Abstract
Angioimmunoblastic T-cell lymphoma (AITL) is a major type of peripheral T-cell lymphoma (PTCL). To elucidate the clinicopathologic characteristics and prognosis of AITL in Japan, we retrospectively analyzed 207 patients with AITL. The median patient age was 67 years (range, 34-91 years), with 73% of patients older than 60 years. With a median follow-up of 42 months in surviving patients, 3-year overall survival (OS) was 54% and progression-free survival (PFS) was 38%. The International Prognostic Index (IPI) and the prognostic index for PTCL, not otherwise specified (PIT) were predictive for OS in this analysis. Multivariate analysis found that age older than 60 years, elevated white blood cell (WBC) and IgA levels, the presence of anemia and thrombocytopenia, and extranodal involvement at > 1 site were significant prognostic factors for OS, and IgA, anemia, and mediastinal lymphadenopathy were significant prognostic factors for PFS. A novel prognostic model consisting of the prognostic factors for OS was successfully constructed. In conclusion, IPI and PIT were still useful for prognostication of AITL, and other factors, including those not used in IPI, such as IgA, anemia, WBC count, thrombocytopenia, and mediastinal lymphadenopathy, also significantly affected prognosis. Future investigations for IgA as a unique prognostic factor are warranted.
Introduction
Angioimmunoblastic T-cell lymphoma (AITL) is a major type of peripheral T-cell lymphoma (PTCL).1,2 Most patients with AITL are elderly; the median age of patients is older than 60 years, and the disease shows a male predominance.3 The primary site of disease is usually the lymph nodes, and 80%-90% of patients exhibit advanced disease and extranodal presentations. Laboratory abnormalities such as polyclonal hypergammaglobulinemia, anemia, and positive autoantibodies are also common.3-6
The disease generally displays an aggressive clinical course and poor prognosis after conventional chemotherapies. Previous studies found overall survival (OS) was < 40% at 5 years.3-6 Immunodeficiency secondary to the disease makes it difficult to treat patients with myelosuppressive combination chemotherapies. Because most patients are elderly, high-dose chemotherapy with stem cell transplantation cannot always be used. The current treatment strategy thus needs to be refined to obtain better outcomes.
AITL is characterized by distinct pathologic findings. The architecture of the lymph node practically disappears, and marked proliferation of branching high endothelial venules is prominent. Clonal rearrangement of the T-cell receptor gene was observed in 75%-90% of cases, indicating clonal proliferation of T cells.7-9 In addition to the usual T-cell Ags such as CD3, CD2, CD5, and CD4, the expressions of CD10, CXCL13, and PD-1 are characteristic in AITL, which indicates that the T follicular helper cell is the normal counterpart cell type of AITL.10,11
Several prognostic factors and several predictive models for non-Hodgkin lymphoma have been reported.12,13 Among them, the International Prognostic Index (IPI) prevails as the most useful and powerful predictive model for many types of non-Hodgkin lymphoma.12 However, previous studies on aggressive lymphoma dealt with diffuse large B-cell lymphoma, which is the most common type of aggressive lymphoma. In contrast, prognostic models for T-cell lymphoma are limited. Although a prognostic model for peripheral T-cell lymphoma, not otherwise specified (PIT) has been proposed,13 prognostic factors or predictive models for AITL have not been established. Furthermore, application of IPI and PIT to AITL remains unclear.3 Elucidation of novel prognostic factors and useful prognostic models are vital to establish an optimal treatment strategy for AITL.
As the first step of the future investigations for better outcomes of AITL, we conducted a multicenter, retrospective study in Japan to clarify the clinical and pathologic features and to uncover prognostic factors or markers for AITL.
Methods
Patients
A total of 207 patients with AITL whose condition was diagnosed between January 1990 and September 2008 from 31 participating hospitals were retrospectively analyzed. Patients with AITL were eligible for analysis only if the diagnosis was confirmed by histopathologic and IHC criteria in accordance with the World Health Organization (WHO) classification.1 Of 207 patients, 190 cases (92%) were diagnosed by a hematopathologist (S.N.). Cases have been excluded from this study if not fulfilling the diagnostic WHO criteria. Twelve cases were not diagnosed as AITL by S.N. and were excluded from this study. Clinical features of unreviewed 17 cases were fully documented in case report form and were consistent with those of AITL. Thus, we included these cases in this analysis. According to the pathologic findings of available specimens, 193 patients were pathologically categorized into 2 major categories: “rich in large cells,” defined as including > 10% of large B and/or T cells in the focus of specimens; and “classic,” defined as being rich in clear cells, rich in epithelioid cells, and with hyperplastic germinal centers in the focus of specimens.3 Patients were treated according to each institutional protocol or physicians' decisions. The study protocols were approved by the institutional review board at each participating hospital and complied with all provisions of the Declaration of Helsinki and Ethical Guideline for Epidemiologic Research from Ministry of Health, Labor and Welfare in Japan.
IHC analyses
Formalin-fixed paraffin sections were subjected to immunoperoxidase studies with the use of the avidin-biotin peroxidase complex method. The following mAbs were used: CD3, CD4, CD5, CD7, CD8, L26/CD20, Ber-H2/CD30, KiM4P/FDC (Dako), TIA-1 (Coulter Immunology), and granzyme B (Monosan). In addition, CD10 (Novocastra Laboratories), CXCL13 (R&D Systems), and PD-1 (CNIO Monoclonal Antibody Unit) were evaluated if specimens were available. All Abs were applied after Ag retrieval after microwave oven heating treatment.14-18
ISH study
The presence of EBV small RNAs was evaluated by in situ hybridization (ISH) with EBV-encoded small nuclear early-region (EBER) oligonucleotides on formalin-fixed, paraffin-embedded sections as previously described.19
Response assessment
Clinical data were collected from case report forms. Patients received treatment for AITL according to the respective institutional protocols. Response to treatment was assessed according to WHO criteria.20
Statistical analysis
OS was calculated from the date of diagnosis to death or the last date of follow-up. Progression-free survival (PFS) was calculated from the date of diagnosis to the first date of disease progression, relapse, death as a result of any cause, or the last date of follow-up. OS and PFS were analyzed with the log-rank test, and results were expressed using Kaplan-Meier methods.
Univariate and multivariate analyses were performed with Cox proportional hazards regression models to identify potential independent prognostic factors. Multivariate analysis was built with a forward/backward stepwise method with the use of threshold values for removal from and addition to the model of P = .10 and P = .05, respectively. Results are expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). All probability values were 2-sided and had an overall significance level of .05. All data were analyzed with Stata SE 10 software (StataCorp).
Results
Clinical characteristics of patients
Patient characteristics are summarized in Table 1. The median age was 67 years (range, 34-91 years) with 73% of patients older than 60 years. The female-to-male ratio was 1:1.8. Details of the treatment were available for 194 of 207 patients (93%). Of these 194 patients, 172 (89%) were treated with anthracycline-containing combination chemotherapies, including 112 of 194 (58%) with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP), 46 (24%) with pirarubicin, cyclophosphamide, vincristine, and prednisolone (THP-COP), and 14 (7%) with second- or third-generation multidrug combination chemotherapies. Seven patients (4%) received prednisolone monotherapy. Twenty-seven patients (14%) received high-dose chemotherapy with autologous stem cell transplantation (ASCT) during their clinical course. Twenty-one patients (11%) were in the upfront setting, and the remaining 6 patients (3%) had relapsed or refractory disease.
Pathologic and immunophenotypic characteristics
All cases were characterized by partial or total effacement of lymph node architecture with proliferation of arborizing high endothelial venules and follicular dendritic cells, which was highlighted by KiM4P/FDC immunostaining, according to the diagnostic criteria of the current WHO classification. They were within boundaries of the typical histopathologic and immunophenotypic features for AITL and were subclassified into 45 patients who were “rich in large cells” and 148 patients who were “classic,” as well as previously reported by the GELA (Groupe d'Etude des Lymphomes de l'Adulte) group.3 The latter included 16 patients rich in epithelioid cells, 19 rich in clear cells, and 10 with hyperplastic germinal centers.
CD3 was positive in 100% (189 of 189) of patients examined, CD4 in 90% (112 of 124), CD5 in 95% (121 of 127), and CD7 in 28% (16 of 58). EBV was detected in 106 of 160 patients (66%) by EBER-ISH to various degrees. CD10 expression was clearly found in 39 of 131 tested patients (30%) on tumor cells. CXCL13 and PD-1 were immunohistochemically evaluated in 78 patients. CXCL13 reactivity was mainly cytoplasmic with a perinuclear dot pattern in 71 of 78 patients (91%). PD-1 expression was on the cell surface and cytoplasmic in 48 of 78 patients (62%). Taken together, at least CD10, PD-1, or CXCL13 was positive in 74 of 78 patients (95%). Table 2 shows pathologic characteristics of patients.
Treatment outcomes and survival
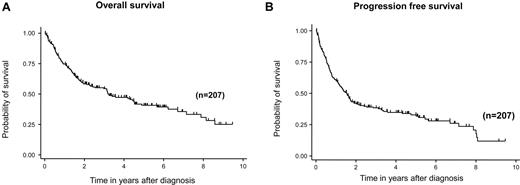
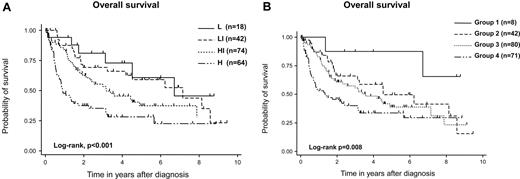
With a median follow-up duration of 42 months in surviving patients, 3-, 5-, and 7-year OS rates were 54% (95% CI, 47%-62%), 41% (95% CI, 33%-48%), and 35% (95% CI, 27%-44%), respectively (Figure 1A). Three-, 5-, and 7-year PFS rates were 38% (95% CI, 31%-45%), 33% (95% CI, 26%-40%), and 26% (95% CI, 18%-34%), respectively (Figure 1B). Both IPI and PIT were predictive for OS (IPI, 3-year OS:, low risk, 81%; low-intermediate risk, 69%; high-intermediate risk, 53%; high risk, 35%; log-rank test, P < .001; PIT, 3-year OS: group 1, 88%; group 2, 66%; group 3, 56%; group 4, 40%; log-rank, P = .008; Figure 2A-B).
Survival of patients with angioimmunoblastic T-cell lymphoma. OS (A) and PFS (B).
Survival of patients with angioimmunoblastic T-cell lymphoma. OS (A) and PFS (B).
OS of patients with AITL. OS according to IPI (A) and PIT (B) are shown. Both IPI and PIT could stratify the prognosis of AITL. IPI categorized patients as follows: L (n = 18), LI (n = 42), HI (n = 74), and H (n = 64). L, low risk; LI, low-intermediate risk; HI, high-intermediate risk; H, high risk. PIT categorized as follows: group 1 (n = 8), group 2 (n = 42), group 3 (n = 80), and group 4 (n = 71).
OS of patients with AITL. OS according to IPI (A) and PIT (B) are shown. Both IPI and PIT could stratify the prognosis of AITL. IPI categorized patients as follows: L (n = 18), LI (n = 42), HI (n = 74), and H (n = 64). L, low risk; LI, low-intermediate risk; HI, high-intermediate risk; H, high risk. PIT categorized as follows: group 1 (n = 8), group 2 (n = 42), group 3 (n = 80), and group 4 (n = 71).
Seventy-two of 106 patients (68%) and 28 of 45 patients (62%) achieved CR after initial CHOP or THP-COP chemotherapies. The 5-year OS rates in patients receiving CHOP and THP-COP chemotherapies were 43% each, and the 5-year PFS rates were 37% and 35%, respectively. In terms of patient outcomes among those receiving ASCT in the upfront setting, 5-year OS was 47% and 5-year and PFS was 39%. According to Fisher exact test, the characteristics of patients treated with ASCT as front line therapy and patients not treated with ASCT were similar, except for age (data not shown).
Prognostic factors
Analyses of prognostic factors are shown in Table 3. On univariate analysis, sex, PS, WBC count, anemia, IgA, albumin, total protein, extranodal involvement, mediastinal lymphadenopathy, sIL-2 receptor, IPI, and PIT were poor prognostic factors for OS. Sex, PS, anemia, IgA, extranodal involvement, mediastinal lymphadenopathy, and sIL-2R influenced PFS.
Multivariate analysis found age (HR, 1.65; 95% CI, 1.03-2.64; P = .039), WBC count (HR, 1.83; 95% CI, 1.18-2.83; P = .006), the presence of anemia (HR, 1.88; 95% CI, 1.57-3.86; P = .004), thrombocytopenia (HR, 1.70; 95% CI, 1.10-2.63; P = .017), IgA level (HR, 1.73; 95% CI, 1.12-2.67; P = .013), and extranodal involvement (HR, 2.46; 95% CI, 1.57-3.86; P ≤ .001) were identified as significant prognostic factors for OS. Anemia (HR, 1.70; 95% CI, 1.18-2.46; P = .005), IgA level (HR, 1.95; 95% CI, 1.32-2.88; P = .001), and mediastinal lymphoadenopathy (HR, 1.75; 95% CI, 1.22-2.52; P = .002) were significant prognostic factors for PFS.
Prognostic model for AITL
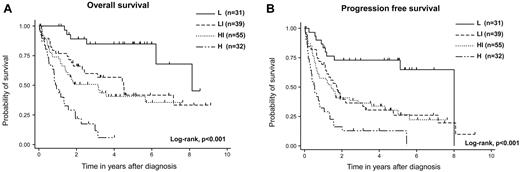
The IPI and PIT could stratify the prognosis of AITL in this analysis; however, several unique factors, including elevated IgA and WBC levels, the presence of anemia, and thrombocytopenia, were identified as prognostic factors for OS. We thus attempted to construct a new prognostic model with these 6 prognostic factors (AITL prognostic index; ATPI). We classified patients into 4 risk groups with the use of the following terms: low risk, 0 or 1 adverse factor; low-intermediate risk, 2 factors; high-intermediate risk, 3 factors; high risk, 4-6 factors. This novel prognostic model for AITL could stratify the prognosis of patients with AITL (P < .001). For 31 patients (20%) classified into the low-risk group, 3-year OS was 85%. For 39 patients (25%) in the low-intermediate group, 3-year OS was 62%; for 55 patients (35%) in the high-intermediate risk group, 3-year OS was 51%; and for 32 patients (20%) in the high-risk group, 3-year OS was 12% (P < .001; Figure 3A). The 3-year PFS in the low-risk group was 73%, that in the low-intermediate-risk group was 37%, that in the high-intermediate-risk group was 36%, and that in the high-risk group was 13% (P < .001; Figure 3B). Compared with IPI and PIT, the receiver operating characteristic (ROC) curve area of our model was superior to those of IPI and PIT (our model ROC area, 0.671; 95% CI, 0.600-0.742; IPI ROC area, 0.615; 95% CI, 0.542-0.688; PIT ROC area, 0.585; 95% CI, 0.511-0.659).
Survival of patients with AITL according to the AITL prognostic index. OS (A) and PFS (B). This prognostic model could efficiently stratify the outcomes into 4 groups: patients with 0 or 1 risk factor (low risk, n = 31), patients with 2 risk factors (low-intermediate risk, n = 39), patients with 3 risk factors (high-intermediate risk, n = 55), and patients with 4-6 risk factors (high-risk, n = 32).
Survival of patients with AITL according to the AITL prognostic index. OS (A) and PFS (B). This prognostic model could efficiently stratify the outcomes into 4 groups: patients with 0 or 1 risk factor (low risk, n = 31), patients with 2 risk factors (low-intermediate risk, n = 39), patients with 3 risk factors (high-intermediate risk, n = 55), and patients with 4-6 risk factors (high-risk, n = 32).
Discussion
In this study, age, elevated WBC and IgA levels, the presence of anemia and thrombocytopenia, and extranodal involvement were significant poor prognostic factors for OS, and anemia, IgA, and mediastinal lymphadenopathy significantly influenced PFS. Although IPI and PIT were still important for the prognostication of patient outcomes, our novel prognostic model, ATPI, including elevated WBC and IgA levels, and the presence of anemia and thrombocytopenia not adopted in IPI could stratify the prognosis of patients with AITL. Considering that male sex, mediastinal lymphadenopathy, and anemia have been reported to influence OS of patients with AITL,3 the presence of anemia, one of our prognostic factors, could be important for patients with AITL. Thrombocytopenia was also reported as one of the prognostic factors in patients with PTCL, not otherwise specified (PTCL-NOS); it could thus be an important prognostic factor for PTCL. IgA, a newly identified prognostic factor, as well as our novel prognostic model ATPI, should be validated in future investigations. Although our analyses were based on a retrospective study, our novel findings could provide helpful information to establish optimal treatment strategies for AITL.
Clinical characteristics of AITL in our current study were similar to those in previous reports.3-6 Distribution of advanced clinical stage, BM involvement, hypergammaglobulinemia, autoantibodies such as Coombs, pleural effusion, ascites, constitutional symptoms, and high serum levels of lactate dehydrogenase were similar to previous reports.3,5,6 Only the incidence of extranodal involvement in organs such as liver, spleen, and gastrointestinal tract seemed to be lower in our series. These results indicate that clinical characteristics of patients with AITL do not differ between patients in Asian and Western countries.
In terms of the clinical outcomes in our analysis, 3-, 5-, and 7-year OS rates were 54%, 41%, and 35%, respectively, and the CR rates in CHOP and THP-COP were 68% and 62%, respectively. These clinical outcomes seem to be slightly better than those of a previous report from GELA.3 Considering that patient background, including age, PS, and clinical stage, and the distribution of IPI and PIT categories were almost similar to those in the GELA report, this difference might be because of treatment protocol. In our analysis, > 80% of patients received CHOP or THP-COP chemotherapy as a first-line treatment, whereas most of the patients in the GELA study, especially younger patients, received more intensive treatment than the CHOP or THP-COP regimens. Actually, the clinical outcome in younger patients in the GELA study was worse than that in older patients. This means intensive chemotherapy might not be beneficial in patients with AITL with potentially immunocompromised status.
We identified elevated serum IgA levels as a novel prognostic factor in AITL; this elevation was observed in 37% of our patients. The significance of serum IgA levels has not been reported, and the mechanism of elevated IgA levels is uncertain with the current understanding of AITL. However, this novel finding is worth investigating in future analyses. In our current study, detailed information about serum IgA levels, for example, whether the elevation of IgA was monoclonal or polyclonal, was not available. According to previous reports, increasing polyclonal IgA may be associated with the biologic aspects of AITL.21,22 Leukocytoclastic vasculitis with IgA deposits and atypical linear IgA dermatosis have been reported in patients with AITL.21,22 A recent biologic finding has found that TGF-β1 and IL-21, produced by T follicular helper cells, are related to the differentiation of IgA-plasmablasts. IgA-plasmablasts induced by neoplastic T follicular helper cells in patients with AITL might produce excessive serum IgA.7 Furthermore, elevated IgA might be associated with the loss of immunocompetence in AITL. The relation between EBER-ISH and IgA was examined, and it was found that IgA levels in the EBER-positive group were significantly higher than those in the EBER-negative group (Student t test; P = .03). Further biologic investigations about the role of IgA should be encouraged for a better understanding of AITL.
We have identified WBC count as a significant prognostic factor in AITL. Because WBC count is composed of various components such as neutrophils and lymphocytes, which component significantly affects the prognosis matters. In the present study, data on neutrophil and lymphocyte counts were not available. However, we noted that lymphoma cells were detected in 21 of 161 patients (12%) and that leukocytosis was found in 11 of 21 leukemic patients with leukocytosis (52%). The leukemic presentation did not influence OS (log-rank test, P = .19) in our patients. However, future studies must clarify which leukocyte component has a significant influence on the prognosis of AITL.
IPI and PIT were useful to predict the prognosis of our patients, although the application of IPI to T-cell and natural killer/T-cell lymphoma, including AITL, remains controversial.3-5,23,24 Only 2 of 6 prognostic factors in our analysis, namely age and extranodal involvement, were adopted in IPI; thus, the attempt to construct a novel prognostic model with newly identified factors might be reasonable. Our novel prognostic model that used 6 factors might be composed of too many factors. However, the presence of anemia and thrombocytopenia and the elevated IgA and WBC levels are quite common parameters in clinical medicine; thus, our model would be practical. In particular, the elevated IgA and WBC levels have not been reported as prognostic factors in AITL. Our model is superior to IPI and PIT in terms of ROC area; future investigations are thus warranted to determine whether our novel prognostic model can be applied to other clinical cohorts.
We evaluated specific markers for AITL, including CD10, CXCL13, PD-1, and EBER-ISH, in addition to routine immunostaining. The positivity of these markers, except for CD10, was similar to previous studies.3,10,15-18 We identified 106 EBV-positive cases and 54 EBV-negative cases. The OS rates of positive and negative cases were not significantly different (log-rank test, P = .58). These results indicate that the immunophenotypic features and EBV status of AITL are not different between Asian and Western patients even if EBV infection prevails in pan-Pacific areas. The possible reason for our low positivity of CD10 compared with previous reports might reflect the difference of the denominator in terms of ambiguous CD10 positivity. In this study, we only included clear CD10-positive pattern on tumor cells as positive, and excluded patients with CD10-positive cells considered as normal lymphocytes and ambiguous CD10-positive cells even if these were considered tumor cells. CD10 positivity in AITL is still controversial, as is which CD10-positive cells are really considered positive in AITL.3,25 Future consensus about the positivity of CD10 is needed. In 2006, Went et al proposed a modification of the PIT model (so-called mPIT) for PTCL-NOS and showed its prognostic value in a retrospective cohort of patients with PTCL-NOS and AILT.25 The model included Ki-67, so the significance of Ki-67 could have been evaluated. However, we failed to do so because of the limited number of slides for this study. In a future study, other biomarkers, including ki-67, should be studied, for example, with the use of the tissue microarray technique.
Although our study provides novel information about AITL, some limitations should be discussed. First, in this sort of retrospective study, unrecognized biases cannot be excluded, and careful interpretation of results is required. Second, the treatment strategies were based on respective institutional protocols and physicians' decisions, so treatment outcomes might have been overestimated or underestimated. Third, the unity of pathologic diagnoses of AITL and interpretations of IHC stains might be difficult even if an expert hematopathologist diagnosed the disease. CD10 positivity of our series might have been underestimated. Nonetheless, we conclude that our findings, including application of IPI to this disease and our novel prognostic model that used IgA levels and anemia, provide enough of a basis to warrant future analysis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Hiroshi Miwa, MD (Aichi Medical University Hospital); Takae Kataoka, MD (Nagoya Memorial Hospital); Atsushi Wakita, MD (East Medical Center Higashi Municipal Hospital City of Nagoya); Hiroshi Ogawa, MD (Seirei Mikatahara General Hospital); Masahide Kobayashi, MD (Hamamatsu Medical Center); Takahiko Ito, MD (Gifu Social Insurance Hospital); Keiki Kawakami, MD (Suzuka Central General Hospital); and Fumihiro Ishida, MD (Shinsyu University Hospital) for providing cases; Mr Hiroshi Yamada (Nagoya University) for performing IHC staining; Keitaro Matsuo, MD (Aichi Cancer Center) for statistical review; and Masao Seto, MD (Aichi Cancer Center) for critical reading of the manuscript.
This work was supported in part by a Grant-in-Aid for Cancer Research (21-6-3) from the Ministry of Health, Labour and Welfare of Japan.
Authorship
Contribution: T.T., K.S., S.N., and T.K. designed the study; K.Y., T. Ichihashi, R.O., M.T., T. Iwasaki, A.I., A.S., H.K., K.K., K.M., M.T., S.T., Y.S., T.M., and T.K. provided cases; S.N. reviewed the pathologic materials; T.T., K.S., S.N., and T.K. collected data; T.T., K.S., and T.K. analyzed and interpreted data; T.T., K.S., and D.C. performed the statistical analysis; and T.T., K.S., T.N., S.N., and T.K. wrote the manuscript. All authors have read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tomohiro Kinoshita, Department of Hematology and Cell Therapy, Aichi Cancer Center, 1-1 Kanokoden, Chikusa-ku, Nagoya 464-8681, Japan; e-mail: kinosita@aichi-cc.jp.