Abstract
Transplantation of whole bone marrow (BMT) leads to engraftment of both osteoprogenitor cells and hematopoietic cells; however, the robust osteopoietic chimerism seen early after BMT decreases with time. Using our established murine model, we demonstrate that a post-BMT regimen of either granulocyte-colony stimulating factor, growth hormone, parathyroid hormone, or stem cell factor each stimulates greater donor osteoblast chimerism at 4 months posttransplantation than saline-treated controls and approximates the robust osteopoietic chimerism seen early after BMT; however, only growth hormone led to significantly more donor-derived osteocytes than controls. Importantly, there were no adverse hematologic consequences of the different treatments. Our data demonstrate that these cytokines can stimulate the differentiation of transplanted donor marrow cells into the osteopoietic lineage after BMT. Post-BMT cytokine therapy may generate durable osteopoietic engraftment, which should lead to sustained clinical benefit and render BMT more applicable to bone disorders.
Introduction
Both hematopoietic and osteopoietic progenitor cells reside within bone marrow and engraft in recipients after bone marrow transplantation (BMT).1-4 In principle, BMT should be effective therapy for disorders of osteoblasts and for hematopoiesis. We tested this notion by undertaking the first clinical trial of BMT for a genetic disorder of bone formation, severe osteogenesis imperfecta (OI). We demonstrated donor-derived osteoblasts that were associated with marked clinical benefits,5,6 findings recently corroborated in an animal model of BMT for OI.3 Follow-up of our patients, however, revealed that the rate of improvement slowed after the first 6 to 12 months after BMT.6
Studies in mice have shown that BMT leads to robust donor-derived osteopoiesis early after transplantation, but the osteopoietic chimerism declines to negligible levels between 6 and 12 months after BMT,7 a time course that closely correlates with the observed slowing of clinical improvement. These data suggest that the underlying explanation for the impermanent rate of improvement in OI patients is transient donor osteopoietic engraftment. A durable osteopoietic graft, then, should lead to substantially improved long-term clinical outcomes. Here, we report the use of cytokines/hormones, selected for their capacity to stimulate hematopoietic and/or mesenchymal cells, to recruit transplanted donor marrow cells into the osteopoietic differentiation pathway after BMT.
Methods
BMT and cytokine treatment
Bone marrow (3 × 106 cells/mouse) obtained from green fluorescent protein (GFP)–transgenic donors (FVB/N background) was transplanted into lethally irradiated (1125 cGy) FVB/N recipients by tail vein injections as described previously.8-10 At 8 weeks after transplantation, mediators were administered by daily intraperitoneal injection for 4 consecutive weeks as follows: Recombinant human G-CSF (Amgen), 250 μg·kg−1·d−1, 4 d/wk; rat parathyroid hormone (PTH; Bachem Americas Inc), 80 μg·kg−1·d−1, 5 d/wk; porcine growth hormone (GH; National Hormone and Peptide Program), 4 mg·kg−1·d−1, 6 d/wk; recombinant mouse SCF (Prospec Bio), 100 μg·kg−1·d−1, 4 d/wk; and sterile saline (control). Mice were killed for analysis at 1 or 4 weeks after completion of the treatment regimen. All animal protocols were approved by the Institutional Animal Care and Use Committee of The Children's Hospital of Philadelphia.
Complete blood counts
Peripheral blood was collected by retro-orbital bleed, and counts were obtained with a Hemavet hematology analyzer (model HV950FS; Drew Scientific).
Immunohistochemistry
Sections were immunostained for GFP expression as described previously.8-10 GFP+ osteoblasts and osteocytes were enumerated by visual examination of stained slides by 2 investigators who were blinded to the experimental conditions. See supplemental Methods for additional details (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Flow cytometry
Peripheral blood and bone marrow were analyzed for GFP expression and lineage-specific markers with commercially available antibodies as described previously.7 See supplemental Methods for additional details.
Histomorphometry
Bone histomorphometry was performed at the Bone Histomorphometry Core Laboratory at the M. D. Anderson Cancer Center according to standard protocols.
Statistical methods
Data are presented as mean ± SE. Differences were considered statistically significant by 1-way or 2-way ANOVA followed by Bonferroni multiple comparison test if they attained P < .05 (Prism Version 4; GraphPad Software Inc).
Results and discussion
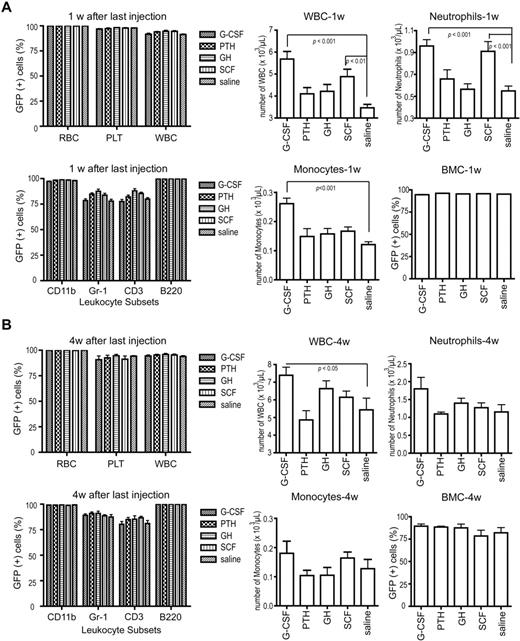
Eight weeks after BMT, before cytokine treatment, all 4 experimental groups (G-CSF, PTH, GH, and SCF) showed similar complete blood counts, complete (> 90%) donor hematopoietic chimerism, and donor contribution to the leukocyte subsets (data not shown). One week after completion of the mediators (13 weeks after BMT), donor contributions to all peripheral blood lineages and leukocyte subsets were not statistically different from controls. G-CSF and SCF stimulated an increase in total leukocytes, neutrophils, and monocytes, but none of the cell counts were substantially greater than physiologic cell counts for healthy mice (Figure 1A). Bone marrow cellularity and GFP expression among marrow cells was > 90% in all groups and controls (Figure 1A). Four weeks after completion of mediator treatment (16 weeks after BMT), the donor contribution to all peripheral blood lineages, leukocyte subsets, and marrow cells remained similar to controls, and the G-CSF–stimulated increase of total leukocytes persisted (Figure 1B). As expected, these data show that hematopoietic reconstitution was robust and that the mediators were not detrimental to marrow function, which supports their clinical use after BMT.
Hematopoiesis and hematopoietic chimerism. (A) Hematopoietic chimerism, determined by flow cytometric analysis for GFP expression, of peripheral blood (top left; n = 10 for each cytokine group) and leukocyte subsets (bottom left; n = 10) at 1 week after cytokine treatment (13 weeks after BMT). Absolute cell counts of leukocytes (top middle; n = 10), neutrophils (top right; n = 10), and monocytes (bottom middle; n = 10) and donor chimerism of total bone marrow cells (bottom right; n = 5 for each cytokine group), all at 1 week after cytokine treatment. (B) The same analyses as in panel A with data obtained at 4 weeks after cytokine treatment (16 weeks after BMT; n = 5 for each cytokine group). All data are presented as mean ± SE. Statistically significant relationships are indicated. RBC indicates red blood cells; PLT, platelets; WBC, white blood cells; and BMC, bone marrow cells. CD11b, Gr-1, CD3, and B220 are lineage markers for monocytes, neutrophils, T lymphocytes, and B lymphocytes, respectively.
Hematopoiesis and hematopoietic chimerism. (A) Hematopoietic chimerism, determined by flow cytometric analysis for GFP expression, of peripheral blood (top left; n = 10 for each cytokine group) and leukocyte subsets (bottom left; n = 10) at 1 week after cytokine treatment (13 weeks after BMT). Absolute cell counts of leukocytes (top middle; n = 10), neutrophils (top right; n = 10), and monocytes (bottom middle; n = 10) and donor chimerism of total bone marrow cells (bottom right; n = 5 for each cytokine group), all at 1 week after cytokine treatment. (B) The same analyses as in panel A with data obtained at 4 weeks after cytokine treatment (16 weeks after BMT; n = 5 for each cytokine group). All data are presented as mean ± SE. Statistically significant relationships are indicated. RBC indicates red blood cells; PLT, platelets; WBC, white blood cells; and BMC, bone marrow cells. CD11b, Gr-1, CD3, and B220 are lineage markers for monocytes, neutrophils, T lymphocytes, and B lymphocytes, respectively.
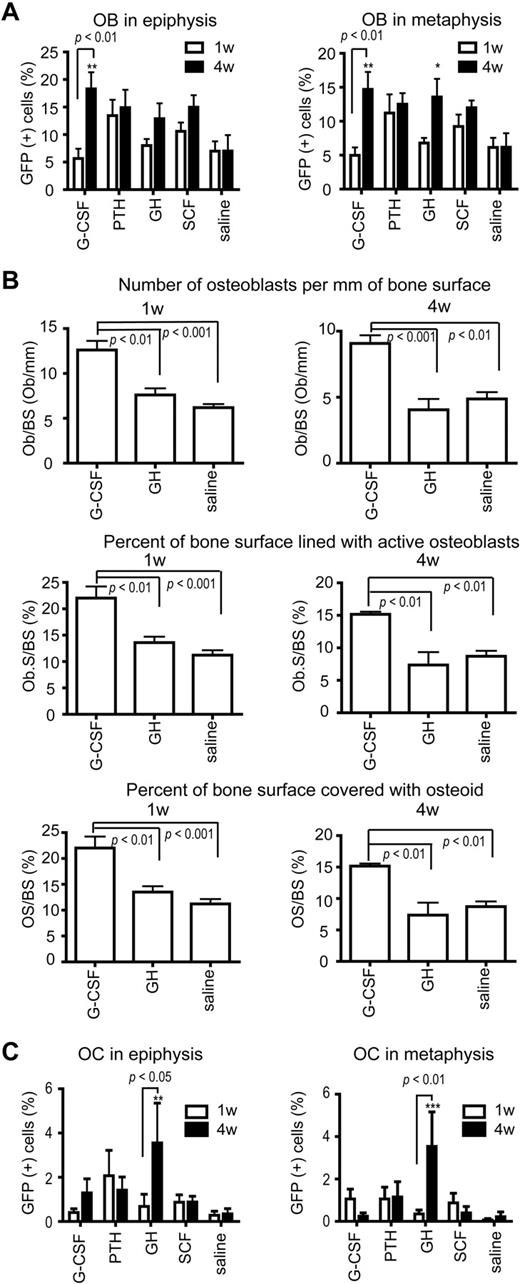
Collectively, cytokine treatment led to significantly increased osteoblast chimerism in epiphysis and metaphysis at 4 weeks (P < .05), and chimerism was significantly greater at 4 weeks than at 1 week after the regimens (P < .001; Figure 2A). Specifically, the G-CSF– and GH-treated mice showed significantly greater chimerism than controls at 4 weeks after treatment. The osteoblast chimerism in the controls at 1 and 4 weeks after the treatment regimens was low and stable, consistent with prior time course studies7 (Figure 2A). The PTH-treated mice also showed a stable donor osteoblast chimerism at 1 and 4 weeks (Figure 2A). In contrast, G-CSF–treated mice showed significantly increased donor osteoblast chimerism at 4 weeks compared with 1 week, and the GH group showed a trend toward the same effect, which suggests that a surge of osteopoietic differentiation followed the cessation of the G-CSF and GH treatments (Figure 2A). Christopher and Link reported that G-CSF suppressed osteoblast maturation and increased the osteoprogenitor pool, which led to a rebound in osteoblast number after G-CSF treatment that resulted from recruitment of new osteoblasts.11 The present data, which are consistent with those findings, suggest that the new osteoblasts are, in part, recruited from transplanted, donor-derived marrow cells. The overall range of donor chimerism (12%-18%) among the 4 groups paralleled the chimerism observed 3 weeks after BMT in mice.7,9 The clinical importance of this observation is that the level of donor osteopoietic chimerism at the later time points approximated that found early after BMT, which has proved beneficial in children with OI treated with BMT.5,8 We anticipate that the cytokine-induced heightened osteopoietic chimerism will decrease over time so that repeated cycles of cytokine treatment will likely be required to maintain high-level osteopoietic chimerism in patients long-term and sustain the associated clinical benefits.
Osteopoietic chimerism and histomorphometry. (A) Osteoblast (OB) chimerism, determined by immunohistochemical staining for GFP expression, in the epiphysis (left) and metaphysis (right) at 1 and 4 weeks after cytokine treatment. n = 5 for each cytokine group at each time point; 8 sections per mouse were analyzed. (B) Histomorphometric analyses of bone from G-CSF–, GH-, and saline-treated mice obtained at 1 (left) and 4 (right) weeks after completion of the cytokine regimen. n = 5 for each cytokine group. (C) Osteocyte (OC) chimerism, determined by immunohistochemical staining for GFP expression, in the epiphysis (left) and metaphysis (right) at 1 and 4 weeks after cytokine treatment. n = 5 for each cytokine group at each time point; 8 sections per mouse. All data are presented as mean ± SE. Statistically significant relationships are indicated. ***P < .001, **P < .01, and *P < .05 compared with saline group at the same time point.
Osteopoietic chimerism and histomorphometry. (A) Osteoblast (OB) chimerism, determined by immunohistochemical staining for GFP expression, in the epiphysis (left) and metaphysis (right) at 1 and 4 weeks after cytokine treatment. n = 5 for each cytokine group at each time point; 8 sections per mouse were analyzed. (B) Histomorphometric analyses of bone from G-CSF–, GH-, and saline-treated mice obtained at 1 (left) and 4 (right) weeks after completion of the cytokine regimen. n = 5 for each cytokine group. (C) Osteocyte (OC) chimerism, determined by immunohistochemical staining for GFP expression, in the epiphysis (left) and metaphysis (right) at 1 and 4 weeks after cytokine treatment. n = 5 for each cytokine group at each time point; 8 sections per mouse. All data are presented as mean ± SE. Statistically significant relationships are indicated. ***P < .001, **P < .01, and *P < .05 compared with saline group at the same time point.
Histomorphometry of the bone revealed that the G-CSF–treated mice had a significantly greater number of osteoblasts per millimeter of bone surface, percent of bone covered with osteoid, and percent of bone surface lined with active osteoblasts than controls at both 1 and 4 weeks (Figure 2B). Using the absolute number of osteoblasts per millimeter of bone surface in the metaphysis and the osteoblast donor chimerism, the calculated absolute numbers of donor cells per millimeter of bone surface for the G-CSF–, GH-, and saline-treated mice were 1.26, 0.88, and 0.32, respectively. Thus, G-CSF appears to stimulate a 4-fold increase and GH a 3-fold increase in the absolute number of donor-derived osteoblasts. Although long-term continuous treatment with G-CSF results in osteopenia in children,12 the intermittent dosing schedule of G-CSF used in the present studies may be anabolic for bone, analogous to use of PTH.13
Osteocyte chimerism was most improved in the GH group, with these animals showing the greatest increase between 1 and 4 weeks in both the epiphysis and metaphysis (Figure 2C). Moreover, GH-treated mice were the only group to show significantly more donor-derived osteocytes than saline controls (Figure 2C). Despite the high donor osteoblast chimerism in the G-CSF group, osteocyte chimerism was not increased, which indicates that these cells do not give rise to osteocytes. Both G-CSF and GH increase the osteoprogenitor pool, but G-CSF is thought to inhibit osteoblast differentiation,11,14 whereas GH promotes osteoblast differentiation.15 Because only approximately 1 of 5 osteoblasts is thought to give rise to an osteocyte,16 one possible explanation for the present data would be that G-CSF leads to the recruitment of osteoblasts that constitute the hematopoietic niche, whereas GH recruits osteoblasts that give rise to osteocytes. If proven true, these cytokines, G-CSF and GH, may find specific clinical applications in therapy directed to the endosteal hematopoietic niche or the structural bone, respectively.
Collectively, the present data suggest that a quiescent stem/progenitor cell resides in the transplanted marrow compartment that can be stimulated to enter the osteopoietic differentiation pathway. Future efforts will be directed toward elucidating the mechanism of the cytokine-induced osteopoietic differentiation of transplanted marrow cells and developing approaches to harness these pathways to advance bone marrow cell transplantation for disorders of bone.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Alan Flake for assistance with flow cytometry and Ilaria Castelli for her assistance with the immunohistochemistry.
This work was supported in part by a grant from the National Institutes of Health (R01 HL077643), the Associazione per il Sostegno dell'Ematologia e dell'Oncologia Pediatrica (ASEOP), and the Fondazione Cassa di Risparmio di Modena.
National Institutes of Health
Authorship
Contribution: S.O. designed, performed, and analyzed research and assisted with manuscript preparation; V.R., R.B., and T.J.H. performed research; M.D. designed and analyzed research; and E.M.H. oversaw the entire project, designed and analyzed research, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Edwin M. Horwitz, MD, PhD, The Children's Hospital of Philadelphia, Colket Translational Research Bldg, Office 3010, 3501 Civic Center Blvd, Philadelphia, PA 19104; e-mail: horwitze@e-mail.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal