Abstract
The serine proteases, neutrophil elastase (HNE) and proteinase 3 (PR3), are aberrantly expressed in human myeloid leukemias. T-cell responses to these proteins have been correlated with remission in patients with chronic myeloid leukemia (CML). Human PR3/HNE-specific CD8+ T cells predominantly recognize a nonameric HLA-A2–restricted T-cell epitope called PR1 which is conserved in both Ags. However, CML patients have CD8+ T cells in peripheral blood recognizing an additional HLA-A2 epitope termed PR2. To assess immunologic properties of these Ags, novel recombinant vaccinia viruses (rVV) expressing PR3 and HNE were evaluated in HLA-A2 transgenic (Tg) mice (HHDII). Immunization of HHDII mice with rVV-PR3 elicited a robust PR3-specific CD8+ T-cell response dominated by recognition of PR2, with minimal recognition of the PR1 epitope. This result was unexpected, because the PR2 peptide has been reported to bind poorly to HLA. To account for these findings, we proposed that HHDII mice negatively selected PR1-specific T cells because of the presence of this epitope within murine PR3 and HNE, leading to immunodominance of PR2-specific responses. PR2-specific splenocytes are cytotoxic to targets expressing naturally processed PR3, though PR1-specific splenocytes are not. We conclude that PR2 represents a functional T-cell epitope recognized in mice and human leukemia patients. These studies are registered at www.clinicaltrials.gov as NCT00716911.
Introduction
The serine proteases human neutrophil elastase (HNE) and proteinase 3 (PR3) are degradative enzymes stored in cytoplasmic azurophilic granules of neutrophils and are involved in degrading engulfed intracellular pathogens and breaking down tissues at inflammatory sites.1 These 2 proteins are aberrantly expressed in human myeloid leukemias such as chronic myeloid leukemia (CML).2 Overexpression of PR3 may be important in maintaining a leukemia phenotype because inhibition of PR3 synthesis by PR3-antisense oligonucleotides halts cell division and drives differentiation of the HL-60 promyelocytic leukemia cell line.3 HNE is also overexpressed in leukemia patients and has been shown to suppress tumorigenic hematopoietic progenitors.2 Thus, both proteins are attractive as vaccine targets.
PR3 and HNE contain a conserved nonameric HLA-A2–restricted T-cell epitope called PR1.4-6 Remarkably, T-cell immunity to PR1 is present in healthy donors at low levels (∼ 1 of 100 000 CD8+ T cells), whereas the level of PR1-specific T cells can be one or 2 logs higher in leukemia patients in remission.6-10 Barrett and colleagues have shown in CML patients undergoing allogeneic HSCT that increased leukemia-associated Ag expression in CD34+ leukemia cells may enhance PR1-specific CTL immune responses which can convey a GVL effect.11 Furthermore, the presence of PR1-specific T cells has been correlated with molecular remission in CML patients.6 Based on these observations, a single PR1 peptide vaccine has been clinically evaluated (reviewed in Molldrem12 and Rezvani and Barrett13 ).
PR1-specific T-cell responses are not universally predictive of long-term CML-free survival after HSCT in individuals expressing HLA-A2.6,8,11 We hypothesized that there may be other CD8+ T-cell epitopes within PR3 and HNE that are recognized in patients with myeloid leukemias. Peptide vaccines such as the PR1 peptide may be limited in clinical efficacy by their induction of a narrowly focused immune response. We investigated whether other epitopes of PR3 and HNE are also immunogenic by expressing differing forms of PR3 and HNE, including full-length and truncated polypeptides, in recombinant vaccinia viruses (rVV). We quantified CD8+ T-cell responses to these Ags in Tg mice expressing HLA-A2 (HHDII). Murine studies were complemented with clinical measurements of PR2 CD8+ T-cell responses that showed strong correlation with remission post-HSCT in CML patients.
Methods
Human subjects
Peripheral blood samples were obtained by venipuncture from CML patients under treatment at City of Hope National Medical Center (COH) after written informed consent in accordance with the Declaration of Helsinki and with approval from the institutional review board. The characteristics of these patients are shown in Table 1. Mononuclear cells were isolated from the blood samples by Ficoll-Hypaque density gradient centrifugation and cryopreserved in 90% FCS (Bio Express) and 10% DMSO (Sigma-Aldrich).
Cell lines
HLA-A2+ T2 cells were obtained from Dr Peter Cresswell (Yale University). T2 cells require exogenous peptide to rescue surface expression of MHC-I.14 K562 genetically modified to express HLA-A2 (K562-A2) and to function as artificial APCs (aAPCs) were obtained from Dr Carl June (University of Pennsylvania).15 A K562-A2 derivative overexpressing PR3 (K562-A2PR3) was developed by electrotransfer of a DNA plasmid expressing full-length codon optimized FLAG-tagged PR3 fused via T2A sequence to a triple fusion of enhanced green fluorescent protein (EGFP), hygromycin phosphotransferase, and thymidine kinase (TK) derived from herpes simplex virus-1. EBV-immortalized B cells (LCL-A2) were derived from HLA-A2 subjects as previously described.16 LCL and K562-A2PR3 were irradiated before use for in vitro propagation of T cells. Table 2 summarizes the key features of the APCs.
Generation of rVVs
We generated a panel of rVV expressing several forms of the granule proteases (Figure 1). rVV-EGFP-PR3 contains a gene-encoding EGFP fused to a human PR3 polypeptide that lacks the N-terminal signal peptide, but retains the pro-dipeptide and the 8-aa C-terminal propeptide sequence. To abolish enzyme activity and facilitate expression, an inactivating mutation changing a serine residue to an alanine at position 176 (S176A) was introduced at 1 of the 3 residues of the catalytic triad characteristic of serine proteases. A second rVV was generated that encoded EGFP fused to a C-terminal truncated PR3 (rVV-EGFP-PR3-T). Finally, 2 rVV expressing HNE were prepared: the first, rVV-EGFP-HNE, being analogous to the rVV-EGFP-PR3 virus, and the second, rVV-HNE, expressing the HNE gene without fusion to EGFP. Expression of the EGFP-PR3, EGFP-PR3-T, and EGFP-HNE fusion proteins was verified by transient transfection of COS cells followed by Western blot analysis. rVV were generated on CV1 cells via homologous recombination followed by screening for the GUS marker gene on chromogenic substrate on TK− cells in the presence of bromodeoxyuridine. All viruses used in this study were expanded on CV1 cells and purified by 36% sucrose density gradient ultracentrifugation.
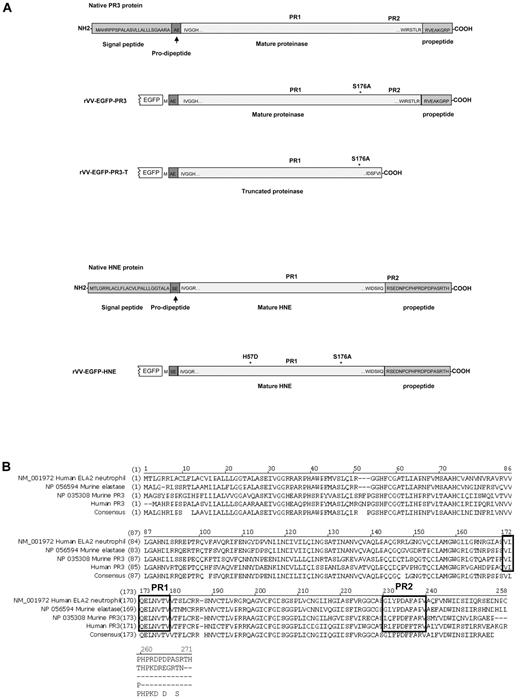
Structures and sequences of PR3 and HNE proteins. (A) Native forms of PR3 and HNE are shown as well as versions engineered for expression as fusions to the C-terminal of EGFP in rVV. Amino acid sequences are shown at the termini of the mature proteases as well as within the signal peptide, pro-dipeptide, and propeptide segments cleaved off during cellular processing (reviewed in Korkmaz et al1 ). The positions of the PR1 and PR2 epitopes are indicated in addition to amino acid substitutions introduced to inactivate the enzymatic activity. (B) Alignment of amino acid sequences and consensus of human and murine proteinase 3 and neutrophil elastase ORFs. The alignment uses the unprocessed forms of the polypeptides with N-terminal signal peptides, prodipeptides, and C-terminal propeptides still present. The regions of the PR1 epitope (completely conserved across the 4 proteins) and the PR2 epitopes (nonconserved) are indicated in black boxes.
Structures and sequences of PR3 and HNE proteins. (A) Native forms of PR3 and HNE are shown as well as versions engineered for expression as fusions to the C-terminal of EGFP in rVV. Amino acid sequences are shown at the termini of the mature proteases as well as within the signal peptide, pro-dipeptide, and propeptide segments cleaved off during cellular processing (reviewed in Korkmaz et al1 ). The positions of the PR1 and PR2 epitopes are indicated in addition to amino acid substitutions introduced to inactivate the enzymatic activity. (B) Alignment of amino acid sequences and consensus of human and murine proteinase 3 and neutrophil elastase ORFs. The alignment uses the unprocessed forms of the polypeptides with N-terminal signal peptides, prodipeptides, and C-terminal propeptides still present. The regions of the PR1 epitope (completely conserved across the 4 proteins) and the PR2 epitopes (nonconserved) are indicated in black boxes.
Mice and immunizations
HLA-A2 Tg mice (HHDII) were obtained from F. Lemonnier (Institut Pasteur, France) and bred and maintained under pathogen-free conditions at the AAALAC-approved animal care facility at COH. All mouse studies were approved by the City of Hope Institutional Animal Care and Use Committee. Ten- to 12-week-old mice were immunized intraperitoneally with 5 × 107 infectious units of various rVV as described in the figure legends.
Peptides
The following peptides were generated in our laboratory as previously described16 : PR1 9-mer (VLQELNVTV), PR2 10-mer (RLFPDFFTRV), PR2 9-mer (LFPDFFTRV), PADRE-PR1 fusion (AKXVAAWTLKAAAVLQELNVTV where X = β-cyclohexylalanine), PADRE-PR2 fusion (AKXVAAWTLKAAARLFPDFFTRV), HLA-A2–restricted CMV pp65495-503 (NLVPMVATV), PADRE-CMV fusion (KSSAKXVAAWTLKAAANLVPMVATV), HLA-B7–restricted CMV IE-188-96 (QIKVRVDMV), and the HIV-1 p17 Gag77-85 epitope (SLYNTVATL). The 256-aa sequence from the PR3 protein was divided into 15-mer stretches that overlap successive peptides by 4 aa, using an online program which excludes impermissible amino acids at the N and C terminus of each 15-mer peptide, based on synthetic consideration (http://www.hiv.lanl.gov/content/sequence/PEPTGEN/Explanation.html). A peptide library derived from PR3 was generated as 66 peptides divided into 4 pools of 16 or 17 peptides per pool. Pool A corresponded to amino acid residues aa6-76 of PR3, pool B = aa65-136, pool C aa125-195, and pool C aa189-256. Peptide pools were subsequently combined into one superpool at a concentration of 200 μg/mL and dissolved in 50% DMSO/water.
Harvesting spleens and ex vivo stimulation using peptides or in vitro stimulation
Three weeks after immunization, spleens were aseptically removed, and splenic suspensions were teased through a sterile nylon mesh using PBS containing 2mM EDTA (pH 7.4). DNase I (120 Kunitz units; Sigma-Aldrich) per milliliter of splenic suspension were then added for 15 minutes at 18-25°C, mixing vigorously. Cell suspensions were further washed with PBS/EDTA and passed once more through a nylon mesh. Splenic single-cell suspensions from immunized mice were either evaluated by ex vivo intracellular cytokine staining, or stimulated by standard methods with LCL-A2 or syngeneic naive mouse splenocytes as APCs,17 exogenously loaded either with PR1 or PR2 epitope peptide or with PR3 peptide library (all at 4 μg/mL).
Chromium release assay
The cytotoxic activity of murine cell cultures was determined by a standard 4-hour chromium release assay (CRA) as previously described,16,18 following 1 or 2 in vitro stimulation (IVS). To measure peptide-specific responses, human LCL-A2 were pulsed with 10μM PR2 peptide or with 10μM gag peptide for 1 hour. Recognition of vaccinia-expressed PR3 was evaluated using LCL-A2 infected overnight at a multiplicity of infection (MOI) 5 with rVV-EGFP-PR3 or rVV-EGFP. K562-A2PR3 and K562-A2 targets were pretreated with IFN-γ.16 Target cells were labeled with 200 μCi (7.4 MBq) Na51CrO4− (ICN) for 1 hour at 37°C, washed extensively, and plated in 96-well round-bottom plates at a concentration of 2000 target cells per well. The radioactivity in the supernatants was determined using a Cobra II auto γ-counter (Packard), and percent-specific lysis was determined as described.16 Experimental determinations were performed in triplicate, and assay data were taken in consideration only if spontaneous release was < 30%.
Intracellular cytokine staining
Splenic cell suspensions were either evaluated ex vivo or following IVS.17 Briefly, ex vivo splenic cell suspensions or cell cultures from standard 7-day IVS were harvested and layered over a Ficoll gradient (1:2) to remove cell debris. Subsequently, 106 cells in 1 mL of RPMI were stimulated for 10-12 hours with single T-cell epitope peptides or with PR3-derived peptide library or sublibrary peptide pools. The peptide library diluent was used as control. After restimulation, HLA-A2+ LCL IVS cultures were washed and stained for 30 minutes on ice with anti-CD8-FITC (clone Ly-2; BD Biosciences) Ab, before fixing and permeabilization (Cytofix-Cytoperm; BD Biosciences) and labeling with anti-IFN-APC (Clone XMG1.2; BD Biosciences) mAb. Flow cytometric acquisition was performed on a FACSCanto (BD Immunocytometry Systems). Between 500 000 and 1 million events were acquired for each sample. FACS analysis was performed using FCS Express Version 2 software (De Novo).
Peptide-binding assay using T2 cells
The binding activity of selected peptides was assayed semiquantitatively by measuring peptide-induced stabilization of HLA-A2 molecules on T2 cells, as determined by flow cytometry. The T2 peptide-HLA stabilization assay has been described previously.19 Briefly, T2 cells (2 × 105/well) were cultured in 100 μL of RPMI serum-free medium (MediaTech) in sterile 96-well U-bottom plates, at 37°C in 5% CO2 for 14-18 hours in the presence of 0.5-200 μmol of synthetic peptides (PR1, PR2, and CMV HLA-A2–restricted epitope peptide as positive control, and a CMV HLA-B7–restricted peptide as a negative control). After the incubation period, the T2 cells were transferred to tubes, washed to remove free peptides, and then incubated with mAb BB7.2, specific for HLA-A2, conjugated to FITC (BD Biosciences), for 30 minutes at 4°C before flow cytometry using a FACSCanto flow cytometer (BD Biosciences). Data were analyzed using FCS Express software (DeNovo).
Peptide-HLA-A2 dissociation assay
T2 cells were cultured in RPMI serum-free media at 37°C overnight with 10 μmol relevant peptides. At time point 0 after culture, cells were washed 3 times and resuspended in 2% FCS RPMI media without peptides. Aliquots of cells were removed at the indicated time points (0, 2, 4, 6, 8 hour), and stained for 30 minutes with HLA-A2–specific BB7.2 Ab before flow analysis. The level of peptide-stabilized and temperature-induced class I molecules at time 0 was defined as 100%.
Ex vivo measurements of PR2 and PR1 T cells in human PBMC
Intracellular cytokine staining (ICC) assays measuring levels of PR1- and PR2-specific CD8 T cells in human PBMC samples were performed as follows. Briefly, 106 PBMC were stimulated for 18 hours with 5 μg/mL peptide in the presence of Abs to CD28 and CD49 days, with brefeldin and monensin added after the first hour of incubation. The cells were then washed, labeled with anti–CD3-PerCP–Cy5.5, and anti-CD8-FITC, fixed and permeabilized (Cytofix-Cytoperm; BD Biosciences), labeled with anti–IFN-γ–PE and analyzed by flow cytometry.
Results
Construction and characterization of rVV-expressing PR3 and HNE
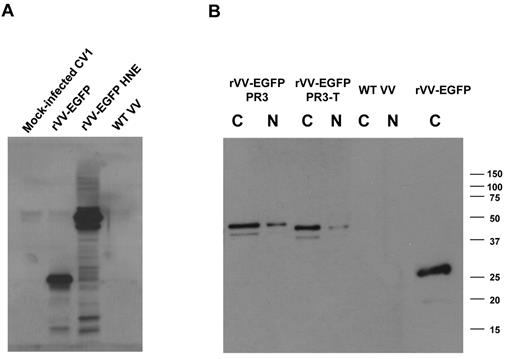
The first objective of these studies was to use a Tg mouse model to characterize T cells specific for PR3 and HNE, Ags important in GVL responses.6,10 For this purpose, we required a robust method for expression of PR3 and HNE, and accordingly developed a panel of rVV-expressing different forms of these granule proteases. Expression of transgenes was monitored by fusing to EGFP (Figure 1). We characterized expression of EGFP-PR3, EGFP fused to truncated PR3 (PR3-T), and EGFP-HNE by Western blot (WB) of nuclear and cytoplasmic extracts from rVV-infected CV1 cell cultures. An Ab to EGFP was used to assess the sizes of fusion proteins (∼ 50 kDa) with unfused EGFP (∼ 27 kDa) expressed by rVV-EGFP (Figure 2B). WB probed with specific Abs to PR3 (data not shown) and to HNE (Figure 2A) confirmed the identity of the fusion proteins. These experiments indicated that expression of the PR3 and HNE fusion proteins in the rVV-infected cells were predominantly expressed in the cytoplasm, and that levels of expression of the different forms of PR3 were comparable with each other (Figure 2).
WB analysis of recombinant PR3 and HNE expression. CV1 cells were infected with rVVs expressing HNE, PR3, and PR3-T fused to EGFP. Cytoplasmic (C) and nuclear (N) fractions were prepared 24 hours after infection with rVVs at an MOI of 1. The blot was probed with an Ab to HNE (A) or to EGFP (B). Apparent molecular weights are indicated in kilodaltons. This image is representative of 3 rVV infection experiments.
WB analysis of recombinant PR3 and HNE expression. CV1 cells were infected with rVVs expressing HNE, PR3, and PR3-T fused to EGFP. Cytoplasmic (C) and nuclear (N) fractions were prepared 24 hours after infection with rVVs at an MOI of 1. The blot was probed with an Ab to HNE (A) or to EGFP (B). Apparent molecular weights are indicated in kilodaltons. This image is representative of 3 rVV infection experiments.
Immunization of HHDII mice with rVV-EGFP-PR3 induces immunodominant responses to PR2
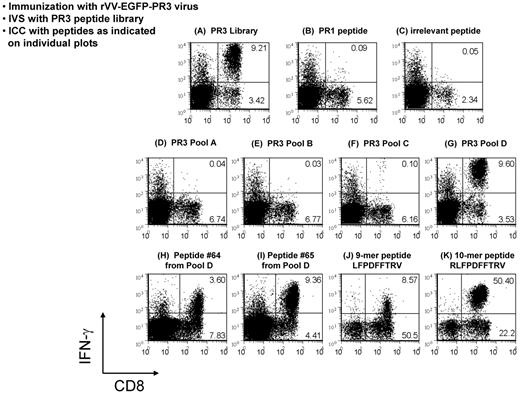
Immune responses to PR3 in HHDII mice were evaluated after injection with rVV-EGFP-PR3, or with rVV-EGFP as a negative control. Splenocytes from these mice were expanded by IVS with syngeneic cells loaded with an overlapping 68-peptide library of our design corresponding to human PR3. T-cell specificities in these cultures were examined by ICC assays using the PR3 peptide library or the PR1 9-mer epitope peptide as Ags. A robust PR3-specific CD8+ T-cell response was present in virtually all (5 of 6) rVV-EGFP-PR3–immunized mice, but paradoxically, there was either no response, or only a minimally detectable response to the PR1 peptide (Figure 3A-C). This PR3-specific T-cell response was deconvoluted by testing with 4 peptide subpools A through D each containing 25% of the PR3 library (Figure 3D-G). The immune response to the library was first mapped to subpool D, and then the 17 peptides of subpool D were tested individually, identifying the overlapping 15-mer peptides 64 GCATRLFPDFFTRVA and 65 RLFPDFFTRVALYVD as being predominantly responsible for the immune response of the immunized mice to the PR3 library (Figure 3H-I). We examined the amino acid sequence common to the 2 peptides for possible HLA-A2–binding epitopes using computerized MHC-I–binding algorithms, and identified the 10-mer RLFPDFFTRV and the 9-mer LFPDFFTRV as being the most likely epitope candidates. Peptides corresponding to these 2 sequences were synthesized and compared with the 15-mer peptides in subsequent ICC assays. We noted variations in the efficiency of expansion of CD8+ T-cell populations between experiments (compare Figure 3H and I with J and K). These results indicated that the response (Figure 3J) to the 9-mer was significantly weaker than to the 10-mer peptide RLFPDFFTRV (Figure 3K), and that this 10-mer sequence (henceforth referred to as PR2) is thus the minimal cytotoxic epitope (MCE) and is immunodominant over PR1 in HHDII mice following immunization with rVV-EGFP-PR3 (Figure 3). This result was unexpected, because the PR2 epitope has previously been predicted by computer algorithms as a potential HLA-A2–restricted epitope within PR3,20 but it has not been shown to be functionally processed and recognized by T cells, unlike the HLA-A2–restricted 9-mer epitope PR1. We minimized the likelihood of a simple explanation to account for these results because we included a control group of unimmunized mice that were subjected to similar conditions of in vitro evaluation with the PR1 and PR2 peptides as the immunized groups. In contrast to the immunized groups, no measurable differences in PR1 and PR2 T-cell precursor frequency were noted because in both cases, levels were underneath our 0.1% detection limit (data not shown).
Deconvolution of immune responses to PR3. Flow analyses of ICC assays analyzing responses of rVV-EGFP-PR3–immunized HHDII mice to a PR3 peptide library. Splenocytes from immunized mice were expanded for 1 week in culture by stimulation with the whole PR3 library (A) and aliquots of these cultures tested, first by restimulation with peptide pools (D-G), and then in a separate experiment, with single peptides (B-C, H-K) followed by labeling with Abs to CD8 and IFN-γ. The robust response to the PR3 library was shown to be because of 2 peptides (64 and 65) within peptide subpool D (H-I). The minimal cytotoxic epitope common to these 2 peptides was shown to be a 10-mer peptide termed PR2 (K). This deconvolution of PR3 responses to the PR2 epitope was performed in 2 repeat experiments, each using at least 3 mice.
Deconvolution of immune responses to PR3. Flow analyses of ICC assays analyzing responses of rVV-EGFP-PR3–immunized HHDII mice to a PR3 peptide library. Splenocytes from immunized mice were expanded for 1 week in culture by stimulation with the whole PR3 library (A) and aliquots of these cultures tested, first by restimulation with peptide pools (D-G), and then in a separate experiment, with single peptides (B-C, H-K) followed by labeling with Abs to CD8 and IFN-γ. The robust response to the PR3 library was shown to be because of 2 peptides (64 and 65) within peptide subpool D (H-I). The minimal cytotoxic epitope common to these 2 peptides was shown to be a 10-mer peptide termed PR2 (K). This deconvolution of PR3 responses to the PR2 epitope was performed in 2 repeat experiments, each using at least 3 mice.
Detection of PR2-specific CD8+ T cells in peripheral blood of CML patients
Based on our observation of immunodominant T-cell responses to the PR2 epitope in HHDII mice immunized with PR3, we investigated whether T cells recognizing PR2 are detectable in CML patients. We obtained PBMC from 27 patients and performed ex vivo ICC assays using PR1 and PR2 peptides as Ags. The clinical status of these subjects, the therapy received, and the timing of the PBMC samples are shown in Table 1. HLA-A2–restricted CMV pp65495-503 and HIV gag77-85 epitopes were used as positive and negative controls, respectively. Background responses obtained with the negative control peptide were subtracted thereby ensuring the specificity of the PR1 and PR2 response measurements. We observed responses (> 0.1 of CD8+ T cells) to PR1 in 12 patients (44%) and to PR2 in 17 patients (63%), of whom 11 also responded to PR1 (Table 1, Figure 4A). We noted that significantly higher responses to both PR1 and PR2 were seen in CML patients who had received HSCT (Figure 4A, P < .01). A scatter plot of ex vivo levels of PR2-specific versus PR1-specific CD8+ T cells in samples from CML patients revealed a strong correlation (r = 0.66, P < .0002) of responses to the 2 epitopes (Figure 4B). This unexpected association of heightened responsiveness to both epitopes in most post-HSCT patients is consistent with previous results with PR1 recognition post-HSCT obtained by multiple investigators.7-11,20-22 These results indicate that PR2-specific CD8+ T cells are present in addition to PR1-specific T cells in a subset of patients with CML. Samples from a small number of acute myeloid leukemia (AML) patients were tested for the presence of PR1 and PR2-specific CD8+ T cells. Low level responses to both epitopes were seen in a few subjects (data not shown). Thus, PR2 likely represents a leukemia-associated T-cell epitope that is a component of the immune response to the PR3 Ag in patients with CML.
PR1-specific and PR2-specific T cells are detectable in CML patients. (A) Side-by-side plots showing CD8+ T cells specific for the HLA-A2–restricted epitopes PR1 and PR2 as measured by ex vivo ICC on PBMCs from 27 CML patients using peptides as restimulation Ags (Table 1). For each of the 2 Ags, subjects were divided into 2 groups according to whether they had been treated by HSCT. Background responses to an irrelevant HIV-1 gag HLA-A2 epitope peptide were subtracted. The geometric mean percentage of CD8+ T cells recognizing each of the epitopes is indicated, and the error bars indicate the 95% confidence interval on the log scale. The dashed line at 0.01% indicates the detection limit of the assay. P values were calculated using the Mann-Whitney 2-tailed test. (B) Scatter plot of PR1 IFN-γ responses and PR2 IFN-γ responses. The Pearson correlation coefficient (r = 0.66, P < .0002) is calculated using the logarithmic scale. In both panels A and B, the zero values were set to 0.001 for graphical presentation.
PR1-specific and PR2-specific T cells are detectable in CML patients. (A) Side-by-side plots showing CD8+ T cells specific for the HLA-A2–restricted epitopes PR1 and PR2 as measured by ex vivo ICC on PBMCs from 27 CML patients using peptides as restimulation Ags (Table 1). For each of the 2 Ags, subjects were divided into 2 groups according to whether they had been treated by HSCT. Background responses to an irrelevant HIV-1 gag HLA-A2 epitope peptide were subtracted. The geometric mean percentage of CD8+ T cells recognizing each of the epitopes is indicated, and the error bars indicate the 95% confidence interval on the log scale. The dashed line at 0.01% indicates the detection limit of the assay. P values were calculated using the Mann-Whitney 2-tailed test. (B) Scatter plot of PR1 IFN-γ responses and PR2 IFN-γ responses. The Pearson correlation coefficient (r = 0.66, P < .0002) is calculated using the logarithmic scale. In both panels A and B, the zero values were set to 0.001 for graphical presentation.
APCs process PR3 and present PR2 epitope, but not the PR1 epitope
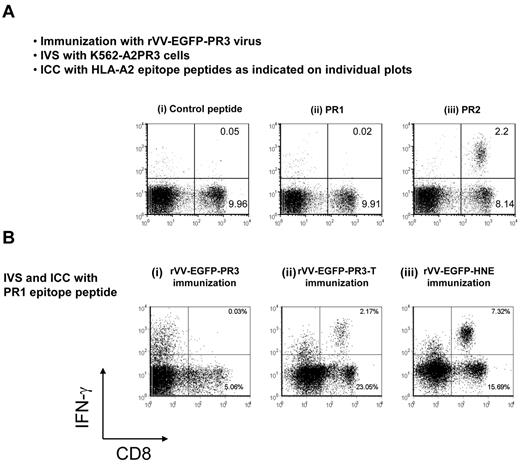
To investigate whether observations of immunodominance of PR2 over PR1 in HHDII mice immunized with rVV-EGFP-PR3 was still present when a naturally processed PR3 polypeptide was used as the Ag in IVS, we conducted additional experiments that used the cell line K562-A2PR3. Splenocytes from mice either immunized with rVV-EGFP-PR3 or rVV-EGFP were expanded by coculture with irradiated K562-A2PR3 or K562-A2 cells and then tested in ICC assays using PR1 and PR2 minimal epitope peptides as Ags. In agreement with the results from peptide IVS experiments, we observed a response to PR2 but not to PR1 (Figure 5A). This result indicates that the K562-A2PR3 cells present the PR2 epitope, presumably from processed PR3 polypeptide. Of note, we detected a weak PR2-specific response when K562-A2 cells were used as APC for IVS, suggesting that these cells produce low levels of PR3 (data not shown). We noted that while the sequence corresponding to the PR1 epitope is absolutely conserved in both human and murine PR3 and HNE, the PR2 epitope is unique to human PR3. The corresponding region of murine PR3 differs from human PR3 by 3 aa, and in addition, the human and murine neutrophil elastases differ by 5 aa (Figure 1B). This raised the possibility that HHDII mice are unresponsive to PR1 because of tolerance or immunodominance.
Immunodominance of PR2 over PR1 in rVV-EGFP-PR3–immunized mice. (A) Naturally processed PR3 protein was used as Ag for IVS. HHDII mice were immunized with rVV-EGFP-PR3, and splenocytes were expanded for 1 week in culture by stimulation with K562-A2PR3 cells. The cultures were then tested in ICC assays using irrelevant HIV-gag control peptide (i), or with PR1 peptide (ii), or with PR2 peptide (iii) as Ags. The IVS cultures contained PR2-specific CD8+ T cells but not PR1-specific CD8+ T cells. (B) Induction of PR1-specific CD8+ T cells by immunization with vaccinia expressing different proteases. Groups of HHDII mice were immunized with rVV-EGFP-PR3 (i), or with rVV-EGFP-PR3-T (ii), or with rVV-EGFP-HNE (iii). Splenocytes from all 3 groups were expanded by cocultivation with K562-A2 cells loaded with PR1 peptide and after expansion compared in ICC assays using PR1 peptide as Ag. These results are representative of 2 experiments using 3 or 4 mice.
Immunodominance of PR2 over PR1 in rVV-EGFP-PR3–immunized mice. (A) Naturally processed PR3 protein was used as Ag for IVS. HHDII mice were immunized with rVV-EGFP-PR3, and splenocytes were expanded for 1 week in culture by stimulation with K562-A2PR3 cells. The cultures were then tested in ICC assays using irrelevant HIV-gag control peptide (i), or with PR1 peptide (ii), or with PR2 peptide (iii) as Ags. The IVS cultures contained PR2-specific CD8+ T cells but not PR1-specific CD8+ T cells. (B) Induction of PR1-specific CD8+ T cells by immunization with vaccinia expressing different proteases. Groups of HHDII mice were immunized with rVV-EGFP-PR3 (i), or with rVV-EGFP-PR3-T (ii), or with rVV-EGFP-HNE (iii). Splenocytes from all 3 groups were expanded by cocultivation with K562-A2 cells loaded with PR1 peptide and after expansion compared in ICC assays using PR1 peptide as Ag. These results are representative of 2 experiments using 3 or 4 mice.
To test the effect of the presence of the PR2 epitope on immune responses to the PR1 epitope, we prepared a truncated form of PR3 (termed PR3-T) that retains the PR1 epitope but is terminated at the C terminus just before the position of the PR2 epitope (Figure 1). We generated rVV-EGFP-PR3-T and used it to immunize HHDII mice, followed by PR1 peptide IVS and detection of PR1-specific CD8+ T cells by ICC. The results (Figure 5B) indicated that immunization with rVV-expressing truncated PR3 protein reliably induced PR1-specific CD8+ T cells (Figure 5Bii) albeit lower than immunization with rVV-expressing HNE (Figure 5Biii), but unlike immunization with rVV expressing the full-length PR3 Ag (Figure 5Bi). We propose that competition by the PR2 epitope likely explains the lack of presentation of PR1.
HLA-binding affinity and stability of the peptide-MHC-I complexes on the surface of T2 cells
To better understand PR2 immunodominance, we theorized that the PR2 peptide has a higher binding affinity than PR1 for HLA-A2, even though prior in vitro stabilization studies suggested that PR2 only poorly stabilized HLA-A2.20 We conducted MHC-I stabilization experiments using TAP-deficient T2-A2 cells. This assay is based on incubation of these cells with titrated concentrations of the 2 peptides or with control peptides, followed by labeling of surface HLA-A2 with fluorescently conjugated Ab to HLA-A2. The results (Figure 6A-B) indicated that there was no significant difference between the PR1 and PR2 peptides in their ability to stabilize HLA-A2 on the surface of T2 cells over the peptide concentration range 200μM to 0.7μM. We further reasoned that while binding affinities are apparently similar, the dissociation constant of the peptide-MHC-I complexes might be different, which would lead to rapid loss of PR2 from the complexes on the cell surface, and so influence immunogenicity by reducing presentation of this epitope. Therefore, we performed a time-course experiment to investigate the loss of the peptide-HLA-A2 complex from the surface of the T2 cells after washout of peptide from the culture medium. This experiment was repeated at 3μM, 6μM, 12μM, 25μM, and 50μM concentration of peptides. The results using 25μM peptides are shown in the plots of Figure 6C, and were representative of the data at other concentrations (not shown). These results indicated that the PR2 peptide-MHC-I complex was slightly more stable than the PR1 peptide-MHC complex over an 8-hour time course. In summary, our T2 cell experiments indicate that in contrast to earlier findings, there is little difference between the PR1 and PR2 epitope peptides in their interaction with HLA-A2. Therefore, it is plausible that processed PR3 Ag could generate PR2 peptide that stabilizes MHC-1 sufficiently to be highly immunogenic in humans, as well as in the Tg mouse model, and supporting the relevance of PR2 as a leukemia-associated T-cell epitope.
Comparison of PR1 and PR2 peptides for HLA-A2–binding affinity and stability of the MHC-I complexes on the surface of T2 cells. (A) Flow cytometric histogram showing labeling of HLA-A2 on the surface of T2 assays using FITC-conjugated Ab to HLA-A2 following incubation of the cells with 100μM PR1 or PR2 peptides. A known HLA-A2 binding CMV pp65 CTL epitope peptide and a HLA-B7–restricted CMV peptide were used as positive and negative controls. (B) Titration of peptide concentration in the assay of panel A. The binding affinity of the pp65, PR1, and PR2 epitope peptides for HLA-A2 was not significantly different as determined by 1-way ANOVA tests. (C) Time-course experiment to investigate stability of peptide-HLA-A2 complexes following washout of peptide from the T2 cell cultures. Experiments were performed twice each, at peptide concentrations of 3, 6, 12, 25, and 50μM. Panel C shows the experiment using 25μM peptide concentration. The other conditions gave comparable data (data not shown). For further details, see “Peptide-binding assay using T2 cells.”
Comparison of PR1 and PR2 peptides for HLA-A2–binding affinity and stability of the MHC-I complexes on the surface of T2 cells. (A) Flow cytometric histogram showing labeling of HLA-A2 on the surface of T2 assays using FITC-conjugated Ab to HLA-A2 following incubation of the cells with 100μM PR1 or PR2 peptides. A known HLA-A2 binding CMV pp65 CTL epitope peptide and a HLA-B7–restricted CMV peptide were used as positive and negative controls. (B) Titration of peptide concentration in the assay of panel A. The binding affinity of the pp65, PR1, and PR2 epitope peptides for HLA-A2 was not significantly different as determined by 1-way ANOVA tests. (C) Time-course experiment to investigate stability of peptide-HLA-A2 complexes following washout of peptide from the T2 cell cultures. Experiments were performed twice each, at peptide concentrations of 3, 6, 12, 25, and 50μM. Panel C shows the experiment using 25μM peptide concentration. The other conditions gave comparable data (data not shown). For further details, see “Peptide-binding assay using T2 cells.”
PR2-specific T cells expanded from immunized mice are functional CTL
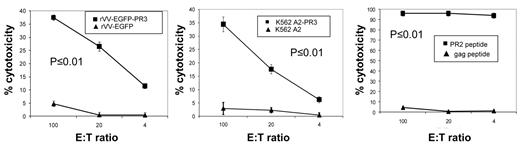
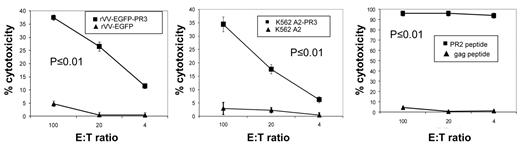
We immunized HHDII mice with PADRE-PR2 peptide and CpG oligonucleotide for 2 weeks followed by a 1-week boost, and then expanded splenocytes by IVS with PR2 peptide loaded autologous splenocytes from naive mice. These cultures were tested after 11 days by ICC with PR2 peptide and were found to contain PR2-specific cells at levels of > 80% of CD8+ T cells. Subsequently, cultures were tested in chromium release assays (CRA) for their ability to lyse human LCL-A2 cells loaded with PR2 peptide (Figure 7 right plot). We observed potent lysis of PR2 peptide-loaded LCL-A2 targets, while LCL-A2 cells loaded with irrelevant peptides were not lysed. We saw modest, but significant, recognition of LCL-A2 targets infected with rVV-EGFP-PR3, which increased after a second round of in vitro stimulation. No lysis was seen of LCL-A2 infected with rVV-EGFP (Figure 7 left plot). PR2-specific effectors were also tested for their ability to lyse K562-A2PR3, cells which endogenously express PR3. As expected, we found significant lysis of K562-A2PR3 cells, though not K562-A2 cells which have only minimal expression of PR3 (Figure 7 central plot).
PR2-specific T cells expanded from PADRE-PR2 fusion peptide immunized mice are functional CTL. PR2-specific murine T cells are cytotoxic to human HLA-A2 LCL infected with rVV expressing PR3 Ag though not HLA-A2 LCL infected with rVV expressing irrelevant EGFP Ag (left panel). PR2 effectors also killed HLA-A2 LCL target cells loaded with PR2 peptide but not an irrelevant peptide (right panel). PR2 effectors were cytotoxic to K562-A2PR3 cells that endogenously express PR3, though not K562-A2 cells (central panel). Each line corresponds to the mean values of 3 individual mice. Error bars indicate the SEM. The specificities of the effector cells for rVV-EGFP-PR3–infected targets versus rVV-EGFP–infected targets, for K562-A2PR3 versus K562-A2 targets, and for PR2 peptide-pulsed targets versus gag peptide-pulsed targets were statistically significant (P < .01) as determined by 1-way ANOVA tests.
PR2-specific T cells expanded from PADRE-PR2 fusion peptide immunized mice are functional CTL. PR2-specific murine T cells are cytotoxic to human HLA-A2 LCL infected with rVV expressing PR3 Ag though not HLA-A2 LCL infected with rVV expressing irrelevant EGFP Ag (left panel). PR2 effectors also killed HLA-A2 LCL target cells loaded with PR2 peptide but not an irrelevant peptide (right panel). PR2 effectors were cytotoxic to K562-A2PR3 cells that endogenously express PR3, though not K562-A2 cells (central panel). Each line corresponds to the mean values of 3 individual mice. Error bars indicate the SEM. The specificities of the effector cells for rVV-EGFP-PR3–infected targets versus rVV-EGFP–infected targets, for K562-A2PR3 versus K562-A2 targets, and for PR2 peptide-pulsed targets versus gag peptide-pulsed targets were statistically significant (P < .01) as determined by 1-way ANOVA tests.
Effectors from Tg mice immunized with PADRE-PR1 were able to specifically lyse target cells loaded with PR1 peptide, but even after 3 rounds of PR1 peptide restimulation, did not efficiently lyse targets infected with rVV-EGFP-PR3-T (data not shown). Inclusion of an umimmunized group that was subjected to IVS with either PR1 or PR2 peptide failed to elicit any Ag-specific effectors, further suggesting that the precursor frequency of T cells from both groups was equivalently small (data not shown). These experiments indicate that CTL from Tg mice immunized with PADRE-PR2 peptide can recognize and kill target cells presenting PR2 generated by processing of endogenously expressed or virus-delivered PR3 Ag, whereas PR1-specific effectors from mice immunized with PADRE-PR1 peptide only efficiently recognize targets pulsed with the PR1 epitope.
Discussion
The objective of these studies was to discover additional CD8+ T-cell responses to the human leukemia associated Ags PR3 and HNE using in vivo murine and in vitro human approaches. Several approaches were taken, including immunization of HLA-A2 Tg mice with rVV expressing PR3 and HNE, in vitro analysis of peptide stabilization of HLA-A2, and ex vivo measurements of human PBMC. When Tg mice were immunized with poxviruses expressing PR3, we observed immunodominant HLA-A2–restricted T-cell responses to an epitope previously named PR2 rather than the expected PR1 T-cell epitope which is therapeutically active in CML patients and recognized in healthy donors.4,5,13,23-25 In contrast, immunization of Tg mice with rVV-expressing HNE induced robust PR1-specific T-cell responses. Although the rVV-EGFP-PR3 and the K562-A2PR3 expressed a form of PR3 corresponding to the mature processed polypeptide, we also developed rVV expressing PR3 with signal peptide and propeptide and without fusion to EGFP. Immunization of Tg mice with this rVV also induced immunodominant T-cell responses to PR2 rather than PR1 (data not shown).
We investigated possible mechanisms for the absence of PR1-specific recognition in the presence of the PR2 epitope using mouse models. We found several lines of evidence that supports our contention of immune unresponsiveness to the PR1 epitope, which could be logically traced to tolerance, because the epitope is found in the endogenous murine PR3 and HNE genes without alteration from the human sequence. Complicating a straightforward conclusion that PR1-specific T cells are deleted or are nonfunctional is the result from a single immunization with rVV-expressing HNE that produced a PR1-specific CD8+ T-cell responses based on IFN-γ expression (Figure 5B). Consequently, it is not difficult to induce PR1 responses in this mouse model, which is consistent with the detection of PR1-specific CD8+ T cells in humans without disease (Figure 4). These data buttress our conclusion that PR1-specific T cells in our mouse model are associated with a lower avidity TCR repertoire which could explain the inability of these cells to lyse targets expressing full-length Ags (data not shown) in contrast to PR2-specific T cells which specifically lysed targets endogenously expressing PR3 as well as targets infected with rVV expressing PR3 (Figure 7). The results of cytotoxicity experiments and ICC assays in Figures 5 and 7 are consistent with each other, and highlight the functional capacity of the PR2 epitope to recognize endogenously processed PR3.
We pursued a mechanistic approach to help explain the immunodominance of the PR2 peptide by comparing to PR1 for binding affinity to HLA-A2. Earlier studies compared PR1 and PR2 peptides in T2 cell stabilization assays similar to those used in the present study.20 In agreement with our data, the 2 peptides were similar in their ability to stabilize HLA-A2 on the cell surface. However, binding assays evaluating the time-dependent stability of PR2-MHC complexes on T2 cells after peptide withdrawal from the culture medium demonstrated greater stability than comparable PR1-MHC complexes (Figure 6C). However, differences of PR1 and PR2 epitopes in T2 binding and stability measurements appeared modest, and while consistent with PR2 being a more strongly recognized epitope in mice, only offered a partial explanation of PR2 immunodominance. In summary, the results from our cell-based binding assays could not distinguish physical differences in the interaction of PR1 or PR2 with APC.
A plausible explanation for the immunodominance of PR2 over PR1 is that the PR2 epitope is not present in murine PR3 or HNE, whereas it is present in human PR3. Thus the PR2 epitope is a foreign epitope in mice immunized with human rVV-EGFP-PR3, whereas the PR1 epitope, being present in murine PR3 and HNE is a self-Ag and may be subject to tolerance mechanisms. Although a deeper mechanistic understanding of the physical basis for the PR2 immunodominance is worthwhile, the mouse model has served an important purpose of focusing attention on the PR2 epitope and its potential significance in human disease settings. One approach to the immunodominance question is to use our rVVs to immunize mice that are knockout for both HNE and PR3 and so would have no PR1-specific T-cell memory. Such mice have been described in the context of autoimmunity models, though unavailable with a humanized immune system.26 Alternatively, a recently published xenograft tumor protection model that proved valuable to study the function of PR1-specific T cells could be adapted to PR2 T cells to establish their functional significance.27 Finally, it would be interesting to investigate whether PR1-specific responses in HHDII mice immunized with PADRE-PR1 peptide or with rVV-EGFP-HNE impact the myeloid compartment. However, PR2-specific responses in mice immunized with rVV-EGFP-PR3 or with PADRE-PR2 peptide would not be expected to impact myeloid cells because the PR2 epitope is not present in mice.
Our data indicates that, at least in this HLA-A2 Tg model, vaccination with recombinant poxviruses expressing PR3 will induce an HLA-A2–restricted T-cell response almost entirely focused on PR2, whereas immunization with rVV-HNE will induce PR1-specific T cells. Extending these results to humans provides a possible explanation for the existence of identical copies of the PR1 epitope in both the HNE and PR3 genes because PR1 recognition could be suppressed from the PR3 Ag. Expression of PR3 and HNE in neutrophils has been reported to be discordant in some CML patients.28,29 If T-cell levels are divergent, this may correlate with PR3 (PR2 epitope) and HNE (PR1 epitope) Ag expression in leukemic cells. Our preliminary investigations in a limited number of CML patients in remission post-HSCT indicate that CD8+ T cells recognizing PR2 are present in a subset of these subjects. The levels of PR1-specific (range 0%-0.21%, median 0.08%) and PR2-specific (0%-0.75%, median of 0.18%) CD8+ T cells were modest but comparable with levels of PR1-specific CD8+ T cells reported by tetramer staining in CML patients in remission after HSCT.6,30
Our results for the first time provide evidence of the recognition of the PR2 epitope in CML (Figure 4) and AML patients (data not shown), and the possibility that immunity to PR2 is involved in the immunologic control of CML. The strong correlation between ex vivo levels of PR1-specific and PR2-specific CD8+ T cells in CML patients (Figure 4B) supports PR2 as a leukemia epitope in humans. It will be important to correlate the phenotype and functionality of such PR2-specific cell populations with leukemia clinical status. Our results also support investigation of PR2 as a peptide vaccine either on its own or included in a mixture of peptides. The HHDII mouse model successfully predicted PR2 to be a second functional human HLA-A2–restricted T-cell epitope within PR3. If PR2 presentation occurs in CML patients, then PR2-specific T cells may contribute to an anti-leukemia effect of a vaccine or a stem cell graft. An Ab (8F4) to a conformational epitope of the PR1/HLA-A2 complex was reported.31 This Ab mediated complement-dependent cytolysis of AML blasts and leukemia stem cells but not normal leukocytes, and so has therapeutic potential. It is conceivable that an analogous PR2/HLA-A2 Ab could be developed.
Beatty and colleagues have reported the presence of CD8+ T cells binding PR2 tetramers as well as T cells binding several other leukemia Ag tetramers in a small number of CML and AML patients after HSCT. However these T cells did not proliferate, produce cytokine, or degranulate on peptide stimulation, leading these workers to suggest that these T cells were senescent.30 This apparent lack of function prevented supporting studies and consequently it is difficult to interpret these observations. Molldrem and colleagues reported similar observations that only low-avidity PR1 specific T cells were observed in newly diagnosed leukemia patients, whereas high-avidity PR1-specific cells were observed in remission leukemia patients, suggesting that leukemia outgrowth may be associated with leukemia-induced selective deletion of high-avidity PR1-specific T cells.21 However, unlike Beatty et al, we present evidence of cytokine stimulation in PR2-specific T cells. Our studies showed functional cytotoxicity of the PR1- and PR2-specific CD8+ T cells from Tg mice, but also suggested lower functionality of the PR1-specific effectors. Other contradictory data are observations in an AML xenograft model which showed potent rejection of human AML cells after introduction of human PR1-specific T cells, suggesting they were functional and capable of tumor killing.27 Similarly, in a clinical study, 66 HLA-A2 myeloid leukemia patients received PR1 vaccine at 3-week intervals with montanide adjuvant and GM-CSF. Twenty-five of 44 evaluable patients responded with higher frequencies of PR1-specific CTLs, and 10 showed clinical improvement that was significantly associated with longer event-free survival.32
The discovery of a functional epitope in a clinically important Ag may lead to new associations with outcome measures and better prediction models of immune response and disease control. In terms of a vaccine model, the properties of the PR2 epitope are unknown in humans, and improved performance of a therapeutic vaccine combining both PR1 and PR2 is a testable outcome. In summary, peptide vaccines based on leukemia Ags show promise as an approach to therapy and may prove useful in combination with HSCT. An improved therapeutic index for CML patients from a PR2-based vaccine should be evaluated by either testing it alone or in combination with the PR1 peptide that has been repeatedly studied in patients in the last several years.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jessica Lo for technical assistance, John A. Zaia and Allen Lin for facilitating access to CML patient samples, Lia Aquino for assembling the patient demographic information, Weimin Tsai for processing patient samples, and Donna Packer and Peter Kwon for assistance with the manuscript.
This work was supported by National Institutes of Health R21DK077374 and Bill and Melinda Gates Foundation grant 52812 (S.F.L.); R01CA95684 (R.B.); PO1CA030206 (S.J.F.), RO1CA124782, R01CA120956, and CA141303 (L.J.N.C.); P50CA100632, LLS SCR7262 (J.M.); R01CA077544 (D.J.D.); MO1RR00043 in support of the General Clinical Research Center; and CA33572 to support the COH Comprehensive Cancer Center.
National Institutes of Health
Authorship
Contribution: S.F.L. designed and performed research, analyzed data, and assisted in writing the manuscript; C.L.R. performed research and analyzed data; T.K., T.S., and R.R. performed research and contributed vital new reagents; A.S., W.Z., K.H., and A.K. performed research; J.L. analyzed data; H.A.A. contributed vital new reagents; L.S.J. performed research; R.B., V.P., and S.J.F. analyzed data and obtained patient consent for immunologic analysis; L.J.N.C. and J.M. contributed vital new reagents and analyzed data; and D.J.D. designed research, analyzed data, and assisted in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Don J. Diamond, PhD, Division of Translational Vaccine Research, Beckman Research Institute of the City of Hope, Duarte, CA 91010-3000; e-mail; ddiamond@coh.org.