Abstract
Recent reports have described complete or major molecular remission in patients with polycythemia vera after long-term treatment with the immunomodulatory agent IFN-α2. Accordingly, there are reasons to believe that the immune system is a key player in eradicating the JAK2 mutated clone in these patients. Foxp3+ regulatory T cells play a pivotal role in maintaining immune homeostasis and, importantly, preventing immune reactivity to self-antigens; however, their suppressive activity can compromise an effective antitumor immune response, and high frequencies of regulatory T cells in peripheral blood have been reported in both hematologic and solid cancers. We have analyzed the number, phenotype, and function of circulating CD4+CD25+Foxp3+ T cells in patients with chronic myeloproliferative neoplasms. Surprisingly, we found a marked expansion of this subset of lymphocytes in patients treated with IFN-α2 (13.0%; 95% confidence interval [CI] 10.8% to 15.2%) compared with healthy donors (6.1%; 95% CI 4.9% to 7.2%), patients with untreated chronic myeloproliferative neoplasms (6.9%; 95% CI 5.8% to 7.4%), or patients treated with hydroxyurea (5.8%; 95% CI 4.3% to 7.4%; P < .0001).
Introduction
The classic Philadelphia-negative chronic myeloproliferative neoplasms—essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF)—are characterized by clonal myeloproliferation, decreased apoptosis of myeloid cells, and progressive myelofibrosis with myeloid metaplasia. The JAK2V617 mutation has a major pathogenetic role, being present in more than 95% of PV patients and approximately half of all patients with ET and PMF.1-4 A higher mutational burden has been correlated to a more severe clinical phenotype and higher risk of progression to myelofibrosis and leukemic transformation.5-7 Recently, reports have described complete or major molecular remissions in PV patients after long-term (years) treatment with pegylated IFN-α2.8-14 Even after discontinuation of therapy, some patients remain in molecular remission, with a JAK2 mutational burden < 1%, for years, which corresponds to a profound suppression of clonal myeloproliferation.12 IFN-α is a very potent immune modulator15-18 and might be able to “burst” immune surveillance and facilitate a stronger antitumor immune response against the JAK2 mutated clone and thus lead to eradication of tumor cells.
Foxp3+ regulatory T cells (Tregs) are a specific subset of CD4+ T lymphocytes that play a pivotal role in regulation and maintenance of immune tolerance to self-antigens; however, their inhibitory activity has been shown to compromise antitumor immune responses.15,18-20 CD4+ Tregs are identified by high expression of surface marker CD25 and the intracellular expression of Foxp3 that is crucial for the induction of a regulatory phenotype.21-23 At present, Foxp3 is thought to be the most specific marker of a regulatory phenotype. Nevertheless, in humans, CD4+ effector T cells (Teffs) can express Foxp3 transiently on activation without possessing suppressive potential.20,23 Other markers (eg, CD49d and CD45RA) have been suggested to aid in the distinction of Teffs and Tregs. Thus, Miyara et al23 recently showed the presence of 3 distinct populations of Foxp3-expressing CD4+ T cells based on their expression of CD45RA: CD45−Foxp3low, CD45+Foxp3low, and CD45−Foxp3high, which represent activated, nonsuppressive Teffs; resting, naive Tregs; and activated Tregs, respectively.
Herein, we present for the first time the frequencies of CD4+CD25+Foxp3+ T cells in patients with PV, ET, and PMF undergoing long-term IFN-α therapy, untreated patients, and patients treated with the cytoreductive agent hydroxyurea.
Methods
Patients and controls
Forty-eight patients (19 women, 29 men; median age 61 years, range 43-83 years) with a diagnosis of PV (n = 31), ET (n = 13), or PMF (n = 4) according to the World Health Organization classification were included in the present study. The study was approved by the regional ethics committee. All patients gave written informed consent according to the Declaration of Helsinki. Eighteen patients were untreated, 11 were treated with hydroxyurea, and 19 were treated with pegylated IFN-α2 long-term (> 1 year; mean 40 months). The dosage ranged between 90-135 μg/wk (IFN-α2a) or 25-50 μg/wk (IFN-α2b). In these groups, 6 patients had blood samples collected before initiation of IFN-α2a therapy. Peripheral blood samples of 9 healthy volunteers (6 women, 3 men; median age 63 years) employed at Herlev University Hospital, Denmark, were used as controls when levels of CD4+CD25+Foxp3+ T cells were evaluated in patients.
Mononuclear cell isolation
Peripheral blood mononuclear cells (PBMCs) were purified from peripheral blood by centrifugation on a Lymphoprep (Nycomed) density gradient. Aliquots of isolated cells were frozen immediately in 10% DMSO and 90% FCS and stored at −140°C.
Antibodies, staining, and flow cytometric analysis
PBMCs were thawed and stained for flow cytometric analysis with a FACSCantoII cytometer from Becton Dickinson. APC-Cy7 anti-CD3, and PerCP anti-CD4 from Becton Dickinson, FITC anti-CD127 and APC anti-CD25 from eBioscience, PE-Cy7 anti-CD45RA from BD Pharmingen, and relevant isotypes were used for surface antigen staining. PE anti-Foxp3 (PCH101) and isotype PE rat IgG2a from eBioscience were used for intracellular staining of Foxp3. For dead cell markers, we used 7-AAD (BD Pharmingen) for surface-stained cells and Fixable Viability Dye eFluor 450 (eBioscience) for permeabilized cells. The staining procedure was performed according to the manufacturer's protocol. Data were analyzed with FACSDiva Version 6.1.3 software from Becton Dickinson.
Proliferation assay
CD4+CD25+CD127− T cells were isolated from thawed PBMCs with a single-cell sorter (FACSAria; Becton Dickinson) after surface staining with FITC anti-CD4, PE anti-CD127, and APC anti-CD25 (eBioscience; Figure 1E-G). Briefly, 5 × 106 autologous PBMCs (responder cells) were stained with PKH26 (Sigma-Aldrich) according to the manufacturer's protocol. A total of 5 × 104 responder cells/well were incubated with CD4+CD25highCD127− T cells, CD4+CD25intermediateCD127− T cells, or CD4+CD25−CD127+ T cells (control cells) and stimulated with anti-CD3. The cells were in culture for 5 days at 37°C in a humidified 5% CO2 atmosphere in X-VIVO medium (Lonza). Subsequently, the cells were harvested and stained with APC anti-CD4, FITC anti-CD8 (BD Biosciences), and 7-AAD (BD Pharmingen), and flow cytometric analysis of proliferation was performed. As positive and negative controls, we used PKH26-labeled mononuclear cells cultured with and without anti-CD3 stimulation, respectively.
Statistical analysis
Statistical analyses were performed with unpaired Student t test and 1-way ANOVA for comparison of groups. Results are given as means with 95% confidence intervals (CIs). P < .05 was considered significant. For serial data, results are reported as median and range. Wilcoxon signed rank tests were used to compare paired data.
Results and discussion
We analyzed the frequency of CD25+Foxp3+ T cells in the CD4+ lymphoid compartment of 48 patients with chronic myeloproliferative neoplasms. We found that patients treated long-term with IFN-α had a marked increase in circulating CD4+CD25+Foxp3+ T cells (13.0%; 95% CI 10.8% to 15.2%) compared with healthy subjects (6.1%; 95% CI 4.9% to 7.2%), untreated patients (6.9%;95% CI 5.8% to 7.4%), or patients treated with hydroxyurea (5.8%; 95% CI 4.3% to 7.4%; P < .0001; Figure 1A-B). We did not observe any significant difference in the level of CD4+CD25+Foxp3+ T cells between the clinical phenotypes (ET vs PV vs PMF) in the various treatment groups, nor did we find any correlation to the hematologic response in ET and PV patients (complete remission [n = 12] 12.91%; partial remission [n = 5] 12.88%; no response [n = 1] 12.50%) or the JAK2 allele burden in IFN-α–treated patients. In 6 patients (4 ET, 2 PV), serial data confirmed a significant expansion of CD4+CD25+Foxp3+ T cells caused by IFN therapy (before treatment: 6.55%, range 3.8%-8.8%; during treatment: 11.35%, range 8.1%-12.90%; P = .03; Figure 1C). In this small subset of patients, the results showed no correlation to molecular response over the course of approximately 1 year of treatment.
Frequency of CD25+Foxp3+ cells in the CD4+ T cell population in patients with chronic myeloproliferative neoplasms. (A) Frequencies of CD25+Foxp3+CD4+ T cells in patients with chronic myeloproliferative neoplasms who were untreated (4 ET, 11 PV, and 3 PMF patients), treated with hydroxyurea (Hydrea; 1 ET and 10 PV patients), or treated with IFN-α (8 ET, 10 PV, and 1 PMF patient) compared with healthy donors. In the IFN-α–treated group, 12 patients were in complete hematologic remission, 5 had a partial hematologic response, and 1 patient did not respond to therapy. No patients were in major molecular remission. The gating strategy is shown in panel B, and frequencies are reported as number of cells in gate CD25++ and CD25+ as percentage of CD4+ T cells. (B) Flow cytometric analysis showing the frequency of CD4+CD25+Foxp3+ cells in a 63-year-old woman with PV before and after 12 months of IFN-α treatment. The plot shows CD4+ cells previously gated on CD3+ cells in the lymphoid compartment. Gates represent CD25highFoxp3+cells and CD25intermediateFoxp3+ cells, respectively. Both Foxp3+ cells with CD25high and CD25intermediate expression expanded significantly during IFN-α2a treatment. (C) The frequency of CD4+CD25+Foxp3+ cells before and after 12-14 months of treatment with IFN-α2a in 6 JAK2-positive CMPN patients (4 ET and 2 PV). (D-E) Gating strategy for single cell sorting. Fraction A: CD4+CD25highCD127−; fraction B: CD4+CD25intermediateCD127−; Fraction C: CD4+CD25−CD127+. The histogram shows that > 90% of cells in fractions A and B expressed Foxp3; however, Foxp3 expression was discretely higher in fraction A. (F) Diagram showing inhibition of proliferating CD8+ T cells in the different fractions in 3 patients undergoing IFN-α2 treatment. Results are given as percentage inhibition of CD8+ T cells compared with positive controls. The suppressor/responder ratio was 1:3 in patient 2 and 1:2 in patients 1 and 3. In patient 1, more inhibitory activity was observed in fraction A than in fraction B. In patient 2, the inhibitory activity was very strong in fraction B compared with fraction A. Patient 3 had no or minimal suppression, and in fact, a discrete increase in proliferation was observed in fraction B.
Frequency of CD25+Foxp3+ cells in the CD4+ T cell population in patients with chronic myeloproliferative neoplasms. (A) Frequencies of CD25+Foxp3+CD4+ T cells in patients with chronic myeloproliferative neoplasms who were untreated (4 ET, 11 PV, and 3 PMF patients), treated with hydroxyurea (Hydrea; 1 ET and 10 PV patients), or treated with IFN-α (8 ET, 10 PV, and 1 PMF patient) compared with healthy donors. In the IFN-α–treated group, 12 patients were in complete hematologic remission, 5 had a partial hematologic response, and 1 patient did not respond to therapy. No patients were in major molecular remission. The gating strategy is shown in panel B, and frequencies are reported as number of cells in gate CD25++ and CD25+ as percentage of CD4+ T cells. (B) Flow cytometric analysis showing the frequency of CD4+CD25+Foxp3+ cells in a 63-year-old woman with PV before and after 12 months of IFN-α treatment. The plot shows CD4+ cells previously gated on CD3+ cells in the lymphoid compartment. Gates represent CD25highFoxp3+cells and CD25intermediateFoxp3+ cells, respectively. Both Foxp3+ cells with CD25high and CD25intermediate expression expanded significantly during IFN-α2a treatment. (C) The frequency of CD4+CD25+Foxp3+ cells before and after 12-14 months of treatment with IFN-α2a in 6 JAK2-positive CMPN patients (4 ET and 2 PV). (D-E) Gating strategy for single cell sorting. Fraction A: CD4+CD25highCD127−; fraction B: CD4+CD25intermediateCD127−; Fraction C: CD4+CD25−CD127+. The histogram shows that > 90% of cells in fractions A and B expressed Foxp3; however, Foxp3 expression was discretely higher in fraction A. (F) Diagram showing inhibition of proliferating CD8+ T cells in the different fractions in 3 patients undergoing IFN-α2 treatment. Results are given as percentage inhibition of CD8+ T cells compared with positive controls. The suppressor/responder ratio was 1:3 in patient 2 and 1:2 in patients 1 and 3. In patient 1, more inhibitory activity was observed in fraction A than in fraction B. In patient 2, the inhibitory activity was very strong in fraction B compared with fraction A. Patient 3 had no or minimal suppression, and in fact, a discrete increase in proliferation was observed in fraction B.
To evaluate the suppressive capacity of the expanded Foxp3+ cells that expressed high and intermediate surface levels of CD25, we performed proliferation assays in 3 patients treated with long-term IFN-α2. We found inhibitory activity in both CD25highFoxp3+ and CD25intermediateFoxp3+ CD4+ T cells in 2 patients. In 1 patient, discrete inhibition was observed in the CD25high population only (Figure 1D-F).
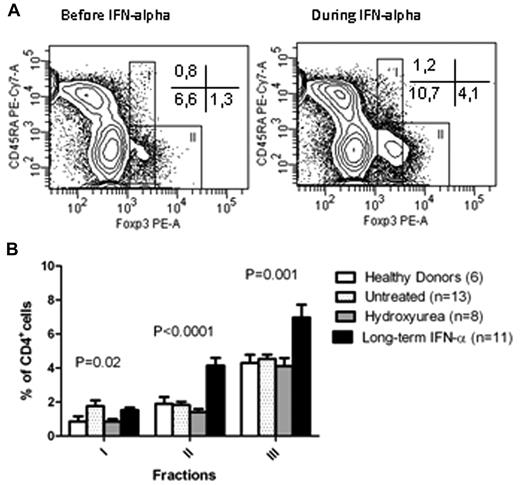
To further characterize the CD4+Foxp3+ population phenotypically, cells were stained with CD45RA to distinguish between naive Tregs, activated Tregs, and cytokine-producing Teffs (Figure 2A). There was a significant increase in both activated Tregs and cytokine-producing Teffs in patients treated with IFN-α (Tregs: 4.2%, 95% CI 3.1% to 5.1%; Teffs: 7.0%, 95% CI 5.3% to 8.6%) compared with untreated patients (Tregs: 1.8%, 95% CI 1.4% to 2.3%; Teffs: 4.5%, 95% CI 4.0% to 5.1%), patients treated with hydroxyurea (Tregs:1.4%, 95% CI 1.0% to 1.8%; Teffs: 4.1%, 95% CI 3.0% to 5.2%), or healthy donors (Tregs:1.9%, 95% CI 0.9% to 2.9%; Teffs: 4.3%, 95% CI 3.1% to 5.5%; P < .0001 for Tregs, P = .001 for Teffs; Figure 2B).
Phenotypic characterization of Foxp3+ CD4 T cells based on CD45RA. (A) Contour plots showing the distribution of cells based on CD45RA and Foxp3 expression in the same patient as in Figure 1B before and after 12 months of IFN-α2a treatment. Fraction I indicates naive, resting, and suppressive Tregs; fraction II, active, suppressive Tregs; and fraction III, cytokine-producing, nonsuppressive effector T cells. There was a marked increase in both CD45RA+Foxp3low (fraction III) and CD45RA+Foxp3high (fraction II). (B) Frequencies of Foxp3+ cells in fractions I, II, and III as described in panel A in patients with chronic myeloproliferative neoplasm according to treatment compared with healthy donors. An increase in both activated Tregs and cytokine-producing Teffs was observed in patients treated with IFN-α2 compared with any other group.
Phenotypic characterization of Foxp3+ CD4 T cells based on CD45RA. (A) Contour plots showing the distribution of cells based on CD45RA and Foxp3 expression in the same patient as in Figure 1B before and after 12 months of IFN-α2a treatment. Fraction I indicates naive, resting, and suppressive Tregs; fraction II, active, suppressive Tregs; and fraction III, cytokine-producing, nonsuppressive effector T cells. There was a marked increase in both CD45RA+Foxp3low (fraction III) and CD45RA+Foxp3high (fraction II). (B) Frequencies of Foxp3+ cells in fractions I, II, and III as described in panel A in patients with chronic myeloproliferative neoplasm according to treatment compared with healthy donors. An increase in both activated Tregs and cytokine-producing Teffs was observed in patients treated with IFN-α2 compared with any other group.
To date, IFN-α is the only therapy that is able to induce minimal residual disease with low-burden JAK2 V617F; however, because of side effects in a proportion of patients, controversy still exists regarding the use of IFN-α. Novel targeted treatments, JAK2 inhibitors and HDAC inhibitors (histone deacetylase inhibitors), have been studied in clinical trials. So far, the results are promising, with cytoreduction and reduction in spleen size and hypermetabolic symptoms. However, JAK2 inhibitors have not significantly reduced the JAK2 allele burden,24,25 and their combination with IFN-α might be beneficial in inducing molecular remission. Treg-mediated immune suppression has been shown to be crucial for tumor evasion and cancer progression, and therefore, the marked increase of CD4+CD25+Foxp3+ T cells in patients treated with IFN-α is surprising and of particular interest. However, an expansion in both nonsuppressive Teffs and activated Tregs supports our hypothesis of a pronounced immune activation accompanied by a counterresponse during treatment with IFN-α, which is possibly responsible for the significant decrease of JAK2 burden in some patients. In serial data from 6 patients before and after 1 year of IFN therapy, we did not detect a consistent pattern of decrease in the JAK2 allele burden and accordingly no correlation to the levels of Tregs. However, on the basis of observations of a steady decline in JAK2 V617F in most IFN-treated patients after several years of treatment,8-14 a correlation between Tregs and the molecular response might be found when larger series of patients are studied for a longer time period.
In conclusion, we have for the first time described a CD4+ T-cell response during IFN-α treatment in patients with chronic myeloproliferative neoplasms. We report a marked expansion of activated Tregs but also of nonsuppressive Teffs. Further immunologic studies are needed to determine whether this immune response is of crucial importance for the induction of minimal residual disease, which has been observed in a subset of patients after long-term treatment with IFN-α2.8-14
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kirsten Nikolajsen for technical assistance and support and Tobias Wirenfeldt Klausen for performing statistical analyses.
This study was supported by research funding from the Danish Cancer Research Foundation and Herlev University Hospital (C.H.R.).
Authorship
Contribution: C.H.R., M.K.J., M.K.B., H.C.H., and I.M.S. designed the study; C.H.R. performed research and analyzed data; M.K.B. and I.M.S. contributed vital new reagents and analytical tools; C.H.R., M.K.B., P.t.S. and I.M.S. interpreted data; C.H.R. wrote the paper; M.K.J., H.C.H., and O.W.B. provided patient samples and collected data; and all coauthors edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Caroline Hasselbalch Riley, Department of Hematology, Center for Cancer Immune Therapy, Herlev University Hospital, Herlev Ringvej 75, 2730 Herlev, Denmark; e-mail: riley@dadlnet.dk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal