Abstract
Patients with relapsed chronic lymphocytic leukemia (CLL) and high-risk features, such as fludarabine refractoriness, complex karyotype, or abnormalities of chromosome 17p, experience poor outcomes after standard fludaradine-based regimens. Alemtuzumab is a chimeric CD52 monoclonal antibody with activity in CLL patients with fludarabine-refractory disease and 17p deletion. We report the outcome for 80 relapsed or refractory patients with CLL enrolled in a phase 2 study of cyclophosphamide, fludarabine, alemtuzumab, and rituximab (CFAR). All patients were assessed for response and progression according to the 1996 CLL-working group criteria. For the intention-to-treat analysis, the overall response rate was 65%, including 29% complete response. The estimated progression-free survival was 10.6 months and median overall survival was 16.7 months. Although we noted higher complete response in high-risk patients after CFAR compared with a similar population who had received fludarabine, cyclophosphamide, and rituximab as salvage therapy, there was no significant improvement in progression-free survival and overall survival appeared worse. CFAR was associated with a high rate of infectious complications with 37 patients (46%) experiencing a serious infection during therapy and 28% of evaluable patients experiencing late serious infections. Although CFAR produced good response rates in this highly pretreated high-risk group of patients, there was no benefit in survival outcomes.
Introduction
Chronic lymphocytic leukemia (CLL) is a disease of progressive accumulation of clonal B-lymphocytes in peripheral blood, marrow, and lymphoid organs. This hematologic malignancy is generally considered incurable, with the exception of patients who remain disease-free after allogeneic stem cell transplantation (SCT). Frontline chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab (FCR) is associated with an overall survival (OS) advantage compared with FC as reported by German CLL study group in the CLL8 trial and improvement in progression-free survival (PFS) in first relapse of CLL in the REACH trial.1,2 We demonstrated that FCR is effective in patients with CLL beyond first relapse; however, patients with poor-risk cytogenetics, including abnormalities of chromosome 17p, patients with fludarabine-refractory CLL, or heavily pretreated patients with more than 3 prior treatments continue to have poor outcomes after this therapy.3
Alemtuzumab is a chimeric CD52 monoclonal antibody, which is effective as monotherapy via intravenous and subcutaneous administration in untreated, previously treated, and refractory patients with CLL.4-7 Studies of alemtuzumab demonstrate good responses for heavily pretreated patients with CLL with overall response rate (ORR) reported between 31% and 65%, including 2% to 27% complete response (CR).5,8-14 Alemtuzumab monotherapy is effective regardless of cytogenetic risk group, including high-risk chromosome 17p-deleted and fludarabine-refractory patients8,9,12,14 ; however, PFS has been short after alemtuzumab monotherapy with median PFS of 5 to 8 months for all patients and 10 to 13 months for responders.5,8,9,12-14 In addition, patients with bulky lymphadenopathy generally have poor responses after alemtuzumab monotherapy,5,9 although this finding has not been universally supported.14
We postulated that the addition of alemtuzumab to FCR chemoimmunotherapy may improve response rates for patients with relapsed and refractory CLL by targeting high-risk groups traditionally responding poorly to FCR. An early report of a combination study of fludarabine and alemtuzumab for 6 CLL patients refractory to both single agents achieved a high response rate (ORR = 83%), including 1 patient with minimal residual disease (MRD)-negative CR.15 A preliminary trial exploring the combination of alemtuzumab and rituximab in heavily pretreated patients with lymphoid malignancies demonstrated an ORR of 63% of patients in patients with relapsed CLL, suggesting synergistic activity between the 2 monoclonal antibodies, although the response duration after this antibody combination was only 6 months.16 Because the addition of rituximab to fludarabine and cyclophosphamide (FC) was well tolerated both in frontline and salvage patients with little additional clinically significant toxicity, we thought that the addition of alemtuzumab to FCR may lead to improved responses and remission duration in high-risk relapsed CLL.
Methods
The M. D. Anderson Cancer Center Institutional Review Board approved this study, patients provided written informed consent per institutional guidelines, and this study was conducted in accordance with the Declaration of Helsinki.
Patients
Eighty patients with relapsed or refractory CLL were enrolled in this phase 2 trial of cyclophosphamide, fludarabine, alemtuzumab, and rituximab (CFAR) between December 2002 and October 2006. All patients had a National Cancer Institute-Working Group (NCI-WG) indication for treatment.17 Patients must have had performance status (Eastern Cooperative Oncology Group) 0 to 3, adequate liver and renal function (creatinine < 2 mg/dL, bilirubin < 2.5 mg/dL) unless related to organ infiltration by CLL. Patients with uncontrolled life-threatening infections were excluded. Patients with HIV or carriers of hepatitis B or C were not excluded.
Treatment
Treatment consisted of cyclophosphamide (C) 250 mg/m2 intravenously on days 3 to 5, fludarabine (F) 25 mg/m2 intravenously on days 3 to 5, alemtuzumab (A) 30 mg intravenously on days 1, 3, 5, and rituximab 375 mg/m2 for cycle 1 and 500 mg/m2 for cycles 2 to 6 on day 2 for up to 6 courses. Courses were repeated monthly or at recovery of hematologic parameters if longer than 28 days. Dose reduction was permitted for grade 3 or 4 infections or other organ toxicity or if patients did not have adequate hematologic recovery by 35 days after the last cycle (dose −1: F 20 mg/m2, C 200 mg/m2; dose −2: F 17.5 mg/m2, C 175 mg/m2). Patients were premedicated with acetaminophen 650 mg and diphenhydramine 25 to 50 mg before antibody administration and hydrocortisone 100 mg intravenously before alemtuzumab administration at the treating physician's discretion. Infection prophylaxis was administered, including trimethoprim/sulfamethoxazole DS 1 tablet orally twice daily and valacyclovir 500 mg orally daily or valgancyclovir 450 mg orally twice daily for prevention of herpes viridae infections. Allopurinol was recommended for tumor lysis prophylaxis for week 1 of cycle 1. Pegfilgrastim 6 mg subcutaneously was administered routinely to patients on day 6 of each cycle as primary prophylaxis for neutropenia.
Patients were evaluated at enrollment with baseline complete blood count and standard biochemical studies, β2-microglobulin, quantitative immunoglobulins, bone marrow aspirate, and biopsy, including flow cytometry with CD38 expression on B-lymphocytes and cytogenetic studies by standard metaphase karyotype analysis. In addition, genomic abnormalities detected by fluorescence in situ hybridization using standard CLL probes (Abbott Molecular), ZAP-70 (by immunohistochemistry or flow cytometry18 ), and immunoglobulin variable heavy chain gene (IGHV) mutational status by PCR19 were routinely performed before initiation of therapy in 2004 and later. Complete blood count and biochemical assessment were performed before each cycle and response assessment with physical examination, complete blood count, and bone marrow aspirate and biopsy were performed after 3 cycles and after the last cycle of therapy. Patient response assessment used 1996 NCI-WG criteria.17 Computed tomography scans were not routinely performed for response assessment. Toxicity was assessed according to Common Toxicity Criteria for Adverse Events Version 3.0 (http://ctep.cancer.gov). Cytomegalovirus (CMV) monitoring was routinely performed before each course of therapy by CMV antigen assay. Patients were followed for toxicity until progression of disease or subsequent therapy.
Flow cytometric evaluation (flow) of bone marrow aspirate was performed to estimate MRD by evaluating for CD5+/CD19+ lymphocytes with light chain restriction by 3-color analysis. Flow MRD negativity was defined as < 1% of CD5+/CD19+ coexpressing cells with normal κ-λ ratio. Molecular monitoring for MRD was also performed using a PCR-based ligase assay for patient-specific IGHV gene.20 The ratio of IGHV to RAS (PCR ratio) was calculated and used to quantify the levels of residual disease. A IGHV/RAS ratio of 0.001 to 0.10 was borderline, higher ratios were considered to be positive, and ratios < 0.001 were considered negative.
Statistical analysis
The primary objective was to evaluate the therapeutic efficacy, in particular to estimate the CR rate of CFAR in previously treated patients with CLL. OS, PFS, and time-to-progression (TTP) were secondary outcomes calculated from the first day of therapy. Other objectives included: assessing the toxicity profile, monitoring for infection including cytomegalovirus, and evaluating molecular remission by PCR.
The overall goal of this study was to improve the CR rate with a total of 6 cycles of treatment from the 25% expected for FCR to 40%. The statistical design estimated an enrollment of 80 patients with an expectation of at least 30 CRs for a statistical certainty of 95% (α = 0.05) and power of 80% (β = 0.2) to reject the null hypothesis. Early stopping rules were incorporated into the design such that accrual would stop if: (1) it was highly unlikely that the responder rate would increase by at least 15%, or (2) it was highly likely that rate of nonresponding patients with severe toxicity would increase by at least 5% above the expectation of 12% after FCR.
Actuarial survival, PFS, and TTP were estimated using the methods of Kaplan and Meier, and survival estimates were compared among subgroups of patients using the log-rank test. Fisher exact test (2-tailed) was used to analyze differences in response outcomes by pretreatment characteristics. Association between CR and OR and patient pretreatment characteristics were evaluated using univariable and multivariable logistic regression models. Association between patient pretreatment characteristics and time-to-event endpoints were analyzed using Cox proportional hazards regression models. There were 18 pretreatment variables analyzed; therefore, a negative stepwise analysis was performed with a P value cut-off of P < .05. All computations were carried out using SAS, S-plus, and Statistica (Stat-Soft).
This protocol was open concurrent with the M. D. Anderson Cancer Center study of FCR for patients with relapsed or refractory CLL.3 There was no systematic approach to enrollment between the trials, although patients who had previously received FCR were refractory to fludarabine or had high-risk cytogenetic abnormalities (such as complex or 17p chromosomal abnormalities) typically were enrolled on this CFAR trial.
Results
Patient characteristics
Eighty patients were entered into this phase 2 trial of CFAR for relapsed or refractory CLL. The median age of patients was 59.5 years (range, 39-79 years). Forty-five (56%) patients had Rai stage III or IV CLL. Metaphase karyotype was obtained in 67 patients (84%), 15 patients had chromosome 17 abnormalities, 8 patients had complex karyotype, 6 patients had isolated deletion of 11q, and 27 patients had normal karyotype or deletion 13q. Fluorescence in situ hybridization results were available for 45 patients, including 14 patients with 17p deletion and 14 patients with 11q deletion. Newer prognostic markers were available in a smaller proportion of patients (Tables 1 and 2).
Median number of prior treatments was 3 (range, 1-14); 60 patients (75%) received prior fludarabine-based combination chemotherapy (FC, FND, and FCM) with or without rituximab, including 46 patients (58%) who received prior FCR. In addition, 17 patients (21%) received multiple (3 or more) agent chemotherapy regimen (ie, cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP]; etoposide, methylprednisolone, high-dose cytarabine, and cisplatin [ESHAP]; or hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and cytarabine [hyper-CVAD]) with or without rituximab. Twenty patients (25%) received prior alemtuzumab and 71 patients (89%) were previously exposed to rituximab-containing regimen. Thirty-one patients (39%) were refractory to fludarabine.
Responses
By intent-to-treat (80 patients), there were 23 CR (29%; 95% confidence interval [CI], 20%-40%), 3 nodular partial response (nPR 4%), and 26 partial response (PR) (33%) for an ORR of 65% (95% CI, 54%-75%). One patient was not evaluable for response. Variables associated with a higher likelihood of achieving a CR included smaller lymph nodes (< 5 cm), < 5 prior treatments, absolute neutrophil count (ANC) ≥ 1.5 × 109/L, no fludarabine or alkylator refractoriness, β2-microglobulin < 4 mg/L, absolute lymphocyte count (ALC) < 100 × 109/L, or absence of chromosome 17 or complex abnormality on metaphase karyotype. Patients with β2-microglobulin < 4 mg/L (odds ratio [OR] = 0.3, P = .036), ALC < 100 × 109/L (OR = 0.3, P = .047) or patients not refractory to fludarabine (OR = 0.2, P = .010) had a higher probability of achieving CR by multivariable regression analysis. In addition, we noted that none of the patients who were 70 years or older (n = 7) nor those who had a pretreatment serum albumin below 3.5 g/dL (n = 7) achieved a CR, but these associations were not significant by logistic regression analysis.
PFS
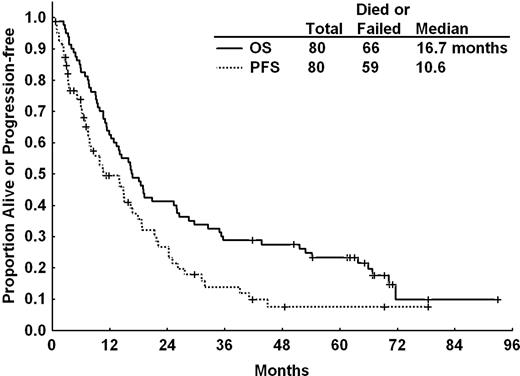
The median PFS was 10.6 months (95% CI, 7.9-16.8 months) for all patients (Figure 1); PFS was 28 months for patients who achieved CR compared with 10 months for patients who achieved PR (Figure 2A). Patient pretreatment characteristics associated with longer PFS by univariate analyses included age younger than 70 years, smaller maximum lymph node size identified by examination (in centimeters), higher hemoglobin, ALC < 100 × 109/L, normal serum albumin (≥ 3.5 g/dL), absence of fludarabine refractoriness, ≤ 5 prior therapies, and absence of complex karyotype or chromosome 17 abnormalities. Patient pretreatment characteristics independently associated with longer PFS by multivariable Cox regression analysis included age younger than 70 years, smaller maximum lymph node, normal serum albumin, no fludarabine refractoriness, and absence of complex karyotype or chromosome 17 abnormalities (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
PFS and OS for all patients after CFAR. The actuarial median PFS was 10.6 months and median OS 16.7 months for all patients after CFAR salvage therapy.
PFS and OS for all patients after CFAR. The actuarial median PFS was 10.6 months and median OS 16.7 months for all patients after CFAR salvage therapy.
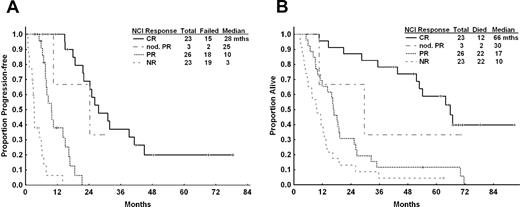
PFS and OS for patients after CFAR according to NCI-WG response. (A) PFS and (B) OS for all patients according to 1996 NCI-WG response criteria. Median PFS for patients who achieved CR was 28 months compared with 10 months for those who achieved PR. Estimated median OS was 66 months for patients who achieved CR, compared with 17 months for those who achieved PR and 10 months if there was no objective response.
PFS and OS for patients after CFAR according to NCI-WG response. (A) PFS and (B) OS for all patients according to 1996 NCI-WG response criteria. Median PFS for patients who achieved CR was 28 months compared with 10 months for those who achieved PR. Estimated median OS was 66 months for patients who achieved CR, compared with 17 months for those who achieved PR and 10 months if there was no objective response.
At the time of analysis, 14 of 80 (18%) patients were alive with a median OS from initiation of treatment of 16.7 months (95% CI, 12.8-26.0 months; Figure 1). The median observation time of surviving patients was 66 months (95% CI, 42-93 months). The median OS was 67 months for patients who achieved CR compared with 17 months for patients who achieved PR and 10 months for nonresponders (Figure 2B). Variables associated with longer survival included age younger than 70 years, Rai stage 0, I, or II disease, no fludarabine or alkylator refractoriness, ≤ 5 prior therapies, ANC ≥ 1.5 × 109/L, albumin ≥ 3.5 g/dL, absence of deletion 17p by fluorescence in situ hybridization, and abnormal chromosome 17 or complex cytogenetic abnormalities by karyotype. By multivariate Cox regression analysis, pretreatment characteristics independently associated with longer OS included: age younger than 70 years, pretreatment ANC ≥ 1.5 × 109/L, serum albumin ≥ 3.5 g/dL, no fludarabine refractoriness, and absence of complex karyotype or abnormalities of chromosome 17 (supplemental Table 1).
MRD monitoring was performed by flow cytometry (flow) for CD19 and CD5 coexpressing lymphocytes. Flow was available in 33 of 34 patients achieving CR, nPR, or CR with residual cytopenia (PRi), with 29 patients being flow MRD-negative. Twenty of 21 patients achieving CR and 9 of 13 patients whose response was nPR or PRi at completion of therapy were flow MRD-negative. Patients who achieved CR, PRi, or nPR and had no evidence of marrow residual disease by flow had an OS of 53 months and time to disease progression of 31 months. We did not observe a statistically significant difference in OS between flow-positive (n = 29) versus flow-negative cases (n = 5), but median TTP of disease was significantly longer for patients with flow-negative CR, PRi, or nPR (31 months vs 18 months, P = .02). We also analyzed the presence of marrow residual disease using PCR for immunoglobulin rearrangement in 20 patients achieving CR, nPR, or PRi; however, this analysis was limited by small numbers and most patients had borderline positive PCR (n = 12) so that we could not detect significant differences in TTP or OS between PCR-positive (n = 4) or PCR-negative patients (n = 4).
Toxicity
Eighty patients received at least one course of CFAR, 61 patients (76%) received at least 3 courses, and 14 patients (18%) received all 6 courses of therapy (median = 3). Thirteen patients (16%) required at least one dose reduction because of cytopenia or infection. Causes of early cessation of therapy included severe infection (n = 22), prolonged cytopenia (n = 15), hemolytic anemia (n = 2), lack of response or progression of disease (n = 11), second malignancy (n = 3), and one patient with a severe infusion reaction with alemtuzumab and one patient with severe cardiac arrhythmia. Eleven patients (14%) stopped therapy electively before 6 courses after achieving CR or proceeding to stem cell transplant (n = 4).
Hematologic toxicity (Table 3) included neutropenia, with 92% of evaluable patients experiencing at least one grade 3 or 4 episode (57% of 216 evaluable courses), grade 3 or 4 thrombocytopenia in 54% of evaluable patients (28% of 232 courses), and grade 3 or 4 anemia in 33% of evaluable patients (15% of 232 courses). Four patients developed autoimmune hemolytic anemia, 2 patients developed autoimmune thrombocytopenia, and 3 patients developed pure red cell aplasia during therapy.
Infections
Infectious complications were divided into “early infections” occurring within 3 months of completing therapy and “late infections” occurring beyond this time. Thirty-seven patients (46%) experienced at least one early severe (Common Toxicity Criteria for Adverse Events, grade 3 or 4) infection with a total of 53 infectious events (Table 4). The majority of infections were pneumonia (19 patients with 25 events), including 5 fungal pneumonias (aspergillus or probable mold), 5 viral (including respiratory syncytial virus, CMV, or other), 6 bacterial (mostly gram-negative), and 9 with no identified organism. There were 4 episodes of bacteremia, 5 symptomatic CMV infections, and 5 episodes of fever with no documented source. Other infectious events are highlighted in Table 5. Seven patients (9%) died of infectious complications during CFAR therapy or subsequent follow-up, including one patient who died after developing cerebral toxoplasmosis in addition to a probable fungal pneumonia. Advanced age was associated with an increased risk of severe bacterial or fungal infection; 5 of 7 patients 70 years of age or older (71%) experienced at least 1 infection compared with 21 of 73 younger patients (29%, P = .034).
CMV reactivation occurred in 16 patients, including 3 patients with late CMV reactivations after completion of therapy. The majority of CMV reactivation was limited to fevers with CMV antigenemia (n = 8) or asymptomatic CMV antigenemia (n = 3) and responded to oral therapy with valganciclovir. Five patients required hospitalization during therapy because of fever (n = 3) or CMV pneumonitis (n = 2), and a further 2 patients were hospitalized with CMV reactivation at least 3 months after the end of therapy. Patients on prophylactic valganciclovir had a lower rate of CMV reactivation compared with valacyclovir (3% vs 24% respectively, P = .01). We did not find an association between likelihood of CMV reactivation and pretreatment variables, including age, hemoglobin level, platelet count, ANC, ALC, serum albumin, or number of prior treatments.
Patients were followed for late infections until relapse of disease or next therapy for patients who remained on study for at least 3 months beyond the last cycle of therapy. Seventeen of 36 evaluable patients (47%) experienced at least one late infection, including 10 patients (28%) with grade 3 or 4 infections. The most common severe infections were pneumonia, including 5 fungal pneumonias, one Pneumocystis jiroveci pneumonia, and one viral pneumonia (Table 4).
Other nonhematologic toxicity
There were 10 episodes of grade 3 or 4 nonhematologic toxicity on therapy. One patient had progressive disease and fatal congestive cardiac failure. Two patients were hospitalized with rapid atrial fibrillation in the setting of respiratory tract infections. Other grade 3 or 4 toxicity included nausea/vomiting (n = 2), and one patient each with ischemic stroke, small bowel obstruction, and severe post-infusion dyspnea. One patient developed Guillain-Barré syndrome 2 months after completion of therapy, and another patient developed a pulmonary embolus associated with progressive disease and pneumonia, 2 months after one cycle of therapy. Common minor (grade 1 or 2) adverse reactions included infusion-related fevers, chills or rigors (40 patients), rash or pruritus (32 patients), nausea or vomiting (33 patients), and diarrhea (8 patients).
Cause of death
There have been 66 deaths, 13 occurred within 6 months of initiating therapy (early deaths), and another 9 deaths occurred during study follow-up. Of the early deaths, 6 were attributed to progressive CLL, 5 with CLL and concurrent infection, one grade 5 infection, and one death after diagnosis of acute myeloid leukemia. The majority of deaths that occurred during study follow-up were related to infections with or without CLL (n = 7), one death from CLL without infection, and one after the development of a second malignancy (Table 5).
Allogeneic SCT
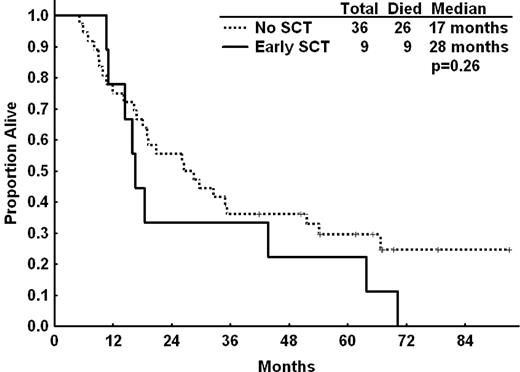
Twenty-two patients underwent allogeneic SCT after enrolment on this study; 10 patients went to SCT immediately after CFAR therapy, whereas the other 12 patients failed CFAR and received at least one other chemotherapy regimen before transplantation. Nine of the 10 patients transplanted immediately after CFAR had achieved a response after CFAR, including 4 patients who achieved CR. These patients received a median of 3 cycles (range, 2-6 cycles) of therapy before transplantation, and the median time from CFAR to transplant was 8 months (range, 3-17 months). There was no significant difference in survival for the 9 patients who had an objective response after CFAR and underwent an SCT compared with 36 patients who achieved a response but did not undergo SCT (Figure 3; OS, 17 vs 27 months, respectively; P = .26).
OS for patients who underwent allogeneic SCT compared with patients who did not proceed to transplantation. Patients who achieved PR or CR and proceeded to transplantation (early SCT, n = 9) were compared with patients who achieved PR or CR but did not proceed to transplantation (n = 36). Seven patients underwent SCT after relapse of CLL and subsequent salvage chemotherapy and were excluded from this analysis. We noted no improvement in median OS for patients who underwent transplantation compared with those who did not proceed to transplantation (17 vs 28 months, respectively, P = .26).
OS for patients who underwent allogeneic SCT compared with patients who did not proceed to transplantation. Patients who achieved PR or CR and proceeded to transplantation (early SCT, n = 9) were compared with patients who achieved PR or CR but did not proceed to transplantation (n = 36). Seven patients underwent SCT after relapse of CLL and subsequent salvage chemotherapy and were excluded from this analysis. We noted no improvement in median OS for patients who underwent transplantation compared with those who did not proceed to transplantation (17 vs 28 months, respectively, P = .26).
Comparison with FCR salvage
We performed a historical comparison of patients who received CFAR as salvage therapy to patients who received FCR as salvage therapy for CLL at our institution.3 Patient characteristics are presented in supplemental Table 2. A number of significant differences were noted in the 2 patient populations, in particular, patients who received CFAR were more likely to have chromosome 17 abnormalities (P = .04), higher number of prior treatments (P < .001), and were more likely to be fludarabine refractory (P < .001) compared with patients who received FCR. In addition, whereas only 4 patients (1%) in the FCR salvage cohort had received prior FCR, 45 patients (56%) in this study had received prior FCR. Other characteristics were not significantly different. When comparing the full cohorts of patients, CFAR was associated with significantly shorter PFS and OS after therapy compared with FCR given as salvage therapy (supplemental Figure 1A-B).
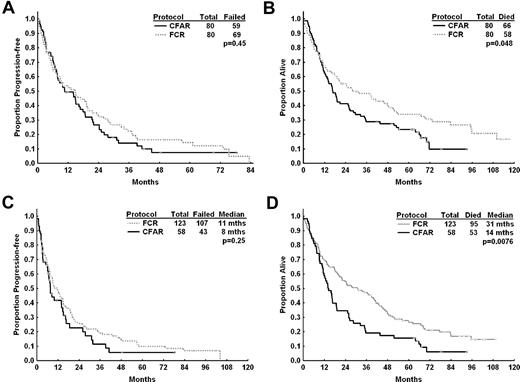
Because these 2 patient populations were not directly comparable resulting from significant differences in pretreatment characteristics, we performed a matched-pair analysis by matching patients who had received CFAR or FCR in a 1:1 ratio according to pretreatment characteristics, including age (> or < 70 years), karyotype (presence of chromosome 17 or complex), number of prior treatments, fludarabine-refractoriness, Rai stage, β2-microglobulin (< or ≥ 4 mg/L), presence of hypoalbuminemia or neutropenia, and ALC (< or ≥ 100 × 109/L). There was no significant difference in PFS for patients who received CFAR compared with FCR in this comparison, although patients who had received CFAR had significantly shorter OS (Figure 4A-B). In addition, because we postulated that CFAR may be of benefit in higher-risk patients, defined as those with complex cytogenetics or chromosome 17 abnormalities, patients refractory to fludarabine or patients who had received 3 or more prior treatments, we compared the PFS and OS for this group of patients. Despite a higher CR rate in high-risk patients after CFAR compared with FCR (20% vs 6%, P = .035), there was no significant difference in PFS, although patients who had received CFAR had significantly shorter OS (Figure 4C-D). After adjusting for differences in pretreatment variables using Cox regression multivariate analysis, there was no significant difference between CFAR and FCR in predicting PFS, but CFAR was associated with shorter OS compared with FCR (supplemental Table 3).
Median PFS and OS for high-risk relapsed CLL patients after CFAR and FCR salvage therapy. (A) Median PFS for all patients with who received CFAR chemoimmunotherapy and 80 patients who received salvage FCR chemoimmunotherapy matched for age, stage, lymphocyte count, cytogenetic risk group, number of prior therapies, and prior fludarabine response. There was no significant difference in PFS between patients who received CFAR as salvage therapy compared with the matched FCR salvage patients (11 vs 15 months, P = .45). (B) Estimated median OS was longer for patients who received CFAR salvage therapy compared with matched FCR patients (17 vs 28 months, respectively, P = .048), although matching could not account for the number of patients who had received prior FCR, which was significantly higher in the CFAR salvage group. (C) PFS for high-risk relapsed patients (defined as patients with complex cytogenetics or chromosome 17 abnormalities, patients refractory to fludarabine, or patients who had received 3 or more prior treatments) who received CFAR as salvage therapy (n = 58) compared with similar patients treated with FCR as salvage therapy at M. D. Anderson Cancer Center (n = 123). There was no significant difference in PFS for these 2 groups of patients. (D) OS for high-risk relapsed CLL patients who received CFAR and FCR. Median OS was significantly shorter for patients who had received CFAR as salvage therapy.
Median PFS and OS for high-risk relapsed CLL patients after CFAR and FCR salvage therapy. (A) Median PFS for all patients with who received CFAR chemoimmunotherapy and 80 patients who received salvage FCR chemoimmunotherapy matched for age, stage, lymphocyte count, cytogenetic risk group, number of prior therapies, and prior fludarabine response. There was no significant difference in PFS between patients who received CFAR as salvage therapy compared with the matched FCR salvage patients (11 vs 15 months, P = .45). (B) Estimated median OS was longer for patients who received CFAR salvage therapy compared with matched FCR patients (17 vs 28 months, respectively, P = .048), although matching could not account for the number of patients who had received prior FCR, which was significantly higher in the CFAR salvage group. (C) PFS for high-risk relapsed patients (defined as patients with complex cytogenetics or chromosome 17 abnormalities, patients refractory to fludarabine, or patients who had received 3 or more prior treatments) who received CFAR as salvage therapy (n = 58) compared with similar patients treated with FCR as salvage therapy at M. D. Anderson Cancer Center (n = 123). There was no significant difference in PFS for these 2 groups of patients. (D) OS for high-risk relapsed CLL patients who received CFAR and FCR. Median OS was significantly shorter for patients who had received CFAR as salvage therapy.
Discussion
The primary aim of this study was to demonstrate an increase in the rate of complete response above our expectation of 25% with FCR to 40% after CFAR. We did not achieve this outcome and therefore are unable to conclude that CFAR is more effective than FCR in relapsed or refractory patients with CLL. We noted an improvement in CR rates compared with FCR in high-risk patients defined by 3 or more prior treatments, fludarabine refractoriness, or presence of complex cytogenetics or chromosome 17 abnormalities by metaphase karyotype: 6% after FCR up to 22% after CFAR. Despite the higher response rates, we did not notice an improvement in PFS or OS after CFAR in this high-risk population.
The CFAR patient population was significantly higher risk than the population treated on our FCR salvage protocol. Patients treated with the CFAR had received a greater number of prior chemotherapy regimens, including a greater proportion of patients who were fludarabine-refractory and greater proportion of patients with high-risk cytogenetics. Furthermore, in the CFAR study, 74% of patients had received prior purine analog and alkylator combinations, including 58% of patients who had received prior FCR; whereas in the FCR salvage population, only 29% had received prior fludarabine-alkylating agent combinations and only 4 patients (1%) had prior FCR. These 2 populations are therefore not readily comparable.
We performed a matched-pair analysis, compared selected high-risk patients, and performed a multivariate regression analysis to account for differences in pretreatment characteristics but noted no improvement in PFS after CFAR compared with FCR. However, no comparison could account for differences in the number of patients who had received prior FC or FCR combinations between the 2 trials. FCR is now considered the standard of care in frontline therapy of CLL21 and management of patients who have short remission duration (< 3 years) after FCR or have bulky disease on relapse remains challenging. The CFAR regimen could be considered for high-risk relapsed patients who are candidates for transplantation; however, because we could not demonstrate a significant improvement in survival in our transplanted patients, this hypothesis remains unconfirmed.
The CFAR regimen was associated with a high rate of treatment-related infections, both during and after completion of chemotherapy compared with the patients who received FCR. A significant number of these were typical opportunistic infections, including protozoan, fungal, or herpes viral infections requiring hospitalization. Antipneumocystis and antiviral prophylaxis was mandated for patients in our trial, but we did not routinely administer continuous antibacterial or antifungal prophylaxis. Opportunistic infections associated with alemtuzumab therapy have been previously well documented.22-25 In addition, immunosuppression in patients with advanced CLL after multiple prior chemotherapy regimen probably contributed significantly to the infection rate observed in this study. Patient compliance with antibiotic or antiviral prophylaxis may also have affected the rate of opportunistic infections; however, this is less probable in view of the close follow-up of patients during therapy. In view of the prolonged immunosuppression and intermittent myelosuppression with CFAR, it is possible that antifungal prophylaxis may also be important in the supportive care of patients receiving alemtuzumab-containing chemoimmunotherapy regimen.
Although the majority of CMV reactivations were asymptomatic, 5 patients required hospitalization for CMV infection. We noted that administration of prophylactic valgancyclovir significantly reduced the incidence of CMV reactivation. We therefore recommend the use of valgancyclovir prophylaxis or CMV antigen monitoring in patients receiving alemtuzumab-containing chemoimmunotherapy regimen following published clinical guidelines.6,26
Disease stage, number of prior therapies, and refractoriness to therapy may have also impacted on the rate of severe infections experienced by patients. The number of prior therapies was demonstrated as an important risk factor in the development of serious infections as is the response status after therapy.27,28 In previously untreated patients with CLL, CFAR did not lead to a higher rate of infections compared with frontline FCR (Parikh S et al, manuscript in press). In contrast, the CLL2007FMP study comparing FCR versus FC and alemtuzumab for frontline treatment of patients with CLL was terminated prematurely because of an increased rate of infectious events in the alemtuzumab-containing regimen.29 In the frontline setting, a high rate of serious infections also was reported with fludarabine and alemtuzumab combinations.30,31 A trial of alemtuzumab consolidation after chemoimmunotherapy prolonged PFS but also was prematurely terminated because of a high rate of opportunistic infections.32 The high rate of infection seen in the study probably reflects the immunosuppression resulting from this combination as well as the reduced baseline immunity of patients who have been heavily pretreated and have active disease.
To improve the remission duration with alemtuzumab monotherapy, a number of clinical trials explored the possibility of chemoimmunotherapy combinations, including fludarabine and alemtuzumab. In a randomized study of 335 patients with relapsed CLL, the addition of alemtuzumab to fludarabine as second-line therapy resulted in higher CR rates associated with an improvement in PFS over fludarabine monotherapy.33,34 In patients with Rai stage III or IV disease, this combination also was associated with an increase in OS.34 The German CLL study group CLL2L FC-Cam study and Italian study group FCC trials investigated chemoimmunotherapy combinations, including fludarabine, cyclophosphamide, and alemtuzumab and reported good response rates (FC-Cam: CR = 22%, ORR = 68%; FCC: CR = 30%, ORR = 67%) with an estimated median PFS of 24 months after FCC.35,36 However, patients in either of these studies were less heavily pretreated than in this report, with a median number of one and 2 therapies before FC-Cam and FCC, respectively. Alemtuzumab also was combined with high-dose corticosteroids in the German Study Group CLL 3O reporting an ORR of 63% in a highly pretreated group of CLL patients who were either fludarabine-refractory and/or had 17p deletion.37 Fludarabine and alemtuzumab combinations are therefore feasible and may have high response rates in selected patients with relapsed or high-risk CLL.
In view of the high infectious toxicity and lack of survival advantage after CFAR in patients with relapsed or refractory CLL, we think that further investigation of this regimen in this setting is not warranted. Alemtuzumab remains a promising therapy for high-risk patients with CLL, including fludarabine-refractory patients, patients with short remission duration after FCR therapy, and patients with deletion 17p in view of its efficacy as a single agent and encouraging results in less intensive combinations either with steroids37 or other monoclonal antibodies.38 Fludarabine and alemtuzumab combinations were demonstrated to be effective in other studies in significantly less pretreated populations but may not be as well tolerated in heavily pretreated patients with significant baseline immunosuppression. Therefore, alemtuzumab chemoimmunotherapy may have a role in selected patients with high-risk cytogenetics or refractory to fludarabine with careful attention to comorbidity status and appropriate infection prophylaxis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the physicians within the University of Texas M. D. Anderson Cancer Center and in the community who assisted with the clinical care of patients on this trial as well as Dr T. Kipps and the CLL Research Consortium, University of California–San Diego, La Jolla, CA for provision of data on ZAP70 and IGHV mutation status.
W.G.W. is a Leukemia & Lymphoma Society Clinical Scholar.
Authorship
Contribution: M.J.K. and W.G.W. designed, performed, and analyzed the trial and coauthored the paper; X.C.B. analyzed results and wrote the paper; S.M.O., A.F., S.F., J.B., C.K., and H.K. provided clinical care to patients, assisted in data analysis and development of critical themes, and coauthored the paper; X.W. performed statistical analysis and coauthored paper; and S.L. collected and verified patient information and analyzed data.
Conflict-of-interest disclosure: M.J.K. served as consultant and received honoraria from Hoffmann-La Roche, Genzyme, and Genentech. S.M.O. received consultancy honoraria and research funding from Biogen-Idec and Genentech. A.F. received speaker's honoraria and research funding from Genentech. W.G.W. is the principal investigator, has received speaker's honoraria from Genentech, Genzyme, and Hoffmann-La Roche, and served as consultant on scientific advisory boards for Genentech and Genzyme and the data safety monitoring board for Genentech. The remaining authors declare no competing financial interests.
Correspondence: William G. Wierda, Department of Leukemia, Unit 428, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: wwierda@mdanderson.org.