysis was performed in all 31 MPN patients for the CD34− compartment. As shown in Figure 3A, compared with controls, we found increased STAT5 activation in PV, MF (P = .01 and P = .03, respectively), and ET patients, and increased STAT3 activation in MF patients (P = .03) compared with the controls. Analysis of entire signaling patterns (for all 4 signaling intermediates) was undertaken using a multivariate ANOVA model (with the 3 factors being disease type (normal, ET, PV, or PMF), signaling protein, and cell type (CD34+ or CD34−). There was no effect of cell type. Globally, there were significant differences in signal intensity across the 4 intermediates by disease status (P = .01). Furthermore, there was significant heterogeneity in the extent to which individual signaling intermediates were affected by disease status (P = .05). To explore the reasons for this heterogeneity in more detail, we undertook t testing. By targeted, pairwise analyses, we found subtle but statistically significant differences between PV and ET signaling for STAT5 (P = .05) and between PV and MF signaling for STAT3 (P = .026) in the CD34− compartment (Figure 3A). No statistically significant differences were apparent in the CD34+ compartment. In CD34− cells, the AKT pathway was active even in normal BM samples and no difference with MPN patients was detected (Figure 3B and supplemental Figure 2A). Figure 3B shows a signaling heat-map table for all normal and MPN CD34− samples analyzed. The JAK2 mutation status and allele burden for individual patients are given. In both the CD34+ and CD34− compartments, obvious heterogeneity of signaling patterns was demonstrated, even between patients with the same disease phenotype and within the controls.
Differences in STAT5 signaling and MF-specific STAT3 signaling in CD34− cells from patients with MPN. (A) Representative histogram profiles of phospho-ERK (top row) and phospho-STAT5 (middle row) staining in a control and a PV patient (left and center panels, respectively). Phospho-STAT3 staining (bottom row) is shown for an MF patient and a control. The bar graph on the right shows increased STAT5 signaling in MPN patients, which reached statistical significance in PV and MF patients compared with controls. There was also a statistical significant difference in STAT5 signaling between PV and ET patients. Statistically significant STAT3 signaling compared with controls and PV occurred only with MF patients. (B) A signaling heat-map table is shown for all control and MPN CD34− samples analyzed. The patient numbers correspond to those in Figure 2B plus additional patients for whom CD34− cells were available for analysis. Where the patient is JAK2 V617F positive, we have also documented the allele burden of the CD34−/GPA+/CD71+ compartment.
Differences in STAT5 signaling and MF-specific STAT3 signaling in CD34− cells from patients with MPN. (A) Representative histogram profiles of phospho-ERK (top row) and phospho-STAT5 (middle row) staining in a control and a PV patient (left and center panels, respectively). Phospho-STAT3 staining (bottom row) is shown for an MF patient and a control. The bar graph on the right shows increased STAT5 signaling in MPN patients, which reached statistical significance in PV and MF patients compared with controls. There was also a statistical significant difference in STAT5 signaling between PV and ET patients. Statistically significant STAT3 signaling compared with controls and PV occurred only with MF patients. (B) A signaling heat-map table is shown for all control and MPN CD34− samples analyzed. The patient numbers correspond to those in Figure 2B plus additional patients for whom CD34− cells were available for analysis. Where the patient is JAK2 V617F positive, we have also documented the allele burden of the CD34−/GPA+/CD71+ compartment.
Altered signaling does not correlate with JAK2 genotype or allele burden in human MPNs
Having correlated signaling with disease phenotype, we next assessed signaling pattern by JAK2 genotype by comparing our patient samples based on the presence or absence of a JAK2 mutation irrespective of the disease phenotype of the patient. In contrast to our findings of some correlation between signaling patterns and disease phenotype, we found no obvious positive correlation between the presence of a JAK2 mutation and signaling in either the CD34+ or CD34− compartments, with most comparisons of signaling nonsignificant between MPN patients who carried or lacked a JAK2 mutation (Figure 4A-B). Indeed, we were actually able to demonstrate a statistically significant increase in basal signaling through ERK and STAT3 in the CD34− compartment for MPN patients who lacked a JAK2 mutation (Figure 4B). These findings demonstrate that altered signaling is a feature of all MPN patients, and suggest the presence of as-yet-unexplained signaling abnormalities in MPN cases that lack a JAK2 mutation.
Signaling analyses in the CD34+ and CD34− compartment of MPN patients based on mutant JAK2 status. (A) Bar charts of phospho-ERK and phospho-STAT5 staining in CD34+ comparing control and MPN patients are shown. No significant differences in signaling are demonstrated between JAK2 wild-type MPN patients and those with mutant JAK2. (B) Signaling patterns were analyzed by mutant JAK2 status in CD34− cells. Bar graphs of ERK and STAT3 signaling are shown. A significant difference was demonstrated in both pathways by JAK2 genotype. (C) A correlation graph comparing the relationship of the JAK2 V617F mutant allele burden in Lin−/CD34+/CD38− HSCs with the phopho-STAT5 and ERK levels within is shown. The correlation is poor, suggesting that factors other than the JAK2 V617F mutant burden contribute to the signaling demonstrated in MPN patients.
Signaling analyses in the CD34+ and CD34− compartment of MPN patients based on mutant JAK2 status. (A) Bar charts of phospho-ERK and phospho-STAT5 staining in CD34+ comparing control and MPN patients are shown. No significant differences in signaling are demonstrated between JAK2 wild-type MPN patients and those with mutant JAK2. (B) Signaling patterns were analyzed by mutant JAK2 status in CD34− cells. Bar graphs of ERK and STAT3 signaling are shown. A significant difference was demonstrated in both pathways by JAK2 genotype. (C) A correlation graph comparing the relationship of the JAK2 V617F mutant allele burden in Lin−/CD34+/CD38− HSCs with the phopho-STAT5 and ERK levels within is shown. The correlation is poor, suggesting that factors other than the JAK2 V617F mutant burden contribute to the signaling demonstrated in MPN patients.
Hematopoiesis is a genetic mosaic in human MPNs, and normal and malignant clones coexist in most patients. In addition, for JAK2 V617F–mutated cases, there is also often the presence of clones homozygous and heterozygous for the mutation within the same patient. Heterogeneity of the mutant JAK2 allele burden has been described previously, and was evident in our cohort (Figures 2B and 3B). We hypothesized that these independent clones may have different signaling properties and that the differences in signaling we have demonstrated may have been masked by the variable size of the JAK2 mutant clone in individual patients. To test this hypothesis, we correlated signaling intensity with JAK2 mutant allele burden. Figure 4C demonstrates 2 representative correlation curves comparing the -fold increase of basal signaling of STAT5 and ERK pathways compared with JAK2 V617F allele burden in the CD34+ compartment. Although the patient numbers are relatively small, these demonstrate no correlation for STAT5 and only a very modest correlation for the ERK pathway. Therefore, in JAK2 V617F patients, our findings suggest that there is no significant correlation between the basal activation of signaling pathways and the JAK2 allele burden (Figures 2B and 3B).
MPN patients demonstrate no incremental signaling on EPO stimulation
Using phospho-flow, patients with other MPNs such as juvenile myelomonocytic leukemia and the majority of AML patients have been shown to be acutely sensitive to further ex vivo stimulation with cytokines.20,24 Having analyzed basal signaling levels, CD34− cells from controls and a subset of our MPN cohort were analyzed before stimulation and after stimulation for 30 minutes with saturating concentrations of EPO (1 IU/mL) to determine whether signaling in our MPN cohort was similarly dynamic on further stimulation. A relative value, “-fold stimulation,” was calculated as the ratio of the stimulated relative to the unstimulated MFI for each sample. Normal BM CD34− cells demonstrated a minor degree of stimulation of the STAT5 pathway after EPO stimulation (Figure 5A). However, CD34− cells from patients with PV, ET, and MF demonstrated no activation of any signaling pathways after EPO stimulation (Figure 5A and data not shown).
No further signaling through the EPO receptor in MPN patients. (A) Representative histogram plots of phospho-STAT5 signaling in control and PV CD34− cells stimulated with 1 IU/mL of EPO (filled histogram) or unstimulated (middle shaded histogram). Dotted line is the isotype control. The relative values are shown in the bar graph (right panel) as the -fold stimulation, which was calculated by dividing the MFI of stimulated cells by that of unstimulated cells. The bar graph demonstrates that CD34− cells from controls demonstrate EPO stimulation. In contrast, PV, ET, and MF patients show no additional stimulation upon EPO addition. (B) Representative histogram plots overlay of the same samples in panel A are shown, this time also gated on the GPA+ population. The bar graph demonstrates that CD34−/GPA+cells from controls demonstrate modest EPO stimulation. In this population, MPN patients show no additional stimulation with EPO. Histogram plots of CD34− /GPA+cells from control and PV patients treated with pervanadate demonstrate that both samples can be further stimulated (P-STAT5) upon addition of a tyrosine-phosphatase inhibitor. (C) Representative histogram plots of CD34−/GPA+cells from a control and a PV patient demonstrate further stimulation of ERK signaling upon treatment with PMA.
No further signaling through the EPO receptor in MPN patients. (A) Representative histogram plots of phospho-STAT5 signaling in control and PV CD34− cells stimulated with 1 IU/mL of EPO (filled histogram) or unstimulated (middle shaded histogram). Dotted line is the isotype control. The relative values are shown in the bar graph (right panel) as the -fold stimulation, which was calculated by dividing the MFI of stimulated cells by that of unstimulated cells. The bar graph demonstrates that CD34− cells from controls demonstrate EPO stimulation. In contrast, PV, ET, and MF patients show no additional stimulation upon EPO addition. (B) Representative histogram plots overlay of the same samples in panel A are shown, this time also gated on the GPA+ population. The bar graph demonstrates that CD34−/GPA+cells from controls demonstrate modest EPO stimulation. In this population, MPN patients show no additional stimulation with EPO. Histogram plots of CD34− /GPA+cells from control and PV patients treated with pervanadate demonstrate that both samples can be further stimulated (P-STAT5) upon addition of a tyrosine-phosphatase inhibitor. (C) Representative histogram plots of CD34−/GPA+cells from a control and a PV patient demonstrate further stimulation of ERK signaling upon treatment with PMA.
Only a subpopulation of CD34− cells, approximating to the erythroid progenitor population, would be predicted to be sensitive to EPO stimulation. The absence of signaling upon addition of EPO in MPN patients was not due to the absence of an EPO-R or a GPA-positive population, because a similar proportion of cells expressed both of these markers in MPN patients and controls (supplemental Figure 3). Moreover, a similar lack of stimulation was evident upon EPO stimulation of the CD34−/GPA+ population in MPN patients, whereas modest stimulation was still evident in controls (Figure 5B). Furthermore, total CD34− and the CD34−/GPA+ fraction could be further stimulated in both MPN patients and controls with either the protein-tyrosine phosphatase inhibitor pervanadate or with PMA (Figure 5C-D and data not shown), suggesting that these cells are capable of stimulation via other pathways.
Inhibition of signaling by selective JAK2 inhibitor occurs independently of JAK2 mutation status in MPN patients and controls
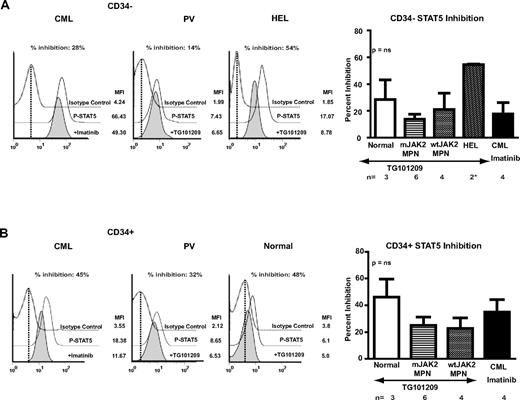
We next investigated whether a JAK2-specific inhibitor would alter the abnormal intracellular signaling demonstrated in MPN patient samples. CD34+ and CD34− samples from MPN patients were treated with the JAK2-selective inhibitor TG101209.20 This inhibitor has been demonstrated to suppress growth of hematopoietic colonies from primary MPN progenitor cells.20 For comparison, inhibition of signaling with TG101209 in the human leukemia cell line HEL (bearing multiple copies of JAK2 V617F) and in control CD34+ and CD34− samples and inhibition of STAT5 signaling in diagnostic samples from CML patients treated with imatinib mesylate were included as controls. HEL cells were markedly inhibited by TG101209 (Figure 6A). In MPN and CML patient samples, the inhibition was more modest, although inhibition was demonstrated in both the early CD34+ and late CD34− compartments. However, the degree of inhibition was similar in MPN and CML patients (Figure 6A-B). In addition, MPN patients with wild-type JAK2 were inhibited to a similar degree as MPN with mutated JAK2. Furthermore, signaling in control CD34+ and CD34− samples was also inhibited to a degree comparable to the MPN patients. These results suggest that JAK2 inhibitors demonstrate modest activity to inhibit signaling in MPN patients that is independent of JAK2 mutation status; however, there is no evidence of a selective effect for MPN patients compared with controls.
Inhibition of signaling in MPN patients with a selective JAK2 inhibitor. (A) Representative plots of inhibition of STAT5 signaling in CML (left panel) and JAK2 V617F–positive PV (middle left panel) patient CD34− cells and the HEL cell line (middle right panel) after exposure to imatinib (CML) or TG101209 (PV and HEL) for 4 hours (filled histogram). DMSO-treated controls are middle histograms in the overlay (solid line). The relative value, percent inhibition was calculated as described in “Methods.” As is demonstrated in the bar chart (right panel), HEL showed marked inhibition of STAT5 signaling. MPNs showed only a modest level of inhibition that was independent of the JAK2 mutant genotype and similar to the level of inhibition of phospho-STAT5 in CML cells treated with imatinib. However, CD34− samples from controls were also inhibited to a similar degree. (B) A similar comparison in CD34+ cells. Representative histograms of a CML patient (left panel), a JAK2 V617F–positive PV patient (middle left panel), and a control (middle right panel) are shown. As is demonstrated in the bar chart in the right panel, the level of inhibition is modest but is independent of JAK2 genotype and is similar to the level obtained in CD34+ cells from CML patients treated with imatinib. However, once again, at least comparable levels of inhibition are seen in normal CD34+ samples.
Inhibition of signaling in MPN patients with a selective JAK2 inhibitor. (A) Representative plots of inhibition of STAT5 signaling in CML (left panel) and JAK2 V617F–positive PV (middle left panel) patient CD34− cells and the HEL cell line (middle right panel) after exposure to imatinib (CML) or TG101209 (PV and HEL) for 4 hours (filled histogram). DMSO-treated controls are middle histograms in the overlay (solid line). The relative value, percent inhibition was calculated as described in “Methods.” As is demonstrated in the bar chart (right panel), HEL showed marked inhibition of STAT5 signaling. MPNs showed only a modest level of inhibition that was independent of the JAK2 mutant genotype and similar to the level of inhibition of phospho-STAT5 in CML cells treated with imatinib. However, CD34− samples from controls were also inhibited to a similar degree. (B) A similar comparison in CD34+ cells. Representative histograms of a CML patient (left panel), a JAK2 V617F–positive PV patient (middle left panel), and a control (middle right panel) are shown. As is demonstrated in the bar chart in the right panel, the level of inhibition is modest but is independent of JAK2 genotype and is similar to the level obtained in CD34+ cells from CML patients treated with imatinib. However, once again, at least comparable levels of inhibition are seen in normal CD34+ samples.
Discussion
The recent discovery of mutations in intermediates involved in cytokine signaling, such as JAK2 and MPL, has greatly enhanced our molecular understanding of MPN pathogenesis. However, the identities of specific signaling pathways that mediate the effects downstream of these mutations are not known. Our study set out to address these questions in a large, well-characterized cohort using predominantly BM from MPN patients, the majority of whom were untreated and at diagnosis.
Phospho-flow has been used previously for the analysis of dynamic signaling in human samples from patients with AML and juvenile myelomonocytic leukemia stimulated with cytokines.22,24 Our data are the first demonstration of the use of this technique to measure basal and dynamic levels of signaling compared with controls in human MPN. To assess basal signaling in both early and late myeloid differentiation compartments, we designed our assay with prior selection of CD34+ cells by immunomagnetic beads. This allowed us to optimize reproducibility by batch-analyzing multiple samples and also optimized the number of events documented for the rare CD34+ population. These technical aspects of our assay may have influenced our results; however, because all patient and control samples were processed in exactly the same manner, our comparisons remain valid. Using this assay, we found phospho-flow to be both reproducible and of similar sensitivity to the current gold standard protein estimation technique, Western blotting. Moreover, it was amenable to limiting numbers of cells present within the CD34+ compartment, as has been reported for measurement of phospho-CRKL, the major substrate of BCR-ABL phosphorylation.23 Further use of similar assays should inform our knowledge of signaling, protein levels, and posttranslational modifications in small numbers of primary human malignant cells.
As expected, the phospho-flow analyses demonstrated increased basal levels of signaling in MPN patients compared with control populations. In addition, increased basal signaling was present in both the early CD34+ and the later CD34− compartments (Figures 2B and 3B). The relative increase in basal signaling for MPN patients over controls was generally modest (between 1- and 2.5-fold increased for P-AKT, P-ERK, and P-STAT3 and 1- to 4-fold increased for P-STAT5). However, this finding would be in keeping with the clinical phenotype and generally indolent nature of MPNs.1 Furthermore, our data demonstrated obvious heterogeneity in the level of basal signaling in MPN patients and controls in both the CD34+ and CD34− compartments, with this heterogeneity even evident between patients within specific disease phenotypes and within the control populations. Our findings both corroborate and contrast with previous studies. For example, our finding of increased P-STAT3 activation in MF patients corroborate the findings of other researchers using Western blotting,25 although P-STAT3 activation has also been described in the granulocytes of some PV patients.10 In addition, our results corroborate the findings of Teofili et al, who demonstrated P-STAT5 activation in PV patients by immunohistochemical analysis of MPN patient BM trephine biopsies. However, the same study found no activation of STAT3 in MF patients.11 It is likely that the altered patterns reported can be explained, at least in part, by differences in phosphoprotein detection method, differences in the cell type analyzed, and, importantly, by the signaling heterogeneity even within individual MPN disease phenotypes, as we have quantitatively demonstrated (Figures 2B and 3B).
We had anticipated that the basal activation of signaling pathways might be different between those patients with a JAK2 mutation and those without, and we would have perhaps predicted a priori that basal activation levels might be higher in patients who carried a JAK2 mutation. However, when basal signaling was correlated with JAK2 mutation status, to our surprise, we found either no difference in signaling between patients who carried or lacked a JAK2 mutation or an increased activation of basal signaling in the patients who lacked JAK2 mutations (for P-ERK and P-STAT3 signaling in the CD34− compartment; Figure 4). These data confirm altered signaling as being central to the MPN phenotype. Furthermore, they suggest that those patients who lack a known mutation have as-yet-undetected mutations in, or dysregulation of, critical signaling intermediates such as those regulating signaling through the MAPK/ERK and STAT3 pathways, which warrant further scrutiny. In support of this notion, 3 of the 13 patients with wild-type JAK2 were documented to have a C-MPL mutation (Table 1). To further assess the role of mutant JAK2 in altered basal signaling and as a possible explanation for the heterogeneity of signaling in JAK2 mutant cases, we also found that signaling activation was correlated with JAK2 mutant allele burden. Although the numbers of patients included in the analysis was relatively small and the increase in basal signaling modest, we could find no obvious correlation between basal signaling and mutant JAK2 burden. These data are corroborated by Western blot analyses in MF patient CD34+ cells and granulocytes for P-STAT5, P-ERK, and P-AKT, although an association was found between JAK2 allele burden and P-STAT3 signaling in the same study.25 Our data for JAK2 mutant genotype and allele burden suggest that factors other than mutant JAK2 also significantly contribute to increased basal signaling in MPN patients.
Whereas an obvious association between JAK2 genotype and basal signaling was lacking, our data do suggest subtle differences between signaling in the individual MPN phenotypes. Global analysis of signaling patterns demonstrated differences in signal intensity across the 4 intermediates by disease status (P = .01). Furthermore, there was significant heterogeneity in the extent to which individual signaling intermediates were affected by disease status (P = .05). In addition, significant differences were demonstrated in P-STAT5 activation between PV and ET patients and in P-STAT3 activation between PV and MF patients, and these differences may contribute to the disease phenotype. For example, the level of STAT5 activation is known to be critical in determining the balance between erythroid and megakaryocytic differentiation downstream of the megakaryocyte-erythroid progenitors, with increasing STAT5 activation associated with erythroid differentiation.26 In addition, MF can be modeled in mice that express low levels of GATA1 in megakaryocytes. GATA1 has been shown to interact with and reduce the transcriptional activity of STAT3,27 and its expression is reduced in the megakaryocytes of MF patients. Therefore, attenuation of STAT3 signaling may be lost in GATA-1lo mice and MF patients, with hyperactive STAT3 signaling contributing to the development of MF. In addition, STAT3 may also promote megakaryocyte growth by suppressing the inhibitory effects of TGF-β on megakaryocytes.28
Cells from patients with MPN have been described to be either cytokine independent or cytokine hypersensitive. Our data demonstrate a lack of further signaling in MPN primary cells stimulated with EPO. One interpretation of these data is that cells from MPN patients are already maintained in a state of tonic maximal signaling downstream specifically of the EPO receptor, either from hypersensitivity to trace levels of endogenous cytokines or because of true cytokine independence. An alternative theory is that EPO signaling is dislocated from downstream signaling partners in MPN. Further experiments will be necessary to determine the exact mechanism and to detail the response of primary MPN cells to other cytokines.
Treatment of the related disorder CML has been revolutionized with the introduction of the selective BCR-ABL1 inhibitor imatinib mesylate,29 and similar hopes have been extrapolated to JAK2 inhibition in MPN. It is likely, however, that the impressive results of imatinib are reflective of the status of BCR-ABL1 as an almost “perfect target.” Not only is chronic-phase CML exquisitely dependent on BCR-ABL1 signaling,30 but normal hematopoiesis appears to be relatively independent of normal ABL1 signaling. This contrasts with the situation in MPN, in which levels of basal signaling are poorly correlated with JAK2 genotype and allele burden. Moreover, it is likely that JAK2 plays a much more prominent role in normal hematopoiesis than ABL1, as is suggested by the phenotypes of the respective knockout mice.31,32 Our data demonstrate inhibition of signaling in CD34+ and CD34− cells from MPN patients, which was independent of JAK2 mutation status and comparable to the degree of inhibition of STAT5 phosphorylation in CML cells after imatinib treatment. In MPN patients who lack a JAK2 mutation, this suggests that wild-type JAK2 at least partially mediates the increased basal signaling and may be therapeutically targeted, or that TG101209 inhibits physiologic targets important for MPN other than JAK2. Regardless of mechanism, on a practical note, our data predict the utility of JAK2 inhibitors in MPN patients independently of JAK2 genotype. However, our findings also raise concerns about potential toxicity for normal hematopoiesis that may potentially limit the use of JAK2 inhibitors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Simon McCallum and Anna Petrunkina-Harrison for expert flow sorting and the patients and staff of the Addenbrooke's Hospital MPN specialist clinic.
This study was funded by an Medical Research Council (United Kingdom) Senior Clinical Research Fellowship (to B.H.), and by the Kay Kendell Leukaemia Fund, Cancer Research UK, the Leukaemia and Lymphoma Research, and the Wellcome Trust. B.H. and A.R.G. receive funding from an Leukemia & Lymphoma Society Specialized Center of Research grant and the NIHR Cambridge Biomedical Research Center.
Authorship
Contribution: S.A. helped to design the study, performed the experiments, analyzed the data, and helped to write the manuscript; F.S. performed inhibitor experiments on the CML samples; E.G. collected and analyzed the patient data; P.C. performed the statistical analyses; P.B. and A.R.G. provided the patient samples; B.J.P.H. designed and guided the study, analyzed the data, and wrote the manuscript; and all authors commented on and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brian J. P. Huntly, Department of Haematology, University of Cambridge, Cambridge Institute for Medical Research, Hills Road, Cambridge CB2 0XY United Kingdom; e-mail: bjph2@cam.ac.uk.