Abstract
Rapidness of leukocyte engraftment in patients receiving peripheral blood stem cell transplantation is clinically important because the risk of fatal opportunistic infections increases with time to engraftment. Adhesion receptor molecules on hematopoietic stem cells (HSCs) have been shown to modulate homing and engraftment of HSCs. Therefore, we correlated expression levels of α4 (CD49d) and α6 (CD49f) integrins in the CD34+ HSC compartment with time to engraftment. Leukapheresis products from 103 patients were retrospectively analyzed for CD34, CD38, CD3, CD49f, and CD49d surface molecules by multiparameter flow cytometry. High expression levels of α4 integrin, but not α6 integrin on CD34+ cells, were associated with regular engraftment of leukocytes (days 8-19), whereas low surface expression correlated with delayed recovery (> 19 days; P < .0005). We show that α4 integrin expression levels on HSCs in leukapheresis products predict the engraftment capacity of mobilized peripheral blood stem cells in peripheral blood stem cell transplantation patients.
Introduction
The very late antigen (VLA) molecules, first described in 1987 by Takada et al,1 are heterodimeric molecules that are involved in embryogenesis, leukocyte adhesion, cell migration, inflammation, and extracellular matrix interaction. α4β1/VLA4 (CD49d/CD29), counter-receptor for VCAM-1 and fibronectin, as well as α6β1/VLA6/laminin receptor (CD49f/CD29) are ubiquitously expressed in human and mouse hematopoietic stem and progenitor cells, and both play a role in homing and engraftment of hematopoietic stem cells (HSCs).2-6 Recent evidence from murine transplantation models suggests that α4-chain deletion5 or treatment with function-blocking antibodies to α4 or α6 integrin4 may impair hematopoietic engraftment. In contrast, it is reported that blockage with α6 integrin antibodies improves engraftment efficiency in human HSCs.2 These data suggest that surface levels and function of VLA4 and VLA6 on stem cells may modulate stem cell engraftment.
However, all these experiments refer to stem cell engraftment in isogenic or immunodeficient (HSC) mouse transplantation models. Until now, there is lack of evidence about the role of HSC α4 and α6 integrin expression levels for human peripheral blood stem cell transplantations (PBSCTs) so far.
Here we demonstrate that low levels of α4 integrin expression on HSCs are associated with a longer engraftment period in patients receiving autologous PBSCT. We found no correlation of α6 integrin expression on HSC engraftment velocity in this patient collective.
Methods
Patients and sample preparation
All patients were treated with autologous PBSCT at the Department of Hematology, Oncology, and Immunology of the University Hospital Marburg, Marburg, Germany. Patients had given written informed consent to donate aliquots of the leukapheresis product according to the guidelines of the local Ethics Committee and in compliance with the Declaration of Helsinki. Frozen leukapheresis product samples were thawed and immediately dissolved in PBS plus 4% human albumin (CLS Behring) on ice. Mononuclear cells were isolated by density centrifugation and washed with PBS plus 4% human albumin (4°C).
Flow cytometric analysis
After washing with PBS plus human albumin buffer, cells were resuspended to a concentration of 106/mL. The following antibodies were used: CD34 phycoerythrin-Cy7, CD38 peridinin chlorophyll protein-Cy5.5, CD3 Pacific blue; isotype control antibodies (all BD Biosciences) included α4 integrin fluorescein isothiocyanate (clone 44H6, Serotec) and α6 integrin allophycocyanin (clone GoH3, BioLegend). After incubation for 15 minutes at room temperature, cells were washed with PBS and resuspended in 400 μL FACSFlow (BD Biosciences) solution. A total of 4 μL 4,6-diamidino-2-phenylindole (10 μg/mL; Invitrogen) was added to each sample for dead cell exclusion, followed by immediate analysis on an LSRII cytometer (BD Biosciences). Data were exported from DIVA Version 6.1 software (BD Biosciences) and analyzed with FlowJo Version 7.6 software (TreeStar).
Statistical analyses
Geometric means of the fluorochrome signals were determined with FlowJo Version 7.6 software, and data were transferred to GraphPad Prism (GraphPad) Version 5.01 software. The primary data of α4 integrin levels were checked for normal distribution, and analysis of variance and Student t tests were conducted. In addition, we performed a logistic regression analysis to analyze the association of CD34+ cell doses and α4 integrin expression levels with engraftment time and to answer the question whether high doses of CD34+ cells may compensate for low α4 integrin expression levels.
Results and discussion
We determined the surface expression levels of α4 integrin and α6 integrin on CD34+ stem cells in leukapheresis products of 103 patients (Table 1). Patients were classified as slow (> 19 days) and regular (8-19 days) engraftment with regard to leukocyte regeneration time (absolute neutrophil count > 0.5 × 109/L). The median time to engraftment was 23 days (range 20-41 days) in the slow engraftment group. The numbers of CD34+ cells/kg in the leukapheresis product and patient age were equal between the groups of slow and regular engraftment. The percentage of patients receiving radiotherapy before PBSCT was slightly lower in the slow engraftment group (P = .02). Thus, radiation could not be attributed to prolonged stem cell engraftment in that patient group because less history of radiation is associated with better HSC engraftment.7
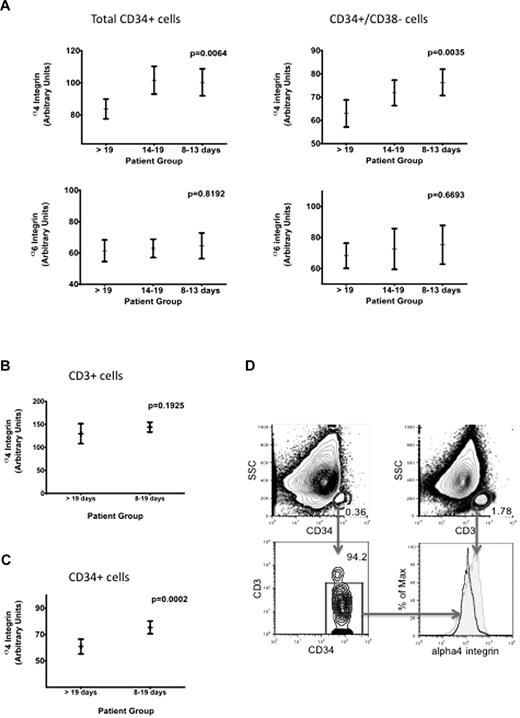
Using multiparameter flow cytometry, median values of fluorescence were first determined in relation to isotype controls. Patients with a prolonged time to engraftment (> 19 days) had significantly lower expression levels of α4 integrin on total CD34+ cells as determined by analysis of variance testing (P = .0064). This correlation was also observed in the more primitive CD34+CD38− stem cell compartment (P = .0035). To analyze whether there was indeed an association between integrin expression level and time to engraftment, we subdivided the originally 2 cohorts into 3 patient groups as slow (< 19 days), regular (14-19 days), and rapid (8-13 days) engraftment. This analysis revealed a dependency of α4 integrin levels and engraftment time in CD34+ cells.
In addition, we performed a logistic regression analysis and found that the α4 integrin expression level had a significant inverse association with the probability of slow engraftment (P = .003). In contrast, such a positive association between the dosage of CD34+ cells and the probability of slow engraftment could not be shown (P = .449). In detail, this analysis revealed that the odds for slow engraftment decrease by 3.7% with an increase of α4 expression by 1 unit. The association of α4 integrin levels and rapidity of engraftment was also significant in 2 uniform cohorts of patients analyzed separately (lymphoma and myeloma; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
For α6 integrin surface expression, we found no correlation with time to engraftment in either CD34+CD38− HSCs or in the total CD34+ stem and progenitor cell compartment (Figure 1A).
Time to PBSC engraftment related to flow cytometric quantification of α4 and α6 integrin levels on total CD34+ cells and the CD34+CD38− stem cell compartment in leukapheresis products. (A) Low expression levels of α4 integrin on total CD34+ or on CD34+CD38− cells correlate significantly with prolonged transplant engraftment time (P = .0064 and .0035, respectively). Geometric mean values of fluorescence intensity were obtained after flow cytometric α4 (top row) and α6 (bottom row) integrin measurement compared with isotype controls and analyzed by analysis of variance testing. There is no correlation between α6 integrin expression levels on stem and progenitor cells and engraftment velocity. (B) In leukapheresis samples, α4 integrin levels on T cells show no significant variation. (C) Normalization of HSC α4 integrin levels on an internal T-cell control is a reliable analysis strategy and confirms the correlation between α4 integrin levels and transplant engraftment time (P = .0002). (D) After dead cell exclusion by 4,6-diamidino-2-phenylindole versus forward scatter gating (not shown), CD34 and CD3 signals were plotted against side scatter (top quadrants). CD34 cells were further plotted against the CD3 signal, so that residual T cells were excluded (left bottom quadrant) for the comparative gating of CD34+ and CD3+ cells for α4integrin expression (right bottom quadrant).
Time to PBSC engraftment related to flow cytometric quantification of α4 and α6 integrin levels on total CD34+ cells and the CD34+CD38− stem cell compartment in leukapheresis products. (A) Low expression levels of α4 integrin on total CD34+ or on CD34+CD38− cells correlate significantly with prolonged transplant engraftment time (P = .0064 and .0035, respectively). Geometric mean values of fluorescence intensity were obtained after flow cytometric α4 (top row) and α6 (bottom row) integrin measurement compared with isotype controls and analyzed by analysis of variance testing. There is no correlation between α6 integrin expression levels on stem and progenitor cells and engraftment velocity. (B) In leukapheresis samples, α4 integrin levels on T cells show no significant variation. (C) Normalization of HSC α4 integrin levels on an internal T-cell control is a reliable analysis strategy and confirms the correlation between α4 integrin levels and transplant engraftment time (P = .0002). (D) After dead cell exclusion by 4,6-diamidino-2-phenylindole versus forward scatter gating (not shown), CD34 and CD3 signals were plotted against side scatter (top quadrants). CD34 cells were further plotted against the CD3 signal, so that residual T cells were excluded (left bottom quadrant) for the comparative gating of CD34+ and CD3+ cells for α4integrin expression (right bottom quadrant).
VLA molecules show high expression levels in bone marrow stem cells, and treatment with granulocyte colony-stimulating factor leads to down-regulation of these integrins on HSCs so that they can be released into the periphery.2 Nevertheless, α4 levels on mobilized HSCs differed significantly between patients with slow and normal engraftment despite down-regulation and consecutively low receptor expression compared with untreated whole marrow HSCs (data not shown). However, the differences of the fluorescence signals (mean channel numbers) measured in log scale were relatively small, leading to overlapping histogram data. This could partially be related to the use of frozen samples because surface antigen expression may decrease in older or thawed material resulting from protein degradation or down-regulation. Therefore, an additional α4 integrin analysis with an internal T-cell control standard was performed because control standards and analysis strategies are crucial in interpretation of flow cytometric data. Testing the constancy of α4 integrin expression levels on T cells, we found that CD3+ cells have a uniform α4 integrin expression pattern (Figure 1B) in leukapheresis samples. Correlation of engraftment and α4 integrin expression by normalization of HSC α4 integrin levels on CD3+ cells proved that this method reliably discriminated for regular versus prolonged engraftment time, indicated by a very low P value of .0002 (Figure 1C). Thus, determination of α4 integrin levels on HSCs compared with T cells should be the preferred analysis method, as shown in Figure 1D.
In line with previous data about the importance of VLA4 for stem cell homing and engraftment derived from conditional deleted knockout mice systems5,6 or antibody blocking experiments,8 we show that the level of α4 integrin expression, but not α6 expression, on CD34+ cells correlated significantly with PBSC engraftment, independent of the total number of CD34+ cells and/or history of radiation therapy. Our finding may have clinical impact because anticipation of PBSC engraftment capacity from α4 integrin determination on HSCs could be valuable information in the clinic. In line with this, drugs that are known to down-regulate these adhesion receptors, such as histone deacetylase inhibitors,9 could influence engraftment and should therefore be handled with care in patients receiving PBSCT. On the other hand, drug-induced up-regulation of adhesion molecules on transplanted HSCs could be a promising strategy to facilitate engraftment in patients receiving PBSCT.
In conclusion, flow cytometric measurement of α4 integrin levels on HSCs is easy to perform and may therefore provide a new diagnostic tool for assessment of homing and engraftment capacity of stem cells from leukapheresis products in patients receiving PBSCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ramona Vietzke for excellent assistance with analysis of patient data.
This work was supported by the German José Carreras Leukemia Foundation (grant DJCLS R06/31) and the Deutsche Forschungsgemeinschaft (DFG KFO210 to C.B. and A.N.).
Authorship
Contribution: C.B., B.H., and T.V. performed the experiments and analyzed the data; C.L. provided the samples and data from our patients' cell bank, and helped with the experiments; S.I. calculated the statistics; and A.N. and C.B. evaluated and discussed the data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cornelia Brendel, MD, Department of Hematology, Oncology, Immunology, Philipps University Marburg and Universitätsklinikum Giessen und Marburg, Baldingerstrasse, 35043 Marburg, Germany; e-mail: brendelc@staff.uni-marburg.de.