Abstract
In this retrospective collaborative study, we have analyzed long-term outcome and donor cell engraftment in 194 patients with Wiskott-Aldrich syndrome (WAS) who have been treated by hematopoietic cell transplantation (HCT) in the period 1980- 2009. Overall survival was 84.0% and was even higher (89.1% 5-year survival) for those who received HCT since the year 2000, reflecting recent improvement of outcome after transplantation from mismatched family donors and for patients who received HCT from an unrelated donor at older than 5 years. Patients who went to transplantation in better clinical conditions had a lower rate of post-HCT complications. Retrospective analysis of lineage-specific donor cell engraftment showed that stable full donor chimerism was attained by 72.3% of the patients who survived for at least 1 year after HCT. Mixed chimerism was associated with an increased risk of incomplete reconstitution of lymphocyte count and post-HCT autoimmunity, and myeloid donor cell chimerism < 50% was associated with persistent thrombocytopenia. These observations indicate continuous improvement of outcome after HCT for WAS and may have important implications for the development of novel protocols aiming to obtain full correction of the disease and reduce post-HCT complications.
Introduction
Wiskott-Aldrich syndrome (WAS, OMIM 301000) is a severe X-linked disorder characterized by microthrombocytopenia, eczema, and immunodeficiency1-4 and is caused by hemizygous mutations in the WAS gene, which encodes the WAS protein (WASp).5 WASp is expressed in hematopoietic cells and mediates rearrangement of the actin cytoskeleton in response to cell activation.2,6 A functional deficit of WASp is often associated with immunologic defects, including reduced number and function of T lymphocytes, impaired antibody production (especially to polysaccharide antigens), defective natural killer cell function, reduced chemokinesis of phagocytes and dendritic cells, functional defects of regulatory T cells, and abnormal induction of apoptosis.3,4 A clinical scoring system has been developed to reflect the variability of the clinical phenotype associated with WAS mutations.1,7 Patients with a typical WAS phenotype (score 3-5) are highly susceptible to severe bacterial, viral, and opportunistic infections. Furthermore, a significant proportion (24%-72% in various series) develop autoimmune and inflammatory complications,8-11 and there is an increased risk of hematologic malignancies, mainly lymphoma and leukemia. In contrast, a score of 1 or 2 is attributed to patients with a milder phenotype, X-linked thrombocytopenia, which is characterized by reduced and delayed occurrence of infections, autoimmunity and malignancies, and prolonged survival.12 These differences in disease severity correlate, albeit imperfectly, with the amount of residual expression of WASp.9,13 Although the WAS scoring system has limited value in infants and young children (who may not yet have developed the full disease phenotype), it can be used to reflect the patients' clinical history at the time of HCT.14
Despite advances in clinical care, patients with classic WAS have a poor prognosis, and the median life expectancy is only 15 years, unless hematologic and immune reconstitution is achieved by hematopoietic cell transplantation (HCT).15 In various series, HCT from human leukocyte antigen (HLA)-matched related donors (MRDs) has consistently resulted in survival rates > 80%.16-20 However, MRDs are available for only a minority of patients. Experience with T cell–depleted HCT from HLA-mismatched family donors (MMFDs) has been less satisfactory, with survival rates between 37% and 55%.16,17,19 More recently, HCT from HLA-matched unrelated donors (URDs) and partially matched unrelated cord blood (UCB) have been increasingly used to treat patients with severe primary immunodeficiencies, including WAS. A large collaborative study of the International Bone Marrow Transplant Registry and the National Marrow Donor Program showed that the 5-year survival rate for WAS patients after matched unrelated donor (MUD)-HCT was 71%,16 and comparable or superior results (with survival rates between 80% and 86%) have been reported subsequently in other studies.17-20 Despite this progress, several problems remain to be addressed.
Age at HCT has been reported to impact overall survival after HCT for WAS. In particular, 2 separate groups reported that 5-year survival was significantly worse for patients who received a transplant after 5 years of age,16,17 with none of the patients undergoing URD-HCT at 5 years of age or older surviving 5 years or more after HCT.16
In most cases, HCT for WAS was performed using a fully myeloablative conditioning regimen, to permit stable donor stem cell and multilineage engraftment, which is expected to result in full correction of the hematologic and immunologic defects. However, with this approach approximately 10% of the patients reject the graft, and even more develop mixed or split chimerism.1 In a European study of 96 patients who survived at least 2 years after HCT, Ozsahin et al estimated that as many as 20% of the long-term survivors developed autoimmunity independent of chronic graft-versus-host disease (cGVHD); furthermore, the risk of autoimmunity was significantly higher for patients who developed mixed and split chimerism after receiving MUD-HCT.14
Analysis of lineage-specific chimerism and correlation with correction of the disease after HCT for WAS has received relatively little attention in the literature. Here we report the results of a retrospective collaborative study on 194 patients who have received HCT for WAS. We analyzed outcome, and the effects of clinical status and age at HCT, donor type, and lineage-specific chimerism with respect to survival and complications of HCT.
Methods
Patients
Data were collected on 194 patients with a clinical diagnosis of WAS who received HCT in 12 European and American centers between 1980 and 2009 (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Eight and 66 of the patients included in this study have been reported previously by Filipovich et al16 and Ozsahin et al,14 respectively. Clinical and laboratory information collected before and after HCT was entered by each center in a deidentified manner in a common electronic spreadsheet (supplemental Methods). Informed consent in accordance with the Declaration of Helsinki was obtained from the parents of all children. The study was approved by the local Institutional Review Board or Ethical Committee at all centers.
HCT
HCT was performed according to the protocols in use at the time at each of the participating Institutions. Transplantation was done using bone marrow, cord blood, or peripheral blood stem cells as the hematopoietic stem cell source. Donor cells were obtained from HLA-matched sibling donors (MSDs), other matched family donors (MFDs), MMFD, URDs, and partially matched UCB. Donor and recipient HLA typing was performed by serology for earlier patients and by molecular DNA typing in more recent years; methods were dependent on each center's practice.
Patients underwent either myeloablative or reduced-intensity conditioning. Busulfan and cyclophosphamide were used most frequently as myeloablative regimen, whereas melphalan or treosulfan in combination with fludarabine was most commonly selected for reduced-intensity conditioning (supplemental Methods). For transplantation from MMFD, the graft was T cell–depleted by E-rosetting and soybean lectin agglutination, in vitro treatment with Campath-1M monoclonal antibody and complement, or more recently by positive selection of CD34+ cells.
GVHD prophylaxis was performed with methotrexate or cyclosporine A or both. Antithymocyte globulin or alemtuzumab was included in the conditioning for GVHD prophylaxis for nondepleted URD-HCTs and for graft rejection prevention of T-depleted transplantations. Acute GVHD (aGVHD) and cGVHD were graded as previously reported.21,22 Occurrence and resolution of aGVHD grade 3 or 4 and/or extensive cGVHD were annotated in the database spreadsheet. Precautions to reduce the risk of infection were based on reverse isolation, antimicrobial prophylaxis, and immunoglobulin replacement therapy, according to the policies adopted at each center.
Mutation analysis and WASp expression
Chimerism analysis
Donor cell chimerism was assessed by the methods in use at the time in each center, including HLA typing, molecular analysis of short tandem repeats, fluorescent in situ hybridization, or cytogenetics for X/Y chromosomes, as well as by flow cytometric analysis of WASp expression in combination with other lineage-specific surface markers. Data on lineage-specific chimerism at the time of last follow-up visit were collected, when possible, for patients who survived at least 12 months after HCT. Longitudinal data of lineage-specific chimerism were available for 92 patients who survived at least 24 months after HCT. Within each lineage, 4 categories of chimerism were established. Full chimerism was defined by the presence of > 95% donor cells, high chimerism as a percentage of donor cells ranging from > 50% to 95%, low chimerism as a percentage of donor cells ranging from 5% to 50%, and null chimerism as less than 5% donor cells.
Statistical analysis
Means were compared by the Student t test. Survival curves were plotted by the method of Kaplan-Meier, and log-rank P values were determined for differences in survival. Spearman rank correlation coefficient was estimated to test the association between myeloid donor engraftment and platelet count. Univariate analyses were performed using the Wilcoxon-Mann-Whitney test. Multivariate analysis was performed using the Cox proportional hazards regression models. Data analysis was performed using the JMP Software Version 5.1.2 (SAS Institute). The level of significance was considered to be P < .05.
Results
Recipient, donor, and transplant characteristics
Among the 194 patients who received a transplant, 27 (13.9%) had been given a WAS clinical score less than 3 at the time of HCT, indicating that they had not experienced any of the following: severe infections, difficult-to-treat eczema, autoimmunity, or malignancy (Table 1). In contrast, the majority of patients (n = 167; 86.1%) had already developed severe clinical features of WAS and hence had a score ≥ 3. In particular, 139 patients (71.6%) had a history of recurrent and/or severe infections, whereas 55 patients (28.3%) had autoimmune disease, especially autoimmune hemolytic anemia (n = 24) and vasculitis (n = 16). Four patients had developed Epstein-Barr virus–mediated lymphoproliferative disease, and one patient each had juvenile myelomonocytic leukemia or embryonal carcinoma of the testis. Twenty-nine patients (14.9%) had been splenectomized before HCT, and 10 of them remained with a platelet count < 50 × 109/L, even after splenectomy.
Data on WAS gene mutations were available for 135 patients (69.6% of the entire cohort). As shown in Table 1, the majority (n = 87) carried severe mutations (frameshift, nonsense mutations, or large deletions). Twenty-six patients carried missense mutations, with 14 of them located in exons 1 to 3, where X-linked thrombocytopenia-associated mutations are clustered. Pretransplantation data on WASp expression were available for 92 patients: 73 of them had undetectable WASp, whereas 19 showed residual WASp expression (data not shown). Compared with patients with residual WASp expression, those who lacked protein expression had a higher clinical score at the time of HCT (mean ± SD, 3.82 ± 1.02 vs 3.16 ± 1.29, P < .05) and received HCT at a younger age (29.3 ± 39.4 vs 35.6 ± 36.5 months, P < .05).
The median age at HCT was 34.6 months (range, 2-240 months). On average, patients with a clinical score < 3 received HCT at a younger age than those with a score ≥ 3 (23.4 ± 28.2 vs 36.4 ± 39.7 months; P < .05). The majority of the patients (n = 119; 61.3%) received HCT at younger than 2 years of age; in particular, 19 of 27 patients with a clinical score less than 3 received HCT at younger than 2 years of age. On the other hand, 32 of the 194 patients (16.5%) were older than 5 years (median, 108 months; range, 63-240 months) at the time of transplantation.
Transplantation characteristics are reported in Table 2. The 194 patients received a total of 204 transplantations; 10 patients (6 of whom had received a MMFD-HCT) required a second transplant because of graft loss or rejection. The majority of the transplantations (62.9%) were performed in the period 2000 to 2009, reflecting wider access to transplantation from URD and UCB. In particular, all 25 UCB-HCTs and 71 of 93 (76.3%) URD-HCTs were performed in 2000 or later. Bone marrow was the primary source of hematopoietic stem cells in 78.4% of all transplantations. The vast majority of the patients (88.1%) received a myeloablative conditioning regimen; reduced-intensity conditioning was used more often in recent years (20 of the 23 HCT with reduced-intensity conditioning were performed since 2000).
Survival
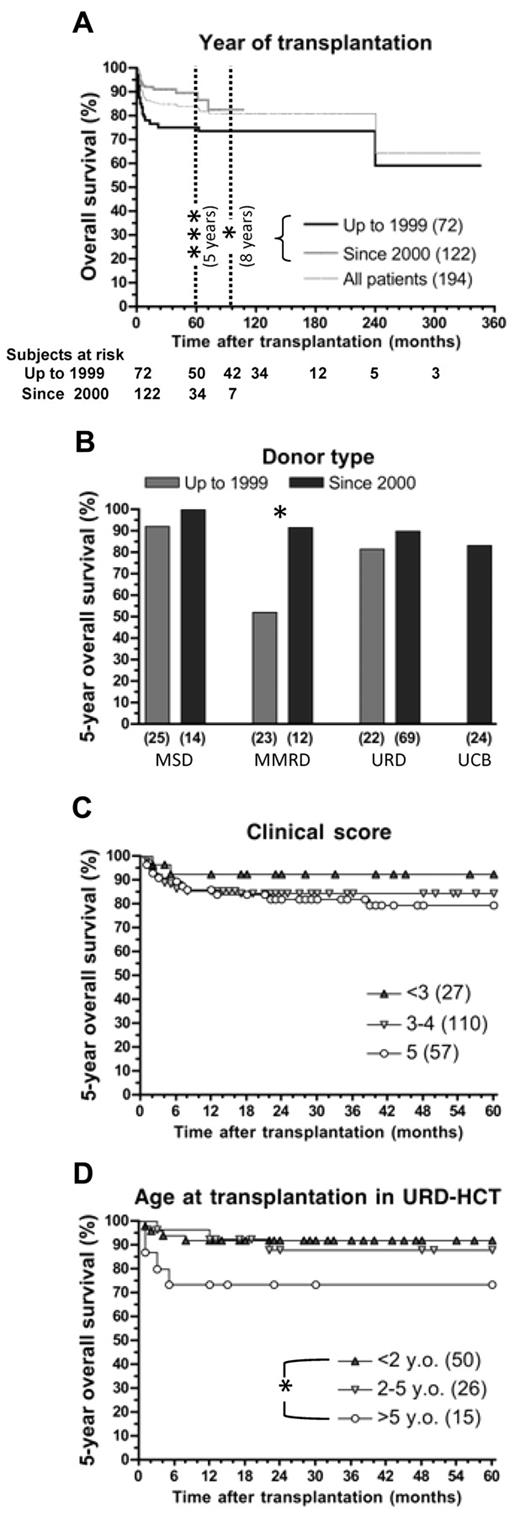
Of the 194 patients who received a transplant, 159 (82%) were alive at the time of the study, with a median follow-up of 76.8 months (range, 12-346 months). As shown in Figure 1A, both 5- and 8-year survival was significantly better for patients who received a transplant since 2000 (89.9% and 83.3% vs 74.9% and 73.4% for HCT performed up to 1999, P < .005 and P < .05, respectively). Improved survival was observed for all donor types in the last decade (Figure 1B) but was particularly significant (P < .05) for recipients of MMRD-HCT, whose overall survival rate increased from 52.2% to 91.7%.
Outcome of 194 patients affected by WAS after HCT. (A) Probability of survival for all patients according to year of transplantation. (B-D) Five-year overall survival for patients who received a transplant up to 1999 and since 2000 and grouped by donor type (B), clinical status before transplantation, as measured by clinical score (C), or for 91 patients receiving URD-HCT and divided in 3 groups according to their age at transplantation (D). *P < .05. ***P < .005
Outcome of 194 patients affected by WAS after HCT. (A) Probability of survival for all patients according to year of transplantation. (B-D) Five-year overall survival for patients who received a transplant up to 1999 and since 2000 and grouped by donor type (B), clinical status before transplantation, as measured by clinical score (C), or for 91 patients receiving URD-HCT and divided in 3 groups according to their age at transplantation (D). *P < .05. ***P < .005
There was a tendency for good clinical status at the time of HCT to result in better survival. In particular, 5-year survival was 92.4% for patients with clinical score < 3 versus 79.3% for patients with a score of 5 (Figure 1C), but this difference did not reach statistical significance. As shown in Figure 1D, younger age at HCT was associated with higher 5-year survival in patients treated with URD-HCT (91.9% vs 73.3% for patients who received a transplant at younger than 2 years of age vs those older than 5 years of age; P < .05). Age at HCT did not significantly affect survival in patients treated by MSD- or MMFD-HCT (data not shown).
Multivariate analysis (Table 3) showed that better survival was associated with HCT performed since the year 2000 (relative risk [RR] = 0.29; 95% confidence interval [CI], 0.11-0.77; P = .017). In contrast, use of MMFD or UCB as source of stem cells was associated with reduced survival (RR = 10.5, P = .003; and RR = 9.98, P = .028, respectively).
Thirty-five of the 194 patients (18%) have died, and causes of death are listed in supplemental Table 1. Most deaths (27 of 35; 77.1%) occurred within the first year, with half of them (n = 17) during the first 3 months after transplantation. Of note, 3 of the 8 patients who died > 12 months after HCT underwent pretransplantation (n = 2) or posttransplantation (n = 1) splenectomy and developed fulminant meningococcal (n = 1) and pneumococcal (n = 2) sepsis at 22, 62, and 72 months after HCT, respectively. Overall, infections (in the absence of GVHD) accounted for death in 15 patients and were more common among recipients of MMFD- and URD-HCT. Fatal lymphoproliferative disease (n = 2) and lymphoma (n = 1) were observed in 3 patients who were treated with MMFD-HCT before the year 2000. GVHD was reported as cause of death in 7 patients, 4 of whom had also developed infections.
Complications
Complications were common within the first year after HCT, affecting 45.9% of the patients, but were observed more rarely (16.8% of the surviving patients) thereafter. Primary graft failure or graft rejection was observed in 13 patients (7%), despite the fact that 11 of them had received myeloablative conditioning. Eight of these 13 patients were given T cell–depleted MMFD-HCT. Patients with graft failure/rejection were equally distributed in the periods 1980 to 1999 (n = 6) versus year 2000 or later (n = 7).
Autoimmune manifestations, predominantly cytopenias and endocrinopathies, were observed in 27 patients (13.9%), 9 of whom had reported episodes of autoimmunity also before HCT. aGVHD grade > 2 was observed in 22 patients (11.3%) and progressed to cGVHD in 11 of them. Infections requiring hospitalization occurred in 55 patients (28.4% of the entire cohort). Five patients developed tumors, and one of them died with relapse of testicular carcinoma.
Of 135 patients who survived at least 2 years after HCT, 39 (28.9%) had active complications at the time of last follow-up visit (median follow-up, 88.9 months; range, 24-346 months) or died (Table 4).
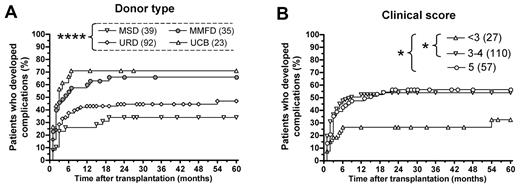
The risk of developing significant complications after HCT was higher among recipients of UCB- or MMFD-HCT than of MSD-HCT (Figure 2A; 71.0% and 66.0% vs 33.6%, P < .005 and P < .05, respectively). In contrast, the proportion of patients who developed complications after URD-HCT (46.7%) was not significantly different from that observed after MSD-HCT.
Probability of clinical and immunologic complications for 194 WAS patients who received HCT. (A) Percentage of patients who developed any complication (graft failure/rejection, acute GVHD grade 3 or 4, extensive cGVHD, severe infections, autoimmune manifestations, tumors, and post-HCT sequelae) up to 5 years after HCT, according to donor type. (B) Percentage of patients who developed any complication up to 5 years after HCT, according to clinical status (measured by clinical score) at the time of HCT. *P < .05. ****P < .001
Probability of clinical and immunologic complications for 194 WAS patients who received HCT. (A) Percentage of patients who developed any complication (graft failure/rejection, acute GVHD grade 3 or 4, extensive cGVHD, severe infections, autoimmune manifestations, tumors, and post-HCT sequelae) up to 5 years after HCT, according to donor type. (B) Percentage of patients who developed any complication up to 5 years after HCT, according to clinical status (measured by clinical score) at the time of HCT. *P < .05. ****P < .001
Within the cohort of 115 patients who received URD- or UCB-HCT, survival was comparable among those who received a transplant from fully matched versus more than one-antigen mismatched donors (57 of 67 vs 16 of 21, respectively; P = not significant; supplemental Table 2). However, the proportion of patients who survived without complications was significantly higher among recipients of transplants from fully matched versus more than one-antigen mismatched (39 of 67 vs 5 of 21, respectively; χ2 = 4.26, P < .05).
The risk of complications was also significantly influenced by the severity of the disease, as assessed by clinical score at the time of HCT (Figure 2B). Only 32.4% of patients with a clinical score less than 3 developed complications compared with 53.6% of patients with a score 3 to 4, and 56.1% of patients with score 5 (P < .05 in both cases).
There was a trend for patients with residual WASp expression to result in lower complication rate (26.3% vs 41.1% for patients lacking WASp; data not shown), but the difference was not statistically significant, possibly reflecting the low number of patients with residual expression of WASp.
In a multivariate analysis, only transplantation from MMFD was identified as a significant risk factor for the development of complications (RR = 2.45; CI, 1.15-5.20), mainly reflecting the high incidence of graft failure/rejection observed in this group, especially before 2000 (Table 5).
Immunologic reconstitution
Information on the absolute number of CD3+ and CD19+ lymphocytes at the time of last follow-up was available in 145 and 143 patients, respectively, who survived at least 12 months after HCT. Normalization of the absolute count of T and B lymphocytes and of T lymphocyte subsets (CD3+ > 1000, CD4+ > 600, CD8+ > 300, and CD19+ > 200 cells/μL) was observed in 68.3% of the patients. Inability to attain normalization of the lymphocyte count was associated with a higher incidence of mixed chimerism and/or autoimmune manifestations (supplemental Table 3).
Information on immunoglobulin replacement therapy was available for 153 patients, 20 of whom (13.1%) required intravenous immunoglobulins at > 12 months after HCT. Data on antibody responses after immunization with tetanus toxoid and pneumococcal polysaccharide antigens were available for a limited number of patients (72 and 53 patients, respectively), and protective titers of specific antibodies were detected in 95.8% and 73.6% of the cases, respectively.
Platelet recovery
Data on pre- and post-HCT platelet number were available in 152 patients, including 25 of the 29 patients who had received pre-HCT splenectomy. Platelet count of these 25 patients was significantly higher than in nonsplenectomized patients at the time of transplantation (mean, 95.5 × 109/L; 95% CI, 53.6-137.5 × 109/L vs mean, 29.0 × 109/L; 95% CI, 24.6-33.4 × 109/L) (P < .001). HCT resulted in a significant increase of the mean platelet count at last follow-up (> 12 months after HCT) among both nonsplenectomized (mean, 235.6 × 109/L; 95% CI, 213.3-257.9 × 109/L) and splenectomized (mean, 318.3 × 109/L; 95% CI, 272-364.6 × 109/L) patients (P < .001 vs pre-HCT values in both cases). However, 36 patients (23.7%) did not achieve normalization of the platelet count (< 150 × 109/L), and 14 of them showed persistent severe thrombocytopenia (< 50 × 109/L; range, 10-42 × 109/L). Among these, one patient developed severe bleeding episodes requiring multiple platelet transfusions and eventually received a second HCT 14 months after the primary transplantation; the remaining 13 patients had mild hemorrhagic manifestations, limited to petechiae. Nonetheless, because of persistent thrombocytopenia, 2 of them received a stem cell boost at 40 and 84 months after HCT, and 5 patients received post-HCT splenectomy, attaining a mean platelet count of 172.8 × 109/L (range, 106-231 × 109/L). Pre-HCT platelet count and clinical score were not significantly different in patients who normalized the platelet count after HCT and those who remained thrombocytopenic (data not shown).
Lineage-specific chimerism
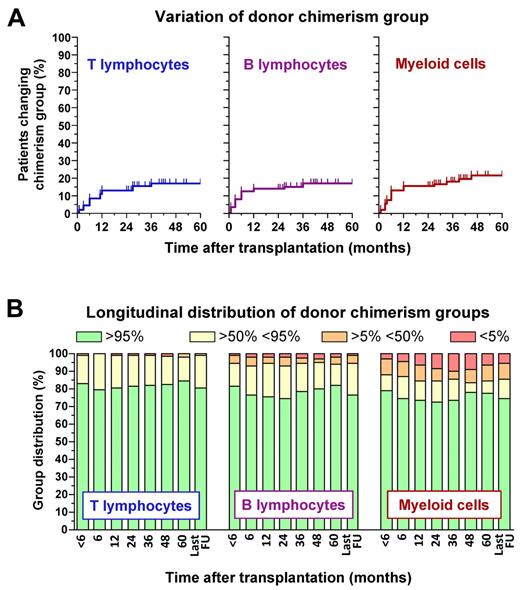
Longitudinal data on donor cell chimerism were available in 92 of the 135 patients who survived at least 24 months after HCT. Analysis of lineage-specific chimerism within this cohort showed that chimerism was relatively unstable in the first year after HCT, with a significant proportion of patients (< 20%) changing chimerism group but became more stable thereafter (Figure 3A). At each time point, donor chimerism was more robust in T than in B and myeloid cells (Figure 3B).
Longitudinal analysis of lineage-specific chimerism after HCT. Data were collected for 92 WAS-transplanted patients with at least 24 months of follow-up after HCT. Chimerism in the T- and B-lymphocyte and in the myeloid compartment was categorized according to the percentage of donor cells in 4 different groups ranging from full (defined by the presence of > 95% donor cells), high (> 50%-95%), low (5%-50%), to null (< 5%) chimerism. These data are reported for each cell type in panel A to show the longitudinal profile of donor chimerism variations, defined as changes in chimerism group, or in panel B to display the distribution of lineage-specific chimerism groups at various time points after HCT.
Longitudinal analysis of lineage-specific chimerism after HCT. Data were collected for 92 WAS-transplanted patients with at least 24 months of follow-up after HCT. Chimerism in the T- and B-lymphocyte and in the myeloid compartment was categorized according to the percentage of donor cells in 4 different groups ranging from full (defined by the presence of > 95% donor cells), high (> 50%-95%), low (5%-50%), to null (< 5%) chimerism. These data are reported for each cell type in panel A to show the longitudinal profile of donor chimerism variations, defined as changes in chimerism group, or in panel B to display the distribution of lineage-specific chimerism groups at various time points after HCT.
Ten patients received a second transplant within 17 months from the first HCT because of primary graft failure or graft rejection; 6 of them are alive with full donor chimerism (supplemental Table 4). Seven additional patients have received stem cell boosts (n = 5) or donor lymphocyte infusions (n = 2) as a result of poor donor chimerism; although all 7 patients are alive, only 2 have achieved full donor engraftment.
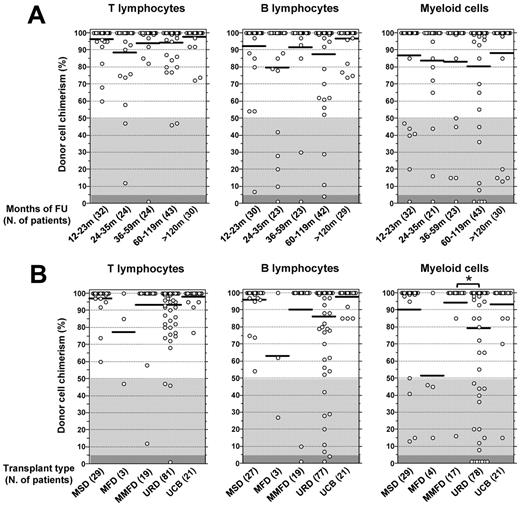
Figure 4A illustrates the results of cross-sectional analysis of lineage-specific chimerism at the time of last follow-up (median, 72.3 months; range, 12-346 months) in 154 of 167 patients (92.2%) who survived at least 12 months after HCT. Multilineage full donor chimerism was observed in 111 patients (72.1%). The remaining 43 patients (27.9%) showed mixed chimerism in at least one of the cell lineages tested. Low or null donor chimerism was more common within myeloid cells (16.5%) than in B cells (7.4%) or T lymphocytes (3.2%).
Quantitative analysis of lineage-specific chimerism at the time of last follow-up in 154 WAS-transplanted patients who had at least 12 months of follow-up after HCT. The percentage of donor-derived T, B, and myeloid cells is reported for each patient. (A) Data of lineage-specific chimerism at the time of last follow-up visit. Patients are grouped according to follow-up interval, and the number of patients studied at each interval is indicated in parentheses. (B) Patients are grouped according to donor type, and the number of patients receiving HCT from a specific type of donor is indicated in parentheses. *P < .05. Horizontal bars represent mean values (A-B).
Quantitative analysis of lineage-specific chimerism at the time of last follow-up in 154 WAS-transplanted patients who had at least 12 months of follow-up after HCT. The percentage of donor-derived T, B, and myeloid cells is reported for each patient. (A) Data of lineage-specific chimerism at the time of last follow-up visit. Patients are grouped according to follow-up interval, and the number of patients studied at each interval is indicated in parentheses. (B) Patients are grouped according to donor type, and the number of patients receiving HCT from a specific type of donor is indicated in parentheses. *P < .05. Horizontal bars represent mean values (A-B).
A significant fraction of the 82 patients treated with URD-HCT attained low or null donor myeloid chimerism (Figure 4B). In this group of patients, no correlation was identified between lack of full donor chimerism and type of conditioning, year of HCT, age and clinical score at HCT, pre-HCT WASp expression, or source of stem cells (data not shown). However, when the analysis was restricted to 36 patients treated by URD-HCT since the year 2000 (and for whom data on the number of CD34+ cells infused were available), poor (ie, < 50%) engraftment of donor myeloid cells was correlated with lower stem cell dose (4.14 ± 1.71 × 106 cells/kg vs 7.18 ± 4.39 × 106 cells/kg for patients with ≥ 50% of donor myeloid chimerism, P < .05).
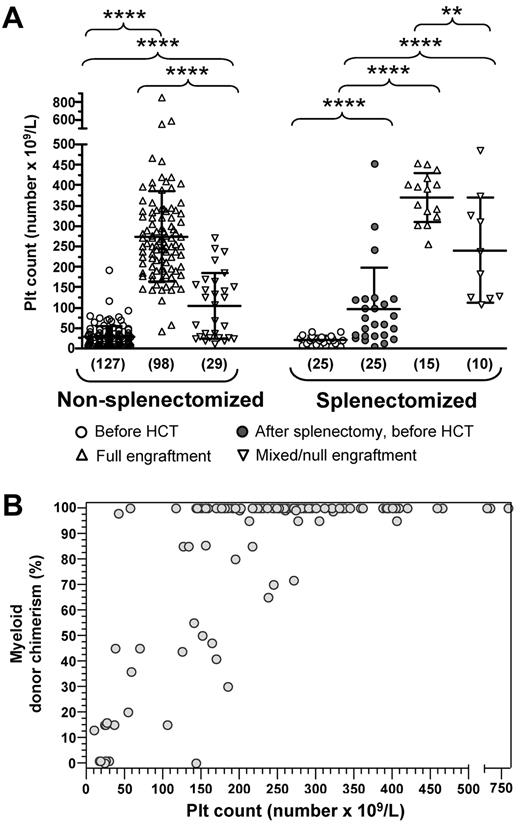
The degree of donor chimerism in the myeloid compartment had a significant impact on the reconstitution of the platelet number (Figure 5A). In particular, higher platelet counts were observed among patients who attained full versus mixed/null donor myeloid cell engraftment both for the cohort of 127 patients who were not splenectomized before HCT (274.5 ± 111.3 × 109/L vs 104.2 ± 79.7 × 109/L, P < .001) and for the 25 patients who underwent pretransplantation splenectomy (370.5 ± 59.7 × 109/L vs 240.0 ± 129.1 × 109/L; P < .005). Nonetheless, a significant increase in the platelet count after HCT was observed also in patients with mixed/null myeloid engraftment (P < .001 for both groups vs platelet counts before HCT). Statistical analysis using the nonparametric Spearman test in the cohort of nonsplenectomized patients confirmed, with few exceptions, the tight correlation between degree of myeloid chimerism and platelet count (Figure 5B; r = 0.5755; P < .0001). Overall, these data indicate that robust (> 50%) and stable myeloid chimerism after HCT is associated with normalization of the platelet count.
Influence of the degree of myeloid cell engraftment on platelet count. Platelet counts before and after HCT were reported for 152 WAS-transplanted patients, who had at least 12 months of follow-up after HCT and for whom quantitative analysis of donor cell engraftment on myeloid cells was available. Pretransplantation splenectomized patients were separated from nonsplenectomized patients. (A) Patients of both groups were further divided according to the degree of donor myeloid cell engraftment (full or mixed/null). For each of them, platelet (PLT) counts at diagnosis and at last follow-up are shown, with pretransplantation values of splenectomized patients reported both at diagnosis and after splenectomy. **P < .01. ****P < .001. (B) Correlation between the platelet (PLT) count of nonsplenectomized patients and the percentage of donor myeloid cell engraftment is shown. A significant correlation between these 2 parameters was observed according to the nonparametric Spearman test (r = 0.584, P < .001).
Influence of the degree of myeloid cell engraftment on platelet count. Platelet counts before and after HCT were reported for 152 WAS-transplanted patients, who had at least 12 months of follow-up after HCT and for whom quantitative analysis of donor cell engraftment on myeloid cells was available. Pretransplantation splenectomized patients were separated from nonsplenectomized patients. (A) Patients of both groups were further divided according to the degree of donor myeloid cell engraftment (full or mixed/null). For each of them, platelet (PLT) counts at diagnosis and at last follow-up are shown, with pretransplantation values of splenectomized patients reported both at diagnosis and after splenectomy. **P < .01. ****P < .001. (B) Correlation between the platelet (PLT) count of nonsplenectomized patients and the percentage of donor myeloid cell engraftment is shown. A significant correlation between these 2 parameters was observed according to the nonparametric Spearman test (r = 0.584, P < .001).
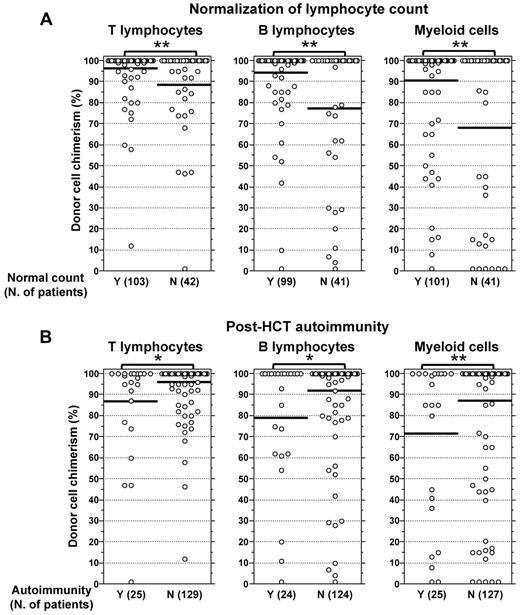
Low donor chimerism in T, B, and myeloid cells was associated with subnormal lymphocyte counts (P < .01; Figure 6A). It has been previously reported that mixed chimerism after HCT for WAS is associated with increased risk of autoimmunity, particularly in patients with URD-HCT,14 but the specific contribution of mixed chimerism in lymphoid or myeloid cells was not investigated. In our series, the 25 patients who developed autoimmune manifestations after transplantation and for whom data on lineage-specific chimerism were available, showed a lower degree of chimerism in each (T, B, myeloid) of the lineages tested compared with patients who did not develop autoimmunity (Figure 6B; T, 87.5% vs 93.8%, P < .05; B cells, 70.8% vs 87.6%, P < .05; myeloid, 63.6% vs 81.2%, P < .01).
Influence of the degree of donor cell engraftment on the reconstitution of lymphocyte counts and autoimmunity after HCT. Data are shown for WAS-transplanted patients who had at least 12 months of follow-up after HCT and for whom data of lineage-specific chimerism were available. (A) The percentage of donor chimerism for each cell lineage at the time of last follow-up is shown for patients who attained (Y) or did not attain (N) normalization of T- and B-cell counts (defined as CD3+ > 1000 cells/μL, CD4+ > 600 cells/μL, CD8+ > 300 cells/μL, and CD19+ > 200 cells/μL). **P < .01. (B) The percentage of donor lineage-specific chimerism is shown for patients who developed (Y) or did not develop (N) autoimmunity. *P < .05. **P < .01. Horizontal bars represent mean values (A-B).
Influence of the degree of donor cell engraftment on the reconstitution of lymphocyte counts and autoimmunity after HCT. Data are shown for WAS-transplanted patients who had at least 12 months of follow-up after HCT and for whom data of lineage-specific chimerism were available. (A) The percentage of donor chimerism for each cell lineage at the time of last follow-up is shown for patients who attained (Y) or did not attain (N) normalization of T- and B-cell counts (defined as CD3+ > 1000 cells/μL, CD4+ > 600 cells/μL, CD8+ > 300 cells/μL, and CD19+ > 200 cells/μL). **P < .01. (B) The percentage of donor lineage-specific chimerism is shown for patients who developed (Y) or did not develop (N) autoimmunity. *P < .05. **P < .01. Horizontal bars represent mean values (A-B).
Discussion
In this study, we analyzed the outcome of HCT in 194 WAS patients, treated in 12 centers between 1980 and 2009. The large number of patients enrolled and the long duration of the study allowed us to demonstrate that there is progressive improvement of outcome after HCT for WAS. In particular, comparable survival rates were observed after MSD- and after URD-HCT performed since 2000, and markedly improved survival rates have been achieved with MMFD-HCT during the same period compared with earlier years. This improved outcome probably reflects larger availability and better selection of unrelated donors (based on high-resolution HLA typing), advances in prevention and treatment of infections and of Epstein-Barr virus–related lymphoproliferative disease, use of less toxic conditioning regimens, and the introduction of more effective immunosuppressive drugs resulting in reduced risk of graft rejection.
Ten years ago, Filipovich et al had reported excellent survival after URD-HCT for WAS in patients younger than 5 years at the time of transplantation, but older patients had a poor outcome.16 Older age at transplantation has been identified as a significant risk factor also for patients with other congenital immunodeficiencies, reflecting increased occurrence of complications and progressive organ damage, reactivation of viral infections, and higher incidence of GVHD in older recipients.18,20,24,25 Although age more than 5 years at HCT was associated with less favorable survival after URD-HCT also in this study, 5-year survival for this group of patients was 73.3%, indicating marked improvement compared with a previous study.16 These data indicate that URD-HCT should no longer be restricted to WAS patients younger than 5 years, especially if clinical conditions are good. However, it should be noted that most (12 of 15) of the patients who received URD-HCT at an age older than 5 years were younger than 10 years. Therefore, it is not possible to assess outcome of URD-HCT for WAS when performed in adolescence or early adulthood.
Although the degree of HLA matching did not affect survival after URD- or UCB-HCT (probably because of the relatively small sample size), survival without complication was better in patients who received a transplant from fully matched donors.
Most complications occurred within the first year after HCT. The occurrence of autoimmunity early after HCT may reflect inadequacy of central and peripheral mechanisms of T-cell tolerance in the setting of T-cell lymphopenia.26 Reactivation of viral infections (cytomegalovirus, Epstein-Barr virus) is common, but careful monitoring of viremia and preemptive treatment has resulted in significantly decreased infection-related mortality in recent years.
Although early complications resolved without sequelae in the majority of the patients, approximately 30% of them had active complications at the time of last visit. In particular, extensive cGVHD, neurologic problems, and autoimmune disease at the time of the last follow-up visit were reported in 20, 10, and 12 patients, respectively. Similar data have been previously reported by Ozsahin et al,14 who found that 7-year event-free survival for WAS patients surviving at least 2 years after HCT was 75% and that 20% of the patients had developed irreversible damage leading to sequelae. Overall, the incidence of late complications and sequelae after HCT for WAS is lower compared with that observed after HCT for SCID.27
One of the main aims of our study was to characterize the stability of chimerism and long-term function of the graft. To establish full donor chimerism, the eradication of host-derived hematopoietic and lymphoid cells is required. Incomplete deletion of host cells may result either in primary graft failure resulting from rejection, with complete and early loss of the graft, or may allow for the development of variable degrees of mixed chimerism. In some cases, progressive loss of donor chimerism may follow, resulting in complete autologous reconstitution. Whereas graft rejection reflects a host-versus-donor immune response, mixed chimerism with coexistence of both autologous and donor blood cells implies mutual tolerance. In a recent study by Ozsahin et al,14 a large fraction of WAS patients who underwent HCT were reported to develop mixed or split chimerism, especially after URD- or MMFD-HSCT, and this condition was associated with increased risk of late autoimmune complications. However, the kinetics and distribution of lineage-specific chimerism were not analyzed.
In the present study, we sought to investigate in greater detail the kinetics and stability of lineage-specific chimerism, to identify risk factors that may favor development of mixed chimerism, and to assess the impact of chimerism on disease correction and posttransplantation complications. Multilineage full donor chimerism was reported in 72.1% of patients who survived at least 12 months after HCT. Retrospective sequential analysis of lineage-specific chimerism in a subcohort of 92 patients who survived at least 2 years after HCT demonstrated that mixed/split chimerism or autologous reconstitution usually becomes apparent within the first year after HCT, and levels of mixed chimerism tend to remain stable thereafter. Lower levels of donor chimerism were observed more often in myeloid than in lymphoid cells; in particular, the highest degree of donor chimerism was detected in T lymphocytes. A similar pattern has been observed in patients with severe combined immunodeficiency after transplantation with a mild conditioning regimen.28-31 These observations are consistent with the notion that autologous stem cell reconstitution does not necessarily affect development of T cells in the thymus once this organ is populated by donor lymphoid progenitor cells. Alternatively, it is possible that expression of the WAS protein confers a stronger and selective advantage in the lymphoid compartment (and especially in T lymphocytes) than in myeloid cells. Data from heterozygous Was+/− mice32,33 and from carrier females of X-linked thrombocytopenia34,35 support this hypothesis. However, patients with poor myeloid donor chimerism often failed to attain normalization of the lymphocyte count. Furthermore, persistent thrombocytopenia after HCT was strongly associated with low or null myeloid chimerism, suggesting that robust and stable engraftment of donor-derived myeloid cells is required to correct this defect because of the lack of selective advantage for WASp-positive cells in the myeloid compartment.32 Alternatively, it is possible that autoimmune thrombocytopenia may contribute to persistence of thrombocytopenia in patients who fail to attain full myeloid chimerism. Indeed, we found that patients with autoimmune manifestations after HCT show a lower degree of donor chimerism. We have also shown that splenectomy may induce normalization of the platelet count in patients who remain significantly thrombocytopenic after HCT. However, the benefits of this strategy have to be weighed against the risk of severe, potentially fatal infections, as confirmed by the finding that 3 patients developed sepsis and died, among the 28 who received pre- or post-HCT splenectomy.
In conclusion, this study confirms that HCT is an effective form of treatment for WAS and should be considered not only for patients younger than 5 years, but also for those older than 5 years with a MRD or MUD, especially if in good clinical conditions. However, robust and stable multilineage donor cell engraftment is required to fully correct the disease, a goal that may be facilitated by infusion of a higher dose of donor stem cells. Previous data favor myeloablative regimens to minimize the chance of autologous reconstitution and recurrence or persistence of the WAS phenotype; therefore, for patients in good conditions, this remains the standard regimen. As less toxic regimens, such as those based on targeted levels of busulfan (or treosulfan) and fludarabine, become available, their efficacy in the treatment of WAS should be tested.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for their trust and cooperation as well as Alexandra Arnold, Chantal Harre, Corinne Jacques, Arnalda Lanfranchi, Stéphanie N′Daga, and Qili Zhu for manipulation of the samples, chimerism, and immunologic studies.
This work was supported in part by the National Institutes of Health (grant 2P01HL059561-11-A1, L.D.N.; 5P01HL059561-12, S.G.; P01 HL036444, L.B.; U54 AI082973-02, L.D.N. and M.J.C.; HD17427-43, H.D.O.), the Manton Foundation, Fondazione Angelo Nocivelli (S.G.), the Jeffrey Modell Foundation, the Dejoria Wiskott-Aldrich Research Fund (H.D.O.), and by Cariplo (grant 2010/0253; A.L.).
National Institutes of Health
Authorship
Contribution: D.M., S.G., and L.D.N. designed research; A.L. performed statistical analysis; D.M., S.G., W.F., and L.D.N. wrote the manuscript; and C.B., E.M., A.F., A.J.C., A.J.T., H.D.O., M.J.C., M.H.A., T.S., S.-Y.P., E.H., M.H., A.S., F.P., M.C.-C., C.P., A.K., J.Z.N., N.M., W.Q., P.V., T.R.T., L.B., K.K., S.H., M.S., W.F., D.M., S.G., and L.D.N. took care of the patients and collected clinical and laboratory data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luigi D. Notarangelo, Division of Immunology and the Manton Center for Orphan Disease Research, Children's Hospital Boston, Karp Bldg, Rm 9210, 1 Blackfan Cir, Boston, MA 02115; e-mail: luigi.notarangelo@childrens.harvard.edu.
References
Author notes
W.F. and L.D.N. contributed equally to this study and share senior authorship.