Abstract
A precise identification of adult human hemangioblast is still lacking. To identify circulating precursors having the developmental potential of the hemangioblast, we established a new ex vivo long-term culture model supporting the differentiation of both hematopoietic and endothelial cell lineages. We identified from peripheral blood a population lacking the expression of CD34, lineage markers, CD45 and CD133 (CD34−Lin−CD45−CD133− cells), endowed with the ability to differentiate after a 6-week culture into both hematopoietic and endothelial lineages. The bilineage potential of CD34−Lin−CD45−CD133− cells was determined at the single-cell level in vitro and was confirmed by transplantation into NOD/SCID mice. In vivo, CD34−Lin−CD45−CD133− cells showed the ability to reconstitute hematopoietic tissue and to generate functional endothelial cells that contribute to new vessel formation during tumor angiogenesis. Molecular characterization of CD34−Lin−CD45−CD133− cells unveiled a stem cell profile compatible with both hematopoietic and endothelial potentials, characterized by the expression of c-Kit and CXCR4 as well as EphB4, EphB2, and ephrinB2. Further molecular and functional characterization of CD34−Lin−CD45−CD133− cells will help dissect their physiologic role in blood and blood vessel maintenance and repair in adult life.
Introduction
The existence of the hemangioblast has been proposed in the first decades of the 20th century.1,2 It was suggested that the cells of the endothelial and hematopoetic lineages might derive from a common precursor, the hemangioblast, present in the blood islands of the yolk sac. Since then, many studies have contributed evidence that the hemangioblast might also be present in the postnatal life. Pelosi et al3 showed that single CD34+KDR+ cells from adult BM or CB can generate both hematopoietic and endothelial cells in vitro and that these cells are endowed with long-term proliferative capacity. The potential of BM-derived hematopoietic stem cells to differentiate in vitro and in vivo into endothelial cells has been confirmed by other investigators.4-6 This has also been confirmed in cancer models that allow cell tracing on the basis of cancer-specific genetic signature. For instance, Gunsilius et al7 obtained in vitro endothelial cells that contained the Bcr/Abl fusion gene starting from blood or BM-derived MNCs of patients with chronic myelogeneous leukemia carrying the Bcr/Abl fusion gene, indicating that both endothelial and hematopietic lineages derive from a common progenitor. Similarly, hemangioblastic precursor cells that may contribute to maintaining both malignant hematopoiesis and angiogenesis have been suggested in other hematologic malignancies.8,9 Importantly, the existence of BM-derived hematopoietic stem cells endowed with bilineage ability to reconstitute both hematopoietic and endothelial cells has been also confirmed at the single-cell level.10 Moreover, transplanted vascular cells from adult thoracic aorta or inferior vena cava have been shown to contribute to the generation of hematopoietic cells in lethally irradiated recipients,11 thus suggesting that bilineage development might reside in endothelial vessels, as well.
Although the above-mentioned studies are convincing in supporting the existence of the hemangioblast in adult life, a precise identification of the hemangioblast with a clear-cut definition of characterizing molecular markers is still lacking. Several studies have shown an extensive overlap in the expression of hematopoietic and endothelial markers during early developmental stages, suggesting a close developmental relationship between the 2 lineages. In particular, markers such as CD34 and vascular endothelial growth factor receptor-2 (KDR), which are expressed on early progenitors of both lineages, are progressively down-regulated during hematopoietic differentiation but are conserved in fully differentiated endothelial cells.12,13 In the present study, to possibly identify precursors in human adult peripheral blood (hPB) endowed with the hemangioblast developmental potentials, we established a new ex vivo long-term culture model aimed to support the differentiation of both the hematopoietic and endothelial lineages. By performing long-term-hematopoietic-endothelial cultures (LTC-HEs), we could identify from PB a cell population lacking the expression of CD34, specific hematopoietic lineage markers, CD45 and CD133 (CD34−Lin−CD45−CD133− cells) that are endowed with the ability to differentiate into both hematopoietic and endothelial lineages, as assessed by immunophenotyping, functional assays, and gene expression analysis of the cells obtained after 6-week culture. We could demonstrate the bilineage potentiality of CD34−Lin−CD45−CD133− cells at the single-cell level. Finally, we demonstrated that CD34−Lin−CD45−CD133− cells transplanted into NOD/SCID mice are able to reconstitute the hematopoietic tissue and to generate functional endothelial cells that contribute to new vessel formation during tumor angiogenesis, thus confirming the bilineage potential of CD34−Lin−CD45−CD133− cells in vivo.
We suggest that the identification of CD34−Lin−CD45−CD133− cells as a circulating population endowed with hemangioblast developmental potentials may provide new insights for understanding the molecular and cellular pathways leading to the differentiation of hematopoietic and endothelial lineages and may support potential future clinical applications.
Methods
Enrichment of hPB Lin− mononuclear cells
HPB Lin− mononuclear cells (MNCs) were obtained by Ficoll density gradient (Ficoll-Hypaque; Sigma-Aldrich) centrifugation from standard buffy coat preparations of healthy donors. Lin− MNCs were isolated with the Human Progenitor Cell Enrichment Kit (StemCell Technologies).
Isolation of a CD34−Lin− subpopulation by cell sorting
To obtain highly purified Lin− subpopulations, Lin− MNCs obtained with the enrichment kit were stained with a pool of lineage-specific PE-labeled mAbs (as reported in supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) also containing CD31 and CD34; FITC-labeled mAb against CD45 (all from BD Bioscience), and allophycocyanin-labeled mAb against CD133 (Miltenyi Biotec). The following primitive subpopulations were isolated by cell sorting with a modified dual-laser FACS Vantage (BD Bioscience): CD34−Lin−CD45− and CD34−Lin−CD45+, CD34−Lin−CD45−CD133−, CD34−Lin−CD45−CD133+, CD34−Lin−CD45+CD133−, and CD34−Lin−CD45+CD133+. Negative controls were isotype-matched irrelevant mAbs (BD Bioscience). Dead cells were excluded from cytometric analysis by propidium iodide staining.
LTC-HE of CD34−Lin−CD45− and CD34−Lin−CD45+ cells
CD34−Lin−CD45− and CD34−Lin−CD45+ cells (1 × 103 to 1 × 104) were added to cultures of MS-5 murine stromal cells as previously described, with some modifications.14,15 In particular, to support the development of endothelial together with hematopoietic lineages we added to the previously described cytokine cocktail stromal-derived factor (SDF; 10 ng/mL), vascular endothelial grow factor (VEGF; 10 ng/mL), and IL-16 (10 ng/mL, all from PeproTech), based on our previous studies16,17 and preliminary experiments.
LTC-IC-HE culture of CD34−Lin−CD45−CD133−, CD34−Lin−CD45−CD133+, CD34−Lin−CD45+CD133−, and CD34−Lin−CD45+CD133+ cells
The presence of LTC-initiating cells (ICs) was assessed by limiting dilution analysis of freshly isolated CD34−Lin−CD45−CD133−, CD34−Lin−CD45−CD133+, CD34−Lin−CD45+CD133−, and CD34−Lin−CD45+CD133+ cells on MS-5 murine stromal feeder layers for 6 weeks, as previously described15 and with the same modifications described for LTC-HEs. Cocultures were initiated by seeding 1-10-100 cells/well with the use of an automated cell deposition unit device on a FACSVantage apparatus (BD Bioscience). Quantitative analysis of precursor frequencies in limiting dilution assays was performed by the maximum likelihood solution method with the use of L-CALC software (StemCell Technologies).
Confocal microscopy of cells obtained from LTC-IC-HE cultures
Cells grown in LTC-HE conditions for 6 weeks in 2-well chamber slides were washed in PBS supplemented with 0.5% BSA. Cells were then indirectly labeled with a primary Ab to human VE-cadherin (eBioscience) for 1 hour at 4°C. After washing in PBS supplemented with 0.5% BSA, samples were incubated with Alexa Fluor 488–conjugated secondary Ab (eBioscience) and ECD/PE-Texas Red–conjugated anti-CD45 (Immunotech) for 1 hour at 4°C. After washing, cells were fixed for 5 minutes with 0.5% paraformaldehyde in PBS. Negative controls were obtained by incubating the samples with isotype-matched IgG controls and were analyzed by confocal microscopy as described as follows.
Flow cytometric analysis of cells obtained in LTC-HEs and LTC-IC-HEs
After 6 weeks of LTC-HEs and LTC-IC-HEs, the cells were harvested and analyzed for the presence of cell surface and intracellular Ags according to standard protocols. The Abs used are described in supplemental data. Cells were analyzed with a FACScan flow cytometer, and data analysis was performed by CellQuest software (BD Bioscience). Cells were electronically gated according to light scatter properties to exclude cell debris.
CFU assay
Highly purified sorted Lin− subpopulations, either freshly isolated or from LTCs, were analyzed for their ability to generate hematopoietic CFU-C colonies (methods are described in supplemental Methods).
Mesenchymal cell culture
Freshly isolated CD34−Lin−CD45− and CD34−Lin−CD45+ cells were cultured in 24-well tissue culture plates in a medium specific for mesenchymal stem cell (MSCs; Mesencult Basal Medium; StemCell Technologies) containing MSC stimulatory supplements (StemCell Technologies).
Evaluation of endothelial progenitor capacity
To ascertain the endothelial progenitor capacity of hPB Lin− subpopulations, freshly isolated CD34−Lin−CD45− and CD34−Lin−CD45+ cells were cultured in conditions commonly used to support the expansion of late-endothelial progenitor cells (late-EPCs).18 To this end, 1-30 × 103 cells were suspended in EGM-2 medium (Cambrex Bio Science) and seeded onto 24-well tissue culture plates precoated with human fibronectin (1 μg/cm2; Sigma-Aldrich). The wells were inspected with an inverted microscope for ≤ 5 weeks.
RT-PCR analysis
Total RNA was isolated from either freshly sorted cells or cells obtained in LTC-IC-HE cultures with the use of a Nano-scale RNA microarray extraction kit (Epicentre Biotechnologies). Complementary DNA was synthesized with SuperScript II reverse transcriptase (Invitrogen). PCR was performed with REDTaq ReadyMix PCR Reaction Mix (Sigma-Aldrich), in a mastercycle gradient PCR (Eppendorf) for 40 cycles (denaturation at 94°C for 45 seconds, annealing at different temperatures according to primers for 45 seconds, and extension at 72°C for 45 seconds). Negative samples were tested in a second PCR, using 3 μL of first PCR as template, and the PCR reactions were performed in the same conditions. Preliminary studies were performed to assess the absence of annealing with the murine cDNA to exclude amplification of MS-5 RNA. The primers and the annealing temperatures used for PCR are listed in supplemental Table 3.
Intra-BM injection of freshly isolated cells
Five-week-old male NOD/Shi-scid/scid (NOD/SCID) mice were obtained from The Jackson Laboratory. All animal experiments were approved by the National Cancer Institute Animal Care and Use Committee and conducted accordingly. All the animals were handled in sterile conditions and maintained in microisolators. Intra-BM injection (IBMI) was performed as previously described.19
SCID-repopulating cell assay
SCID-repopulating cell assay was performed as previously described20 with minor modifications. Freshly isolated CD34−Lin−CD45−CD133− cells suspended in PBS were transplanted into the femural BM (IBMI) of sublethally irradiated mice (250 cGy with the use of a 137Cs-γ irradiator). PBS alone, CD34+ cells, and CD34−Lin−CD45+ cells were transplanted into control mice. The number of cells injected ranged from 1 × 102 to 8 × 103 cells per mouse. The mice were killed 6-8 weeks after transplantation, and BM cells were isolated by repeatedly flushing the pair of femora, tibiae, and humeri of each mouse with α-medium containing 10% FCS. After 8 weeks, the presence of human CD45+ cells in the murine BMs was analyzed by flow cytometry. SCID-repopulating cell assay was scored as positive when > 0.1% of total murine BM cells stained positive for human CD45. Further characterization of the engrafted cells was obtained by standard flow cytometry of BM suspensions with the use of following anti–human mAbs: PCy5-conjugated anti-CD45; PE-conjugated anti-CD34, CD19; FITC-conjugated anti-CD33 (all from BD Bioscience). Appropriate isotype-matched mAbs were used as controls. Cells were analyzed on a FACSCanto, and data were analyzed with FACSDiva software (BD Bioscience).
NOD/SCID mouse model of tumor angiogenesis
To test whether CD34−Lin−CD45−CD133− cells can give rise to both hematopoietic and endothelial cells in vivo, we used a murine model that has the potential to show the engraftment of human cells belonging to both lineages in the same animal. Four weeks after CD34−Lin−CD45−CD133− cell transplantation, when multilineage human hematopoietic reconstitution had occurred, 10 × 106 murine MOPC315 plasmacytoma cells were injected subcutaneously into the left abdominal quadrant, to evaluate the possible contribution of CD34−Lin−CD45−CD133−-derived endothelial-lineage cells to tumor angiogenesis. After an additional 2-4 weeks, the mice were killed for BM cell isolation and for tumor removal, fixation in 4% paraformaldehyde, and cryopreservation.
Immunohistochemistry and immunofluorescence
Tumor sections were stained with H&E or immunostained with anti–mouse CD31/PECAM mAb (BD PharMingen), anti–human CD31/PECAM mAb (R&D Systems), anti–human CD45 (MyBioSource), and 4′-6′-diamidine-2-phenylindole (DAPI; Invitrogen) and were analyzed by confocal microscopy as previously described.21 Microvessel density in tumor sections was measured with the use of NIH ImageJ software (http://rsb.info.nih.gov/ij/download.html). A ratio of CD31 pixel values to DAPI pixel values was calculated for each section to derive a relative mean CD31 pixel count. Cell immunostaining for Oct4 and Nanog was performed on cells fixed with 4% paraformaldehyde and washed in 0.3% Saponin. After incubation with anti-Oct4 (rabbit polyclonal; Abcam) or with anti-Nanog (mouse monoclonal; BD Biosciences) Abs and washing, the cells were stained with goat anti–mouse Alexa Fluor 488 or goat anti–rabbit Alexa Fluor 555 secondary Abs (both from Invitrogen).
Results
Purification of CD34−Lin−CD45− cells
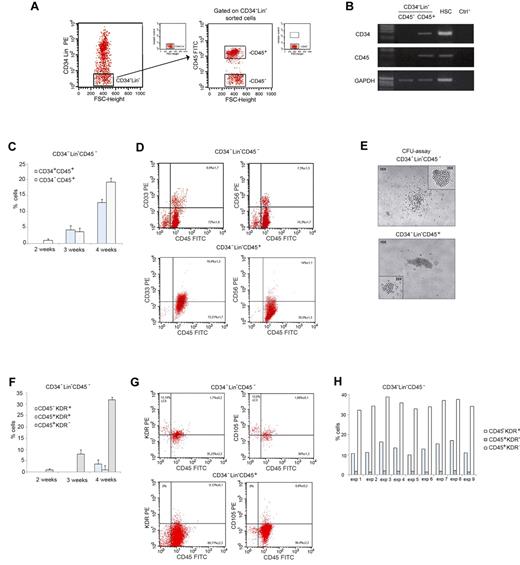
Lin− cells were enriched from human MNCs by magnetic depletion. Starting from this cell population, CD34−Lin−CD45− and CD34−Lin−CD45+ cells were further identified and sorted by FACS (Figure 1A). CD34−Lin−CD45− cells were found at a lower frequency than CD34−Lin−CD45+ cells (0.9% ± 1.3 vs 2.0% ± 2.2% of Lin− MNCs). The purity of sorted CD34−Lin−CD45− cells always exceeded 98%, as assessed by flow cytometry and confirmed by RT-PCR analyses (Figure 1B).
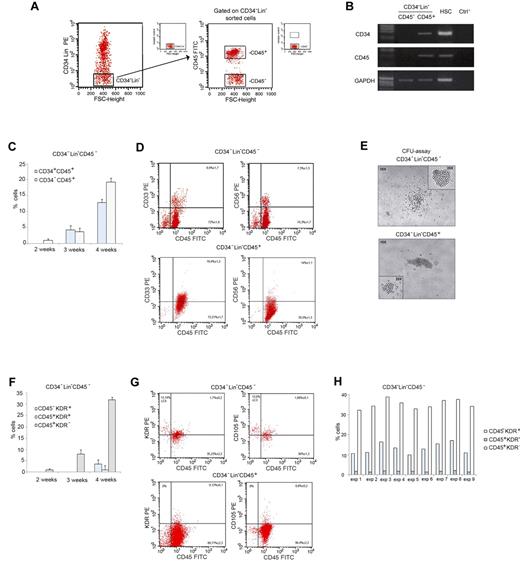
PB CD34−Lin−CD45− cells give rise to hematopoietic and endothelial-lineage cells on LTC-HE. (A) Sorting criteria used to separate fresh adult human CD34−Lin−CD45− and CD34−Lin−CD45+ cells from PB. (B) RT-PCR showing CD34 and CD45 mRNA expression in freshly sorted CD34−Lin−CD45− and CD34−Lin−CD45+ cells, and hematopoietic stem cells (HSCs) as a control. (C) Time-course of immunophenotypic changes in CD34−Lin−CD45− cells during LTC-HE: progressive appearance of CD34+CD45+ and CD34−CD45+ cells. Data shown as mean ± SE of 6 independent experiments. (D) Representative plots showing the expression of hematopoietic markers by CD34−Lin−CD45− compared with CD34−Lin−CD45+ cells after 6 weeks in LTC-HEs. The mean ± SE of 20 independent experiments is shown in each quadrant. (E) After 6 weeks in LTC-HEs, both cell populations showed similar CFU capacity. Represenative bright-field microscopy images (original magnification 40×). (F) Time-course of immunophenotypic changes in CD34−Lin−CD45− cells during LTC-HE: progressive appearance of CD45−KDR+, CD45+KDR+, and CD45+KDR− cells. Data shown as mean ± SE of 6 independent experiments. (G) Representative plots showing that after 6 weeks of culture (under LTC-HE conditions) CD34−Lin−CD45− but not CD34−Lin−CD45+ cells gave rise to CD45+ cells and to CD45− cells coexpressing molecules linked to endothelial development. The mean ± SE of 9 independent experiments is shown in each quadrant. (H) Percentage of CD45−KDR+, CD45+KDR+, and CD45+KDR− cells in LTC-HEs of CD34−Lin−CD45− cells after 6 weeks. The graph shows the results obtained in each experiment. FSC indicates forward scatter.
PB CD34−Lin−CD45− cells give rise to hematopoietic and endothelial-lineage cells on LTC-HE. (A) Sorting criteria used to separate fresh adult human CD34−Lin−CD45− and CD34−Lin−CD45+ cells from PB. (B) RT-PCR showing CD34 and CD45 mRNA expression in freshly sorted CD34−Lin−CD45− and CD34−Lin−CD45+ cells, and hematopoietic stem cells (HSCs) as a control. (C) Time-course of immunophenotypic changes in CD34−Lin−CD45− cells during LTC-HE: progressive appearance of CD34+CD45+ and CD34−CD45+ cells. Data shown as mean ± SE of 6 independent experiments. (D) Representative plots showing the expression of hematopoietic markers by CD34−Lin−CD45− compared with CD34−Lin−CD45+ cells after 6 weeks in LTC-HEs. The mean ± SE of 20 independent experiments is shown in each quadrant. (E) After 6 weeks in LTC-HEs, both cell populations showed similar CFU capacity. Represenative bright-field microscopy images (original magnification 40×). (F) Time-course of immunophenotypic changes in CD34−Lin−CD45− cells during LTC-HE: progressive appearance of CD45−KDR+, CD45+KDR+, and CD45+KDR− cells. Data shown as mean ± SE of 6 independent experiments. (G) Representative plots showing that after 6 weeks of culture (under LTC-HE conditions) CD34−Lin−CD45− but not CD34−Lin−CD45+ cells gave rise to CD45+ cells and to CD45− cells coexpressing molecules linked to endothelial development. The mean ± SE of 9 independent experiments is shown in each quadrant. (H) Percentage of CD45−KDR+, CD45+KDR+, and CD45+KDR− cells in LTC-HEs of CD34−Lin−CD45− cells after 6 weeks. The graph shows the results obtained in each experiment. FSC indicates forward scatter.
CD34−Lin−CD45− cells are not MSCs
To investigate the nature of CD34−Lin−CD45− cells, we first examined their MSC capacity in vitro. In 5 independent experiments, we observed that CD34−Lin−CD45− cells did not adhere to plastic in specific MSC medium and died after 3 days, showing that CD34−Lin−CD45− cells do not contain MSCs (data not shown).
CD34−Lin−CD45− cells do not show hematopoietic progenitor capacity in short-term cultures
In the first series of experiments, we examined whether CD34−Lin−CD45− cells may display hematopoietic progenitor capacity as determined by the CFU assay.22 As expected, after 7-14 days of culture no hematopoietic CFUs were visualized, thus showing that the CD34−Lin−CD45− cells do not contain hematopoietic progenitors (data not shown).
CD34−Lin−CD45− cells do not have prompt endothelial progenitor capacity but show long-term survival in endothelial growth medium EGM-2
We also examined whether CD34−Lin−CD45− cells show endothelial progenitor capacity as determined by the generation of late-EPCs.18 To this end, freshly purified CD34−Lin−CD45− and CD34−Lin−CD45+ cells were cultured in EGM-2, commonly used to support the generation of late-EPC colonies. No late-EPC colonies originated, starting from either CD34−Lin−CD45− or CD34−Lin−CD45+ cells. However, the CD34−Lin−CD45− cells survived ≤ 5 weeks in EGM-2 medium, whereas the CD34−Lin−CD45+ cells died within a few days. Because EGM-2 supports the growth of endothelial but not hematopoietic cells,23 it may be speculated that the long-term survival of CD34−Lin−CD45− but not CD34−Lin−CD45+ cells is suggestive of a possible endothelial commitment of the CD34−Lin−CD45− cell population.
CD34−Lin−CD45− but not CD34−Lin−CD45+ cells give rise to hematopoietic and endothelial lineage cells on long-term coculture with MS-5 murine stromal cells
To further investigate the nature of CD34−Lin−CD45− cells and to compare them with CD34−Lin−CD45+ cells, we designed in vitro conditions that may allow the simultaneous differentiation of human primitive progenitor cells into both the hematopoietic and endothelial lineages. To this end, CD34−Lin−CD45− and CD34−Lin−CD45+ cells were cocultured with MS-5 murine stromal cells, that we had previously demonstrated can support the differentiation of CD34+CD38low LTC-ICs into myeloid and lymphoid hematopoietic cells.14 In the current study, to allow endothelial cell differentiation, the standard hematopoietic conditions used to differentiate myeloid, natural killer (NK), and B-lymphoid cells were modified. In particular, we added VEGF, because it promotes the growth and differentiation of endothelial cells, SDF and IL-16, because they may promote stem cell proliferation, as previously suggested by us and others16,17,24,25 and as observed in preliminary experiments.
CD34−Lin−CD45− and CD34−Lin−CD45+ cells were grown in LTC-HE conditions for 6 weeks and then compared for cell proliferation as well as for differentiation toward the hematopoietic and endothelial lineages. The total number of cells obtained by LTC-HE of CD34−Lin−CD45− cells was similar to the number obtained from CD34−Lin−CD45+ cells. In LTC-HE conditions, CD34−Lin−CD45− cells reproducibly gave rise to both CD45+ and CD45− cells. Figure 1C displays the kinetics of immunophenotypic changes in CD34−Lin−CD45− cells during LTC-HE, showing that CD45 expression was detectable after 2 weeks (1.0% ± 0.5%) and progressively increased with time. After 3 weeks, a substantial proportion of CD45+ cells also expressed the stem cell marker CD34+ (4.3% ± 1.3%). As shown in Figure 1D, after 6 weeks the CD34−Lin−CD45− cells gave rise to a predominant population of CD45+ cells, a proportion of which also expressed the myeloid marker CD33 (8.9% ± 1.7%) and the NK marker CD56 (7.3% ± 1.3%) but not the CD19 lymphocyte cell marker (not shown). In addition, cultured CD34−Lin−CD45− and CD34−Lin−CD45+ cells displayed a similar CFU capacity. CFUs derived from CD34−Lin−CD45− cells were similar in structure and phenotype to those derived from the CD34−Lin−CD45+ hematopoietic cells (Figure 1E). Therefore, although surrogate in vitro assays showed intrinsic differences in hematopoietic progenitor differentiation, the results obtained after culture under LTC-HE conditions suggested that CD34−Lin−CD45− and CD34−Lin−CD45+ cells possess a similar hematopoietic progenitor potential.
To further characterize the cells obtained from LTC-HE, we analyzed their surface expression of KDR and CD105, 2 molecules that are expressed during early hematopoietic development as well as in cells of endothelial lineage.18,26 KDR expression was detected on the surface of CD34−Lin−CD45− cells cultured for 4 weeks (Figure 1F). After 6 weeks (Figure 1G), KDR and CD105 expression was observed on CD45+ cells derived from LTC-HE of CD34−Lin−CD45− and of CD34−Lin−CD45+cells, possibly indicating a recent hematopoietic commitment. KDR and CD105 were also observed on CD45− cells derived from CD34−Lin−CD45− but not on CD34−Lin−CD45+ cells, possibly indicating their development toward the endothelial lineage.27,28 As summarized in Figure 1H, the proportion of CD45−KDR+ cells among nucleated cells ranged from 10.0% to 17.7% in cultures derived from CD34−Lin−CD45− cells. By contrast, CD45−KDR+ cells were absent from cultures derived from CD34−Lin−CD45+ cells. MS-5 murine stromal cells cultured alone in parallel control wells also lacked the expression of KDR and CD105 (not shown).
All together, the results obtained from LTC-HEs indicate that CD34−Lin−CD45− cells represent a unique population of hPB cells endowed with the ability to give rise to CD45+CD33+ and CD45+CD56+ hematopoietic progenitor cells, as well as to CD45−KDR+ and CD45−CD105+ cells that are committed to endothelial development.
Single CD34−Lin−CD45−CD133− cells give rise to hematopoietic and endothelial progenitors on long-term coculture with MS-5 murine stromal cells
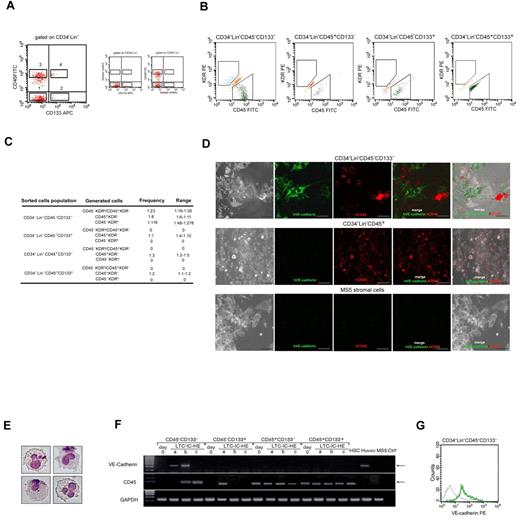
To investigate whether CD45+ hematopoietic progenitor cells and CD45− endothelial-committed cells generated from LTC-HE of CD34−Lin−CD45− cells derived from a common precursor or from distinct progenitor cells, we applied the LTC-HE system to study the phenomenon at the single-cell level. Moreover, because hematopoietic stem cells express CD133 on their surface, although late-EPCs have been shown to derive from CD133− precursors,29,30 we included CD133 expression among the criteria for cell sorting of the initial PB populations to be cultured. Therefore, in 5 independent experiments, CD34−Lin−CD45−CD133−, CD34−Lin−CD45−CD133+, CD34−Lin−CD45+CD133−, and CD34−Lin−CD45+CD133+ cells were sorted as shown in Figure 2A, starting from Lin− human MNCs. The frequency of the 4 sorted cell populations was 0.6 ± 1.35, 0.3 ± 0.18, 1.9 ± 2.1, and 0.1 ± 0.04, respectively, and their purity always exceeded 98%, as assessed by flow cytometry. We detected no correlation between the frequencies of the above-mentioned populations in the blood and donor age or sex. Each sorted population was cultured in LTC-IC-HE cultures by seeding at limiting dilution. In each experiment, 60 wells of each population were seeded. After 6 weeks, all the wells with a sufficient number of cells were harvested and individually examined for the presence of cells expressing CD45 and/or KDR (Figure 2B). The CD34−Lin−CD45−CD133− population was the only one able to give rise, on LTC-IC-HE culture, to CD45−KDR+ cells; it also had the unique ability to give rise, starting from a single cell, to a double population of CD45−KDR+ and CD45+KDR− cells. As summarized in Figure 2C, the results obtained by Poisson analysis of 5 independent experiments indicated that the frequency of CD34−Lin−CD45−CD133− cell precursors capable of giving rise to both CD45−KDR+ and CD45+KDR− cells was 2%; the frequencies of CD45−KDR+ cells only and CD45+KDR− cells only were 0.5% and 5%, respectively. By contrast, CD34−Lin−CD45−CD133+, CD34−Lin−CD45+CD133−, and CD34−Lin−CD45+CD133+ cells gave rise exclusively to populations containing CD45+KDR− cells. One representative experiment is shown in Table 1.
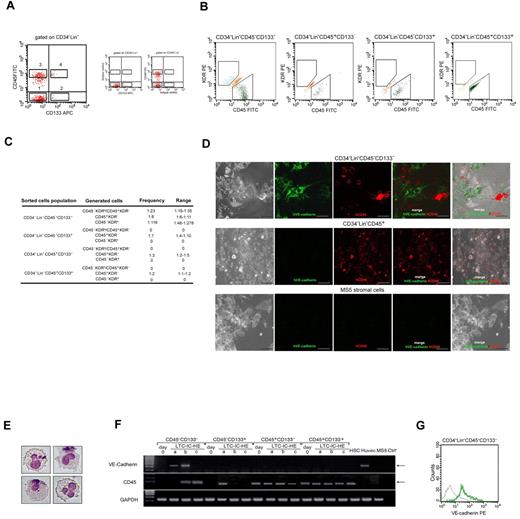
Single CD34−Lin−CD45−CD133− cells give rise to hematopoietic and endothelial lineage cells on LTC-IC-HE culture. (A) Strategy used to sort human CD34−Lin−CD45−CD133− (1), CD34−Lin−CD45−CD133+ (2), CD34−Lin−CD45+CD133− (3), and CD34−Lin−CD45+CD133+ (4) cells. (B) Flow cytometric analysis showing that after 6 weeks in LTC-IC-HEs only CD34−Lin−CD45−CD133− cells gave rise, starting from a single cell, to both CD45−KDR+ (endothelial) and CD45+KDR− (hematopoietic) cells. (C) Poisson analysis of 5 independent experiments, showing the frequency of wells containing CD45+KDR− only, CD45−KDR+ only, or CD45+KDR− and CD45−KDR+ cells, obtained after LTC-IC-HE of the 4 distinct sorted cell populations. (D) Confocal images of cells derived from single CD34−Lin−CD45−CD133− cells after 6-week culture (under LTC-IC-HE conditions). Round CD45+ (hematopoietic-like) and spindle-shaped VE-cadherin+ (endothelial-like) cells in intimate contact with MS-5 murine stromal cells are shown. (E) May-Grunwald-Giemsa staining showing typical myeloid cells generated in CFU assay of LTC-IC-HEs started from single CD34−Lin−CD45−CD133− cells. Original magnification 40×. (F) RT-PCR showing the expression of the hematopoietic CD45 marker and the endothelial VE-cadherin marker in the 4 sorted cell populations at day 0 and after 6 weeks in LTC-IC-HE. Three representative LTC-IC-HE wells (a-c) per cell population after 6 weeks are shown. Only the endothelial-positive control HUVEC line and LTC-IC-HE wells derived from CD34−Lin−CD45−CD133− contained cells expressing VE-cadherin. The concomitant expression of VE-cadherin and CD45 in a single well indicated that, starting from a single cell, CD34−Lin−CD45−CD133− cells gave rise to cells of both endothelial and hematopoietic lineages. (G) Representative flow cytometric histogram showing the expression of VE-cadherin (green line) by cells derived from CD34−Lin−CD45−CD133− single cells (gray line indicates isotype control). At the end of the culture, 11 of 60 seeded wells contained a sufficient number of cells for analysis. Of these 11 wells, 3 tested positive for VE-cadherin expression. APC indicates allophycocyanin.
Single CD34−Lin−CD45−CD133− cells give rise to hematopoietic and endothelial lineage cells on LTC-IC-HE culture. (A) Strategy used to sort human CD34−Lin−CD45−CD133− (1), CD34−Lin−CD45−CD133+ (2), CD34−Lin−CD45+CD133− (3), and CD34−Lin−CD45+CD133+ (4) cells. (B) Flow cytometric analysis showing that after 6 weeks in LTC-IC-HEs only CD34−Lin−CD45−CD133− cells gave rise, starting from a single cell, to both CD45−KDR+ (endothelial) and CD45+KDR− (hematopoietic) cells. (C) Poisson analysis of 5 independent experiments, showing the frequency of wells containing CD45+KDR− only, CD45−KDR+ only, or CD45+KDR− and CD45−KDR+ cells, obtained after LTC-IC-HE of the 4 distinct sorted cell populations. (D) Confocal images of cells derived from single CD34−Lin−CD45−CD133− cells after 6-week culture (under LTC-IC-HE conditions). Round CD45+ (hematopoietic-like) and spindle-shaped VE-cadherin+ (endothelial-like) cells in intimate contact with MS-5 murine stromal cells are shown. (E) May-Grunwald-Giemsa staining showing typical myeloid cells generated in CFU assay of LTC-IC-HEs started from single CD34−Lin−CD45−CD133− cells. Original magnification 40×. (F) RT-PCR showing the expression of the hematopoietic CD45 marker and the endothelial VE-cadherin marker in the 4 sorted cell populations at day 0 and after 6 weeks in LTC-IC-HE. Three representative LTC-IC-HE wells (a-c) per cell population after 6 weeks are shown. Only the endothelial-positive control HUVEC line and LTC-IC-HE wells derived from CD34−Lin−CD45−CD133− contained cells expressing VE-cadherin. The concomitant expression of VE-cadherin and CD45 in a single well indicated that, starting from a single cell, CD34−Lin−CD45−CD133− cells gave rise to cells of both endothelial and hematopoietic lineages. (G) Representative flow cytometric histogram showing the expression of VE-cadherin (green line) by cells derived from CD34−Lin−CD45−CD133− single cells (gray line indicates isotype control). At the end of the culture, 11 of 60 seeded wells contained a sufficient number of cells for analysis. Of these 11 wells, 3 tested positive for VE-cadherin expression. APC indicates allophycocyanin.
To gain insight into the possible endothelial commitment of CD45−KDR+ starting from CD34−Lin−CD45−CD133− cells, we examined cell structure of LTC-IC-HE cultures after 6 weeks of incubation. Using confocal microscopy (Figure 2D), we found that only a few wells derived from single CD34−Lin−CD45−CD133− cells contained both round CD45+ and spindle-shaped VE-cadherin+ cells in intimate contact with MS-5 murine stromal cells. With the use of flow cytometry, the same wells contained both CD45+KDR− and CD45−KDR+, thus suggesting that the round and spindle-shaped cells may represent hematopoietic and endothelial progenitors, respectively. Spindle-shaped cells were not observed in wells derived from the other sorted populations, which instead gave rise exclusively to round cells (data not shown).
The ability of CD34−Lin−CD45−CD133− cells and the other sorted populations to generate hematopoietic progenitors in LTC-IC-HE cultures was confirmed by CFU assays. Typical myeloid cells obtained in CFU culture starting from CD34−Lin−CD45−CD133− cells are shown in Figure 2E.
To further evaluate the ability of CD34−Lin−CD45−CD133− cells to give rise to both hematopoietic and endothelial progenitors, we analyzed the cells obtained after 6 weeks in LTC-IC-HE cultures for the expression of VE-cadherin, which is selectively expressed in endothelial cells,31 and CD45.32 By RT-PCR, we demonstrated that the cells contained in wells derived from CD34−Lin−CD45−CD133− cells expressed either CD45 only or VE-cadherin only or both CD45 and VE-cadherin (Figure 2F), consistent with the development of either CD45+KDR− cells only or CD45−KDR+ cells only or both CD45+KDR− and CD45−KDR+ cells in those wells. Moreover, the observation that cells contained in individual wells derived from single CD34−Lin−CD45−CD133− cells did coexpress CD45 and VE-cadherin confirmed the bilineage hemangioblast potential of CD34−Lin−CD45−CD133− cells. As expected, no expression of VE-cadherin was detected in the cells contained in wells derived from the other sorted populations that were found to express CD45 only, consistent with their ability to generate only hematopoietic progenitors. Representative RT-PCR analyses of the cells contained in wells obtained by LTC-IC-HE culture of the 4 sorted populations are shown in Figure 2F. Expression of VE-cadherin protein on the surface of cells derived from CD34−Lin−CD45−CD133− cells was confirmed by flow cytometry (Figure 2G).
Molecular characterization of CD34−Lin−CD45−CD133− cells shows a stem cell profile compatible with both hematopoietic and endothelial potentials
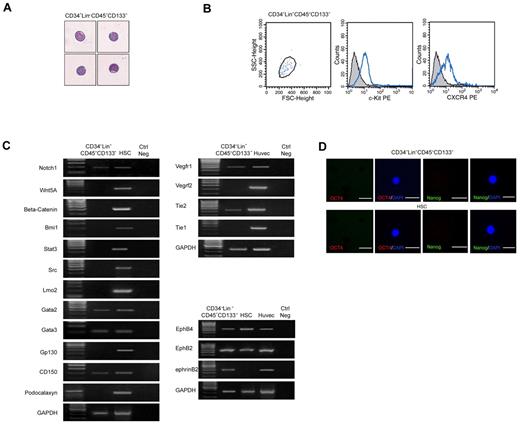
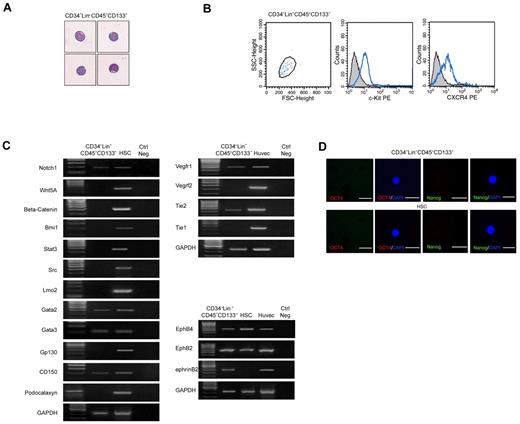
The unique ability of CD34−Lin−CD45−CD133− cells to differentiate into both hematopoietic and endothelial cells prompted us to investigate their molecular profile. Highly purified CD34−Lin−CD45−CD133− cells (≥ 99% purity, as assessed by flow cytometry) that showed blastoid structure characterized by scanty cytoplasm and lack of granules (Figure 3A), expressed c-Kit and CXCR4 (Figure 3B), in accordance with their progenitor nature. To investigate their gene expression signature, RNA from freshly sorted cells was subjected to global amplification of mRNA transcripts.33 The amplified PCR products were analyzed for expression of a wide range of molecules that are either expressed in stem cells or in progenitor cells endowed with both hematopoietic and endothelial potential. A complete list of the primer and experimental conditions for PCR analysis of all the markers is provided in supplemental Table 3. As shown in Figure 3C, gene expression analysis indicated that CD34−Lin−CD45−CD133− cells expressed the stem cell markers Notch1, Gata2, Gata3, and CD150, whereas they lacked expression of Wnt5A, β-catenin, Bmi1, Stat3, Src, Lmo2, or Gp130. By immunofluorescence we found that the CD34−Lin−CD45−CD133− cells do not express Oct4 or Nanog proteins (Figure 3D). These results indicate that the CD34−Lin−CD45−CD133− cells express selected phenotypic markers typical of pluripotent cells.
Freshly isolated CD34−Lin−CD45−CD133− cells have a stem cell profile compatible with hematopoietic and endothelial potential. (A) May-Grunwald-Giemsa staining showing that freshly isolated CD34−Lin−CD45−CD133− cells have blastoid structure typical of stem cells. (B) Flow cytometric analysis showing that freshly isolated CD34−Lin−CD45−CD133− cells express c-Kit and CXCR4, according to their progenitor nature. (C) RT-PCR showing that freshly isolated CD34−Lin−CD45−CD133− cells express selected stem cell markers as well as markers shared by progenitors of hematopoietic and endothelial lineages. HSCs and primary human endothelial cells (HUVECs) were used as positive controls. One representative of 8 independent experiments is shown. (D) Failure to detect expression of Oct4 (red) and Nanog (green) in freshly isolated CD34−Lin−CD45−CD133− cells and CD34+ HSCs, as assessed by confocal microscopy after immunostaining with specific Abs. Nuclei (blue) are stained with DAPI. iPSC served as a positive control (results not shown). FSC indicates forward scatter. Original magnification in panels A and D is 20×.
Freshly isolated CD34−Lin−CD45−CD133− cells have a stem cell profile compatible with hematopoietic and endothelial potential. (A) May-Grunwald-Giemsa staining showing that freshly isolated CD34−Lin−CD45−CD133− cells have blastoid structure typical of stem cells. (B) Flow cytometric analysis showing that freshly isolated CD34−Lin−CD45−CD133− cells express c-Kit and CXCR4, according to their progenitor nature. (C) RT-PCR showing that freshly isolated CD34−Lin−CD45−CD133− cells express selected stem cell markers as well as markers shared by progenitors of hematopoietic and endothelial lineages. HSCs and primary human endothelial cells (HUVECs) were used as positive controls. One representative of 8 independent experiments is shown. (D) Failure to detect expression of Oct4 (red) and Nanog (green) in freshly isolated CD34−Lin−CD45−CD133− cells and CD34+ HSCs, as assessed by confocal microscopy after immunostaining with specific Abs. Nuclei (blue) are stained with DAPI. iPSC served as a positive control (results not shown). FSC indicates forward scatter. Original magnification in panels A and D is 20×.
CD34−Lin−CD45−CD133− cells also lacked expression of podocalaxyn, a marker of adult hematopoietic stem cells34 that we found expressed in our control HSCs. They did not express Tie-1 or KDR, which was expressed after 6 weeks in LTC-IC-HE culture. In addition, as shown in Figure 3D, CD34−Lin−CD45−CD133− cells expressed several transcripts that are shared by progenitors of both hematopoietic and endothelial lineages and are involved in vascular development and angiogenesis-related signaling pathways, namely Vegfr1, Tie2, EphB4, EphB2, and ephrinB2, further supporting the bilineage hemangioblast potential of CD34−Lin−CD45−CD133− cells.
CD34−Lin−CD45−CD133− cells generate cells of both hematopoietic and endothelial lineages in mice
To establish the hematopoietic and endothelial differentiation potential of CD34−Lin−CD45−CD133− cells in vivo, we transplanted these freshly purified human cells in T-cell immunodeficient NOD/SCID mice with the use of the IBMI technique.19 Before transplantation, the recipient mice were irradiated to deplete the host's hematopoietic compartment and to facilitate engraftment. Moreover, to facilitate the detection of a possible contribution of CD34−Lin−CD45−CD133− cells that were transplanted to tumor endothelium, the mice were inoculated subcutaneously with the syngeneic MOPC315 (10 × 106 cell/mouse) plasmacytoma cells 2 weeks before being killed. With the use of this model, we demonstrated that CD34−Lin−CD45−CD133− cells do contribute to blood as well as blood vessel formation in vivo.
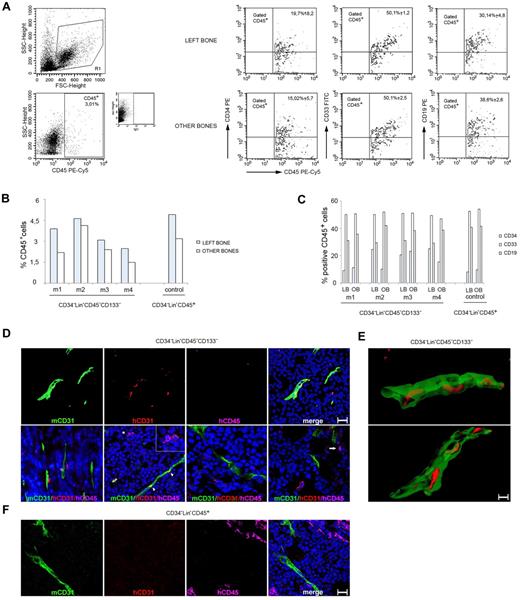
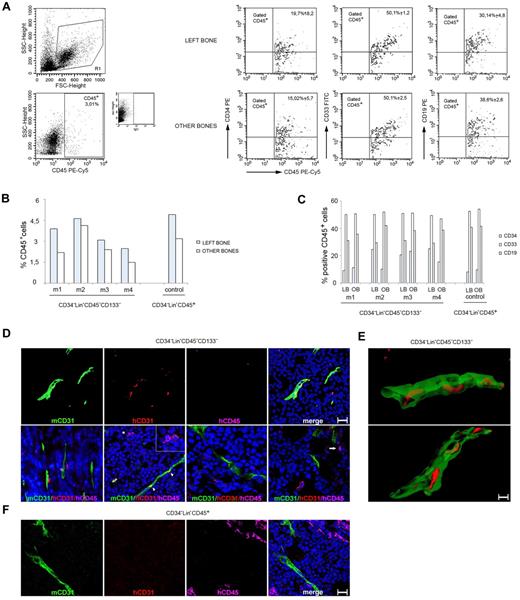
Four mice were transplanted with CD34−Lin−CD45−CD133− cells, one control mouse with CD34−Lin−CD45+ cells, and all of them were killed 6-8 weeks thereafter. As shown in Figure 4A-C, all 5 mice that received a transplant showed evidence of human CD45+cell engraftment within the BM of the femur originally injected and from the combined BM from the contralateral femur and tibias. The presence of human cells at distant sites from the injection provides evidence that the transplanted human cells had disseminated from the original site and homed to other BM compartments. By flow cytometry, the human CD45+ cells coexpressed to varying degrees the human CD34, CD33, and CD19 markers, providing evidence for the presence of hematopoietic precursors (CD34) and differentiation in vivo into the myeloid (CD33) and lymphoid (CD19) lineages. All mice developed subcutaneous tumors. We removed the tumors and looked for evidence of human cell engraftment with the use of human-specific mAbs to CD31 and CD45 and with a mouse-specific Ab to CD31. By confocal microscopy (Figure 4D) we observed that human CD31+/CD45− cells colocalized with mouse CD31+ tumor vessels and that human CD45+/CD31− cells were either proximal or distant from mouse CD31+ vessels. This was observed in the tumors of 2 of 4 mice injected with CD34−Lin−CD45−CD133− cells, which displayed a tumor vessel density of 4.0 ± 1.9 human CD31+ cells/field (1 μm2). By clipping and rotating 3-dimensional confocal images (Figure 4E) we observed that the human CD31+ cells were components of the vessel wall within the mouse tumor vasculature. In the control mouse injected with CD34−Lin−CD45+ cells, we observed clusters of human CD45+ cells but not human CD31+ cells (Figure 4F; supplemental Figure 1). The degree of tumor vascularization was similar in the control mice (mean relative CD31 pixel count, 3.3) and in the mice injected with CD34−Lin−CD45−CD133− cells (mean relative pixel count, 3.9). Together, these results provide evidence that human CD34−Lin−CD45−CD133− cells can differentiate into hematopoietic and endothelial cells when transplanted into mice.
Adult human CD34−Lin−CD45−CD133− cells give rise to both hematopoietic and endothelial lineage cells in NOD/SCID mice. (A) Example of multilineage human hematopoietic (CD45+) reconstitution of BM cells from the IBMI of NOD/SCID recipients receiving CD34−Lin−CD45−CD133− cells that include CD34 (stem/progenitor hematopoietic cells), CD33 (myeloid), and CD19 (B lymphoid) lineage markers. Representative results from 4 injected mice. (B) Frequency of cells positive for human CD45 staining in the BM of mice that received a transplant. Graph showing the results obtained in each experiment; m indicates mouse. (C) Coexpression of human CD34, CD33, and CD19 by CD45+ in BM cells from the same mice. Graph showing the results obtained in each experiment; m indicates mouse; LB, left bone; and OB, other bones. (D) Mouse CD31, human CD31, and human CD45 immunostaining of MOPC315 tumor tissues from mice injected 4 weeks earlier with 2 × 102 CD34−Lin−CD45−CD133− cells. (Top) Confocal microscopy images (3-dimensional rendering) showing the colocalization of mouse tumor vessels (mouse CD31+) and human CD31+ cells, which fail to costain for human CD45. (Bottom) Confocal microscopy images showing human CD31+/CD45− and human CD45+/CD31− cells proximal or distant from mouse CD31+ vessels. The asterisk points to a human CD45+ cell (pink) adjacent to a human CD31+ cell (red); the cells are shown at a higher magnification in the inset. The arrowheads point to the colocalization (yellow) of mouse CD31 (green) and human CD31 (red) immunostaining. The arrow in the far right panel points to a human CD45+/CD31− cell. Scale bars = 50 μm. (E) Images from clipping and rotation of a 3-dimensional confocal image of a tumor vessel from a mouse injected 4 weeks earlier with CD34−Lin−CD45−CD133− cells showing the spatial relationship between the murine endothelial cells (green, mCD31+) and the human endothelial cells (red, hCD31+) contributing to the vessel wall. Scale bar = 50 μm. (F) MOPC315 tumor tissue from a mouse injected with 1 × 103 CD34−Lin−CD45+ cells shows vascular structures expressing mouse but not human CD31. Clusters of human CD45+ cells are observed. Results from immunostaining with specific anti–mouse CD31 (green), anti–human CD31 (red), and anti–human CD45 (pink) are shown. Nuclei are detected by DAPI staining (blue). FSC indicates forward scatter.
Adult human CD34−Lin−CD45−CD133− cells give rise to both hematopoietic and endothelial lineage cells in NOD/SCID mice. (A) Example of multilineage human hematopoietic (CD45+) reconstitution of BM cells from the IBMI of NOD/SCID recipients receiving CD34−Lin−CD45−CD133− cells that include CD34 (stem/progenitor hematopoietic cells), CD33 (myeloid), and CD19 (B lymphoid) lineage markers. Representative results from 4 injected mice. (B) Frequency of cells positive for human CD45 staining in the BM of mice that received a transplant. Graph showing the results obtained in each experiment; m indicates mouse. (C) Coexpression of human CD34, CD33, and CD19 by CD45+ in BM cells from the same mice. Graph showing the results obtained in each experiment; m indicates mouse; LB, left bone; and OB, other bones. (D) Mouse CD31, human CD31, and human CD45 immunostaining of MOPC315 tumor tissues from mice injected 4 weeks earlier with 2 × 102 CD34−Lin−CD45−CD133− cells. (Top) Confocal microscopy images (3-dimensional rendering) showing the colocalization of mouse tumor vessels (mouse CD31+) and human CD31+ cells, which fail to costain for human CD45. (Bottom) Confocal microscopy images showing human CD31+/CD45− and human CD45+/CD31− cells proximal or distant from mouse CD31+ vessels. The asterisk points to a human CD45+ cell (pink) adjacent to a human CD31+ cell (red); the cells are shown at a higher magnification in the inset. The arrowheads point to the colocalization (yellow) of mouse CD31 (green) and human CD31 (red) immunostaining. The arrow in the far right panel points to a human CD45+/CD31− cell. Scale bars = 50 μm. (E) Images from clipping and rotation of a 3-dimensional confocal image of a tumor vessel from a mouse injected 4 weeks earlier with CD34−Lin−CD45−CD133− cells showing the spatial relationship between the murine endothelial cells (green, mCD31+) and the human endothelial cells (red, hCD31+) contributing to the vessel wall. Scale bar = 50 μm. (F) MOPC315 tumor tissue from a mouse injected with 1 × 103 CD34−Lin−CD45+ cells shows vascular structures expressing mouse but not human CD31. Clusters of human CD45+ cells are observed. Results from immunostaining with specific anti–mouse CD31 (green), anti–human CD31 (red), and anti–human CD45 (pink) are shown. Nuclei are detected by DAPI staining (blue). FSC indicates forward scatter.
Discussion
Although the first evidence supporting the existence of the hemangioblast, a common precursor to hematopoietic and endothelial lineages, dates back to the first decades of the last century,1,2 the formal proof providing the molecular identity of the hemangioblast in adult life is still unsatisfactory. The reason for this probably resides in the difficulty to get an adequate model to study this cell.
In this study we developed and optimized an in vitro model able to support the growth and differentiation of cells toward both hematopoietic and endothelial lineages. To this aim, we modified a LTC-culture system that we had previously developed to study hematopietic precursors.14,15,17 In accordance with recent studies,35 to increase cell expansion, we added SDF to the LTC culture system. We also added IL-16 that we had previously demonstrated to stimulate the expansion of CD34+ hematopoietic stem cells.16,17 Indeed, in preliminary experiments we observed that the addition of SDF-1 and IL-16 to the cytokine cocktail of the LTC system significantly improved cell proliferation (data not shown). Finally, we added VEGF to support the growth and differentiation toward endothelial in addition to hematopoietic lineage.36,37 Using this system, we demonstrated that not only CD34−Lin−CD45+ but also CD34−Lin−CD45− cells proliferated and differentiated into hematopoietic cells, giving rise to both myeloid and lymphoid NK cells. Note that CD34−Lin−CD45− cells lacked colony-forming ability immediately after sorting, denoting a stem cell like rather than hematopoietic progenitor-like behavior of these cells.38,39 Similarly, freshly purified CD34−Lin−CD45− cells failed to give rise to late-EPC colonies when cultured in the endothelial-specific medium, but, unlike CD34−Lin−CD45+ cells, they survived for ≤ 5 weeks, possibly denoting a stem rather than endothelial progenitor-like behavior. The long-term survival in endothelial-promoting conditions was specific, because the same cells died shortly after culture in mesenchymal-promoting conditions. Accordingly, on LTC-HE conditions CD34−Lin−CD45− cells gave rise not only to hematopoietic progenitors but also to cells with an immunophenotype compatible with endothelial lineage. The observation that CD34+KDR+ cells can give rise to hematoendothelial colonies under different experimental conditions3 suggests that there could be redundancy in human hemangioblast populations postnatally. Alternatively, the CD34−Lin−CD45−CD133− cell population and the CD34+KDR+ cells may represent different stages of differentiation of the same precursor.
We next applied the LTC-HE system at the single-cell level (LTC-IC-HEs) to investigate whether the hematopoietic progenitor and endothelial cells generated from LTC-HE of CD34−Lin−CD45− cells derived from a common precursor or from distinct progenitor cells. To allow a more precise definition of the PB cells giving rise to both hematopoietic and endothelial lineages, we included the expression of CD133 among the criteria for cell sorting of the starting populations of LTC-IC-HE cultures. Several studies indicate that CD133 is expressed by stem and hematopoietic progenitor cells.40 The expression of CD133 by progenitors giving rise to endothelial cells is more controversial.6,29,30,41 With the use of the LTC-IC-HE system we could successfully demonstrate that CD34−Lin−CD45−CD133− cells plated as single cells give rise to both CD45+KDR− hematopoietic cells and CD45−KDR+ endothelial cells in the same well. The hematopoietic lineage of CD45+KDR− cells was proven by generation of myeloid cells in CFU assay. The endothelial lineage of CD45−KDR+ cells was shown by the expression of KDR and VE-cadherin in the absence of CD45 expression.27,31 The detailed molecular characterization of freshly purified cells further supported that CD34−Lin−CD45−CD133− cells, endowed with the ability to give rise to cells of both hematopoietic and endothelial lineages, may indeed represent putative adult hemangioblasts. In fact these cells expressed c-Kit similarly to an isolated murine aorta-gonads-mesonephros–related CD34−c-Kit+ cell subset that has been shown to be able to generate hemangioblast colonies in vitro.42 Moreover, CD34−Lin−CD45−CD133− cells expressed CXCR4, a key molecule in the migration and homing of both hematopoietic stem cells and endothelial progenitors.43-45 This observation is in accordance with a previous study by Jin et al,46 who demonstrated that nonendothelial hematopoietic progenitors, recruited by SDF-1 to promote revascularization, coexpressed KDR and CXCR4 similarly to the CD34−Lin−CD45−CD133− cells described in the present study. A molecular profile that is consistent with the hemangioblast identity of CD34−Lin−CD45−CD133− cells obtained from gene expression analysis of freshly purified cells showed the coexpression of stem cell markers and the expression of molecules shared by hematopoietic and endothelial cells. Particularly intriguing was the finding that CD34−Lin−CD45−CD133− cells do express EphB4, EphB2, and ephrinB2. In fact, over the past decade a key role for Eph receptor tyrosine kinases and their ligands, the ephrins, has emerged from studies on embryonic development.21,47,48 EphB4 and its cognate ligand ephrinB2 play critical roles in determining vascular networks. The former also participates in adult hematopoiesis involving primitive human CD34+, thus suggesting that EphB4− may be required for proper hematopoietic, endothelial, hemangioblast and primitive mesoderm development.49 To evaluate the hemangioblast potential of CD34−Lin−CD45−CD133− cells in vivo, we transplanted freshly purified cells into NOD/SCID mice. We used the IBMI technique of cell transplantation, a method described to improve human hematopoietic cell engraftment in murine BM and successfully used to identify human CD34− HSCs.46,50 We observed, indeed, that CD34−Lin−CD45−CD133− cells generated a significant number of CD45+ progenies in vivo. More importantly, the transplantation study showed for the first time that CD34−Lin−CD45−CD133− cells were able to reconstitute the hematopoietic compartment. To facilitate the detection of endothelial cells of human origin in mice, 2 weeks after cell transplantation the mice were inoculated intraperitoneally with tumor cells to induce a localized site of tumor angiogenesis. The development of this animal model suitable for the study of hematopoietic and endothelial bilineage engraftment allowed us to demonstrate that CD34−Lin−CD45−CD133− cells indeed contribute to blood and blood vessel formation in vivo. In fact, in the same animals in which CD34−Lin−CD45−CD133− cells were able to successfully reconstitute the hematopoietic compartment, peritumoral vessels contained endothelial cells that stained positively with a human-specific anti-CD31 Ab, thus confirming in vivo the bilineage potential of these cells.
All together, the results presented in this study provide the first evidence that CD34−Lin−CD45−CD133− PB cells may act as putative circulating hemangioblasts, endowed with the ability to give raise to multiple hematopoietic lineages as well as endothelial cells in vitro and in vivo. We believe that the identification of CD34−Lin−CD45−CD133− cells as hemangioblast-like cells will encourage further molecular and functional characterization of these cells and the application of novel techniques of continuous in vitro and in vivo imaging that will lead to a better comprehension of the possible physiologic role of CD34−Lin−CD45−CD133− cells in blood and blood vessel maintenance and repair in adult life.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr J. Dennis, G. McMullen, and Tina Kilts for assistance with animal experiments; Dr S. Civilli for advise on immunostaining; Dr A. Iacone for all his support and help; and Dr B. Papp for critically reading the manuscript.
This work was partially supported by “Associazione Italiana contro le Leucemie” (AIL) Pescara.
Authorship
Contribution: E.C. collected data, assembled analysis of data, and wrote the manuscript; S.D.B. provided study material and wrote the manuscript; O.S. and C.B. provided study material; C.R. collected, assembled, and analyzed data; M.S., A.M., and D.M. collected data; G.T. provided study material and analyzed and interpreted data; and A.C.B. provided the study conception and design, assembled data, analyzed and interpreted data, wrote the manuscript, provided financial support, and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anna C. Berardi, Research Stem Cells Laboratory of Transfusion Medicine, Spirito Santo Hospital, Via del Circuito, Pescara 65100, Italy; e-mail: annacberardi@yahoo.it.