Abstract
We examined the prevalence and prognostic relevance of bone marrow reticulin fibrosis in 526 patients with World Health Organization–defined polycythemia vera evaluated at the time of initial diagnosis. Seventy-four patients (14%) displayed mostly grade 1 reticulin fibrosis, with only 2 cases showing higher-grade fibrosis. Presenting clinical and laboratory characteristics, including JAK2V617F allele burden, between patients with and without fibrosis were similar for the most part, with the exception of a higher prevalence of palpable splenomegaly in patients with fibrosis (P < .01). Patients with fibrosis were less prone to experience thrombosis during their clinical course (1.1 vs 2.7 per 100 patient-years; P = .03) and more prone to develop post-polycythemia vera myelofibrosis (2.2 vs 0.8 per 100 patient-years; P = .01). There was no significant difference between the 2 groups in terms of overall or leukemia-free survival. The present study clarifies the incidence, degree, and prognostic relevance of bone marrow fibrosis obtained at time of initial diagnosis of polycythemia vera.
Introduction
Although histologic bone marrow (BM) features in polycythemia vera (PV) have been described repeatedly,1-3 the incidence and particularly the clinical impact of reticulin fibrosis at diagnosis continue to be controversial issues. According to relevant findings reported by the Polycythemia Vera Study Group (PVSG) on 226 patients with treatment-naive BM biopsy samples, 25% showed a slight and 11% a moderate to marked increase in fibers.1,4,5 In a recently published study, an even more increased incidence was found in 143 PVSG-defined PV patients, including slight reticulin fibrosis in approximately 20% and moderate to marked fibrosis in 51% of cases.6 In contrast to these relatively high frequencies at presentation, other larger series reported lower ranges between 10% and 20%, with a significant prevalence of a minor fiber grade.2,7-9 In this context, scant knowledge is available regarding the precise relationship of initial BM fibrosis and clinical activity of PV.1,6 To date, it has not been clear whether a minor increase in reticulin fibrosis indicates a later stage of disease or a more aggressive course with a more rapid evolution into the so-called spent phase (post-PV myelofibrosis). In the 25 patients of the PVSG study with marked to moderate fibrosis, follow-up examinations failed to reveal a predictive value, and sequential biopsies showed that myelofibrosis may be present for a long time before onset of spent-phase PV.1 On the other hand, although no relationships between BM fibrosis and bleeding or thrombosis could be ascertained, a higher reticulin fibrosis grade was regarded as an independent risk factor for transformation into overt myelofibrosis (post-PV myelofibrosis) or acute leukemia.6
In the present study, we examine the incidence and prognostic role of minor reticulin fibrosis at presentation of PV, diagnosed according to the World Health Organization (WHO) criteria.10 Our study population was derived from an international, multicenter PV database.
Methods
A clinicopathologic database of patients who were diagnosed and treated for PV in their respective institutions was created by clinicians and hematopathologists from 7 international centers of excellence for myeloproliferative neoplasms.
Eligibility criteria included the availability of representative, treatment-naive BM biopsy samples obtained at diagnosis and properly processed BM slides (hematoxylin-eosin staining and silver impregnation after Gomori or Gordon-Sweet staining). Diagnostic criteria of PV were strictly applied according to the updated 2008 WHO criteria.10 The study was approved by the institutional ethics review board of each institution. A total of 526 patients met the stringent criteria for study inclusion. All patients were diagnosed between 1990 and 2011 and were recruited from Pavia, Italy (n = 126), Padua, Italy (n = 94), Florence, Italy (n = 88), Vienna, Austria (n = 87), Bergamo, Italy (n = 80), and Vicenza, Italy (n = 51). All BM biopsy samples were centrally re-reviewed by 1 of the authors (J.T.), who is a coinvestigator in this project as well as the author of the relevant WHO chapter on diagnostic criteria for PV.10 Fiber scoring was performed according to a 3-graded (0, 1, 2, 3) system.11 Both local pathologists and the expert reviewer had access to patient information that was necessary for routine interpretation of BM morphology; however, the review process was completely blinded to outcome data, which were analyzed after completion of the histopathology review. Allele-specific PCR techniques were used to screen for JAK2V617F in each participating center.12
Statistical analysis included an extraction of disease-relevant parameters in each occurrence. These parameters (considered at diagnosis) included data concerning diagnosis, death, leukemic transformation, and progression into overt myelofibrosis. Differences in the distribution of continuous variables between categories were analyzed by the Mann-Whitney test. Patient groups with nominal variables were compared by the χ2 test. Outcomes of interest were reported as rates per 100 patient-years and by cumulative incidences calculated at 5, 10, and 15 years from the date of diagnosis. Incidence rate ratios between groups were estimated and tested. Overt myelofibrosis-free and thrombosis-free survival rates were calculated from the date of diagnosis to the date of progression into overt myelofibrosis and occurrence of thrombosis (all uncensored), respectively, or last contact/date of death (all censored).
Results and discussion
At presentation, 74 (14%) of the 526 WHO-defined PV patients displayed an increase in BM fiber content (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The degree of fibrosis was minor in most instances, with only 2 cases showing moderate (grade 2, n = 1) or marked (grade 3, n = 1) myelofibrosis.
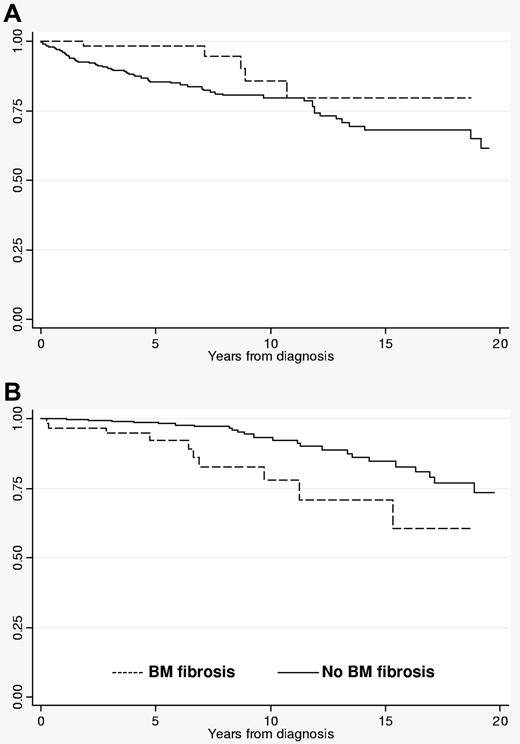
Neither hematologic data nor frequency of previous thrombosis was significantly different between fibrotic versus nonfibrotic PV patients. Patients with fibrosis were significantly more likely to display palpable splenomegaly (P < .0001). JAK2V617F mutation status and allele burden were similar between PV patients with and without fibrosis. During follow-up, patients with initial BM fibrosis showed a significantly lower cumulative incidence of major arterial and venous thrombosis (Table 1), superior thrombosis-free survival (Figure 1A), and inferior myelofibrosis-free survival (Table 1; Figure 1B). The cumulative risks of thrombotic complications, overt myelofibrotic progression, leukemic transformation, and death are presented in Table 1. Leukemic transformation and death rates were similar between the 2 groups.
Thrombosis-free and overt myelofibrosis-free survival rates in polycythemia vera patients with or without BM fibrosis. (A) Thrombosis-free survival; (B) survival free of overt myelofibrosis.
Thrombosis-free and overt myelofibrosis-free survival rates in polycythemia vera patients with or without BM fibrosis. (A) Thrombosis-free survival; (B) survival free of overt myelofibrosis.
The PVSG did not consider BM examination as part of the diagnostic criteria in PV or attribute a predictive value for clinical outcome.1,13 In contrast, the WHO classification system10 has underscored the diagnostic value of BM histology in PV by listing it as one of the minor diagnostic criteria.14 The present findings with regard to the incidence of > grade 1 BM fibrosis are consistent with other large series that reported incidence figures of 3% to 5%7,8 and differ markedly from yet other studies that reported much higher incidence rates (ranging between 11%1 and 51%).6 Development of myelofibrosis is part of the natural evolution of the disease process in PV.1,7,13,15 Consequently, higher grades of reticulin fibrosis suggest a delayed diagnosis, and not surprisingly, such cases were also associated with higher incidence of splenomegaly.6 The diagnostic workup in the present study population was ascertained to be performed early in the disease process, which might explain in part the comparatively lower incidence of moderate to marked fibrosis. This contention is further supported by the fact that JAK2V617F allele burden in the present study was similar between PV patients with and without fibrosis; mutant allele burden in PV increases with disease duration and development of post-PV myelofibrosis.12
The significantly lower occurrence of major thrombotic events during follow-up in PV patients with reticulin fibrosis should be interpreted with caution; the low number of events prevents a reliable conclusion. We can speculate that the positive correlation of reticulin fibrosis with splenomegaly and lower risk of thrombosis suggests an altered cytokine/chemokine profile that promotes extramedullary hematopoiesis and partially abrogates the hypercoagulable state of otherwise florid PV. It is also possible that the presence or absence of reticulin fibrosis reflects a genetic difference in the disease clone, which also might explain the increased risk of transformation to overt myelofibrosis. Additional pathogenetic insight might be obtained from future studies that include information on karyotype and plasma cytokine levels.
In conclusion, the incidence of mostly minor BM fibrosis at presentation of WHO-defined PV patients ranges between 10% and 20%. PV patients with such minor increases in reticulin fibrosis display a higher prevalence of palpable splenomegaly and are more prone to develop overt myelofibrosis. The association between the presence of BM fibrosis and a lower risk of thrombosis needs to be validated by additional studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
A.M.V. and A.R. were supported by a grant from Associazione Italiana per la Ricerca sul Cancro (AIRC, Milano) “Special Program Molecular Clinical Oncology 5x1000” to AGIMM (AIRC-Gruppo Italiano Malattie Mieloproliferative), project number 1005. A detailed description of the AGIMM project is available at http://www.progettoagimm.it.
Authorship
Contribution: T.B., J.T., G.F., A.C., and A.T. designed the research, contributed patients, participated in data analysis and interpretation, and wrote the paper; J.T. reviewed all bone marrow histopathology; all other authors either contributed patients or participated in reviewing bone marrow histopathology; and all authors read and approved the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tiziano Barbui, Ospedali Riuniti di Bergamo, Largo Barozzi 1, Bergamo 24128, Italy; e-mail: tbarbui@ospedaliriuniti.bergamo.it.
References
Author notes
T.B., J.T., and A.T. contributed equally to this study.