Abstract
Pediatric patients with SCID constitute medical emergencies. In the absence of an HLA-identical hematopoietic stem cell (HSC) donor, mismatched related-donor transplantation (MMRDT) or unrelated-donor umbilical cord blood transplantation (UCBT) are valuable treatment options. To help transplantation centers choose the best treatment option, we retrospectively compared outcomes after 175 MMRDTs and 74 UCBTs in patients with SCID or Omenn syndrome. Median follow-up time was 83 months and 58 months for UCBT and MMRDT, respectively. Most UCB recipients received a myeloablative conditioning regimen; most MMRDT recipients did not. UCB recipients presented a higher frequency of complete donor chimerism (P = .04) and faster total lymphocyte count recovery (P = .04) without any statistically significance with the preparative regimen they received. The MMRDT and UCBT groups did not differ in terms of T-cell engraftment, CD4+ and CD3+ cell recoveries, while Ig replacement therapy was discontinued sooner after UCBT (adjusted P = .02). There was a trend toward a greater incidence of grades II-IV acute GVHD (P = .06) and more chronic GVHD (P = .03) after UCBT. The estimated 5-year overall survival rates were 62% ± 4% after MMRDT and 57% ± 6% after UCBT. For children with SCID and no HLA-identical sibling donor, both UCBT and MMRDT represent available HSC sources for transplantation with quite similar outcomes.
Introduction
Primary immune deficiencies (PIDs) are a heterogeneous group of genetic disorders of the immune system. They are characterized by impairment of innate or adaptive immunity and, in some cases, other concomitant disorders. SCIDs are among the most severe T-cell immune deficiencies; sufferers are highly susceptible to infections and invariably die within the first year of life if not treated.1 In 1968, hematopoietic stem cell transplantation (HSCT) from an HLA-identical sibling donor was shown to correct the immune function of a patient with SCID2 and has since become the treatment of choice, with overall survival (OS) at 1 year having risen from 80% to 90%.3-5 Unfortunately, because SCID is a genetic disease, less than 25% of these children will have a healthy, HLA-matched sibling donor available.
The development of techniques for removing mature T cells from BM grafts from mismatched related donors has made HSCT applicable to virtually all patients.6 With better management of supportive care and CD34+ immunoselection, the OS of recipients of mismatched related donor transplantations (MMRDTs) has significantly improved and ranges from 54%-78% in more recent studies.3-5 A study of the period between 2000 and 2005 found that there was no significant difference in survival comparing a well-HLA–matched unrelated adult stem cell with a mismatched relative5 ; however, it is extremely important to bear in mind that the time needed to find an appropriate, unrelated donor is a serious limiting factor in this medical emergency setting.
Umbilical cord blood transplantation (UCBT) was first reported in 1989.7 Since that time, more than 20 000 UCBTs have been performed worldwide (mainly for hematologic disorders).8 One advantage of UCB as a stem cell source is the acceptable degree of HLA mismatch, which increases the likelihood of finding a suitable, unrelated donor. Given the urgency of these medical situations, UCB constitutes a more readily available stem cell source than an HLA-matched, unrelated HSCT donor. It has been shown recently that UCBT in PID patients is a feasible alternative.9-12
A critical end point after HSCT for PID is successful immune reconstitution and the latter's kinetics. In fact, after transplantation of a T cell–depleted graft, circulating T cells may not be detected for several months and normal counts are generally only achieved between 6 and 12 months after transplantation. Slow T-cell recovery is responsible for significant mortality and morbidity and worsens the overall prognosis during the first year after transplantation.13,14 In UCBT, infused T cells are functionally naive and posttransplantation immune reconstitution is slow compared with recipients of nonmanipulated BM.15-17 However, to the best of our knowledge, UCBT and MMRDT recipients have never been formally compared in terms of the time course of immune reconstitution.
In the absence of an HLA-identical sibling donor, the use of immediately available sources of HSCs (such as MMRDs and UCB) is subject to ongoing debate to determine the first-chance therapy for SCID. Therefore, in the present study, we assessed and compared the outcomes (ie, survival, occurrence of GVHD, engraftment, and immune recovery) after MMRDT and UCBT in a large cohort of children with SCID and Omenn syndrome in a registry-based, retrospective study.
Methods
Patients
Data were collected from 2 European registries (Eurocord and Stem Cell Transplant for Immunodeficiencies in Europ [SCETIDE]) operated for the European Group for Blood and Marrow Transplantation (EBMT). Participation was open to European and non-European centers performing HSCT with MMRDs or UCB for primary, severe T-cell immune deficiencies. Data concerning patient and disease characteristics and transplantation outcomes were collected with standardized questionnaires. Additional questionnaires were sent out to collect missing or supplementary data.
Thirty-two centers supplied their data (between 1 and 50 cases per center) for analysis. Each center was responsible for the quality control of its own data. Data from Eurocord and SCETIDE were checked and validated by a physician (J.F.F.) with experience with HSCT in PID patients. The inclusion criteria for this retrospective analysis were as follows: (1) children with severe T-cell deficiencies (SCIDs or Omenn syndrome); (2) consecutive patients undergoing MMRDT or UCBT in participating centers; and (3) transplantations performed between January 1995 and December 2005 inclusive. A total of 249 patients met these inclusion criteria (MMRDT, n = 175; UCBT, n = 74). All participating centers obtained informed consent from the parents to use blinded information for scientific purpose in accordance with the Declaration of Helsinki. The design and the objectives of the study were approved by the scientific board of Eurocord, SCETIDE, and EBMT.
Definition of outcomes
Myeloid and T-cell engraftment.
Engraftment was analyzed in patients having survived for more than 28 days after transplantation and was defined as an absolute neutrophil count above 0.5 × 109/L for 3 consecutive days and/or donor chimerism. Lineage-specific chimerism data for the myeloid (CD3−) or T-cell (CD3+) compartments were assessed for patients surviving 6 months after transplantation, and were available for a subset of patients. For each cell lineage, mixed chimerism was defined as the presence of 5%-95% of donor-derived cells. Engraftment failure was defined by the absence of neutrophil recovery or the presence of at least 95% recipient chimerism (in both CD3+ and CD3− compartments).
GVHD.
OS.
OS time was defined as the time between transplantation and death from any cause.
Immune recovery.
Immune recovery was analyzed in the subgroup of patients with donor chimerism who were alive at least 6 months after transplantation. Total lymphocyte and absolute CD3+ and CD4+ cell counts were assessed at 6, 12, and 24 months after transplantation. The time to B-cell function recovery was estimated from the date of discontinuation of intravenous Ig (IVIg) replacement therapy (ie, the time between transplantation and the last day of IVIg infusion).
Statistical analysis
The MMRDT and UCBT groups were compared in terms of patient-, disease-, and transplantation-related variables using a χ2 test or Fisher exact test for categorical variables and the Mann-Whitney test for continuous variables. The primary end point was OS after transplantation according to the Kaplan-Meier method. Cumulative incidence functions (CIFs) considering death and repeated transplantations as competing events were used to estimate other end points, such as the incidence of GVHD and the discontinuation of Ig replacement therapy. Univariate analyses were performed using a log-rank test for OS and the Gray test for CIFs. Total lymphocyte and absolute CD3+ and CD4+ cell counts measured 6, 12, and 24 months after transplantation were compared using the Mann-Whitney test. Multivariate analyses were performed using a Cox proportional hazard model for OS and a Fine-Gray model for CIFs.20 Factors with significantly different distributions in the 2 groups or that were significantly associated with OS were included in the multivariate model. All tests were 2-sided and the threshold for statistical significance was set to P < .05. Statistical analyses were performed with the SPSS Version 18 and S-Plus Version 8.1 (TIBCO) software packages.
Results
Patient, donor, and disease characteristics
Table 1 shows the characteristics of children with severe T-cell deficiencies who received MMRDT (n = 175) or UCBT (n = 74). A high proportion of the MMRDTs (88%) were performed in 4 European centers. In contrast, UCBTs were performed in 30 different centers, with 19 centers performing 1 or 2 transplantations and 4 centers performing more than 5 transplantations. Only 4 centers performed both types of transplantations. In comparison with MMRDT recipients, UCBT recipients had undergone transplantation more recently (P = .03) and tended to have a longer median time between diagnosis and transplantation (P = .06). The UCBT group had a higher proportion of patients without T and B lymphocytes (T−B−) or Omenn syndrome phenotype than the MMRDT group (P = .01). Before transplantation, there were no intergroup differences in terms of the frequency of failure to thrive, chronic diarrhea, respiratory impairment, or the number of infections. However, pretransplantation viral infections were more common in the MMRDT group (P = .02).
In the MMRDT group, the majority of patients (56%) received BM as the stem cell source; most of the latter (68%) had 3 of 6 HLA mismatches. All MMRD grafts were T-cell depleted (mostly via CD34+ cell selection). In the UCBT group, 50 (67%) patients received grafts with 0 or 1 of 6 HLA mismatches and 24 (33%) received grafts with 2 or 3 HLA mismatches (considering HLA-A and HLA-B in low-resolution typing and DRB1 in high-resolution typing).
Transplantation characteristics
Preparative regimens varied according to the transplantation center, the disease phenotype, and the patient's health status before transplantation (Table 1). Thirty transplantations in the MMRDT group and 7 in the UCBT group were performed in the absence of conditioning. The majority of conditioning regimens contained busulfan, which was combined with cyclophosphamide or fludarabine. Moreover, UCBT recipients were more likely to undergo myeloablative conditioning than were MMRDT recipients (P = .04). Furthermore, the proportion of patients receiving antithymocyte globulin or other mAbs was higher in the UCBT group than in the MMRDT group (P = .001). The majority of MMRDT recipients (n = 119) did not receive any GVHD prophylaxis in addition to T-cell depletion. Seventy-one (95%) of the UCBT recipients received cyclosporine A (combined with steroids in 43 of these patients).
Outcomes
Myeloid and T-cell engraftment.
At 28 days after transplantation, 162 MMRDT recipients and 70 UCBT recipients were evaluable for engraftment. Engraftment was observed in 126 patients (78%) in the MMRDT group and 60 (86%) in the UCBT group (P = .14). Lineage-specific chimerism data were available for a subset of patients surviving 6 months after transplantation (MMRDT, n = 77; UCBT, n = 36). When considering the (CD3+) T-cell compartment, 88% of MMRDT recipients and 97% of UCBT recipients had full donor chimerism (P = .29). In the myeloid compartment (CD3−), full donor chimerism was achieved by more patients in the UCBT group (75%) than in the MMRDT group (33%; P = .001). This difference remained statistically significant when analyzing patients receiving full myeloablative conditioning regimens and those receiving reduced-intensity conditioning regimens separately (data not shown). Two years after MMRDT, 2 patients presented late graft failure (3 and 12 years after transplantation), but none after UCBT.
Acute and chronic GVHD.
There was a trend toward a higher cumulative incidence of grades II-IV acute GVHD in UCBT recipients relative to MMRDT recipients (34% ± 6% and 22% ± 3%, respectively; P = .06). Furthermore, the cumulative incidence of chronic GVHD was significantly higher in the UCBT group than in the MMRDT group (22% ± 5% and 10% ± 2%, respectively; P = .03). Nineteen patients in the MMRDT group had chronic GVHD (limited, n = 11; extensive, n = 8) compared with 17 patients in the UCBT group (limited, n = 9; extensive, n = 8).
Immune recovery.
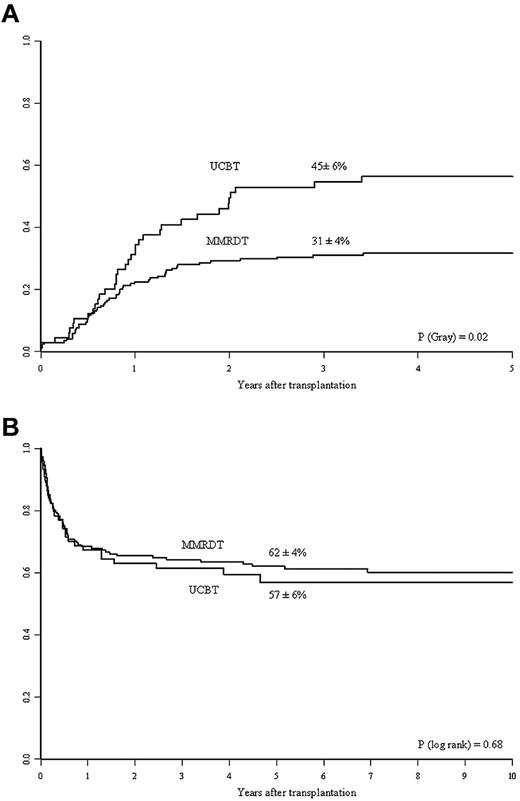
Table 2 shows the number of patients with data on lymphocyte, CD3+, and CD4+ cell counts at 6, 12, and 24 months after transplantation. The UCBT recipients had greater total lymphocyte recovery than the MMRD recipients (P = .043). However, there were no statistically significant differences between the 2 groups in terms of T-cell lymphocyte (CD3+ and CD4+) recoveries at any time point. Compared with MMRDT recipients, the UCBT recipients had a higher cumulative incidence of discontinuation of IVIg replacement therapy. At 3 years, 45% ± 6% of the UCBT recipients had discontinued Ig replacement therapy, compared with 31% ± 4% of the MMRDT recipients (P = .023; Figure 1A). This observation was confirmed in a multivariate analysis after adjustment for intergroup differences (Table 3). Other factors associated with better B-cell function recovery were the absence of pretransplantation infections and use of a myeloablative conditioning regimen (not shown).
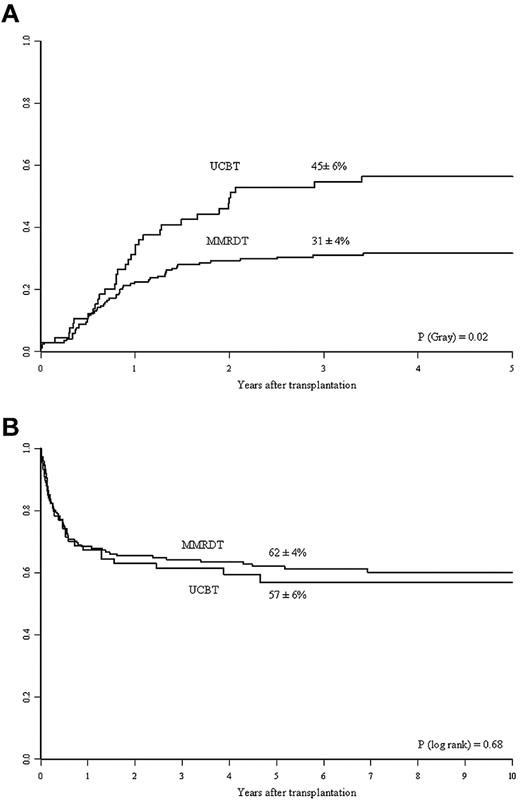
Kinetics of Ig therapy discontinuation and overall survival in the 2 study groups. (A) Three-year cumulative incidence of discontinuation of IVIg replacement therapy in the MMRDT and UCBT groups. (B) Probability of 5-year OS for SCID patients according to the use of MMRDT or UCBT.
Kinetics of Ig therapy discontinuation and overall survival in the 2 study groups. (A) Three-year cumulative incidence of discontinuation of IVIg replacement therapy in the MMRDT and UCBT groups. (B) Probability of 5-year OS for SCID patients according to the use of MMRDT or UCBT.
OS.
In a nonadjusted, univariate analysis, the estimated 5-year OS was 62% ± 4% for MMRDT recipients and 57% ± 6% for UCBT recipients (P = .68; Figure 1B). In the MMRDT group, 46 (28%) patients underwent a second transplantation for nonengraftment compared with 7 (9.5%) in the UCBT group (P = .002); of these, 59% and 47% survived, respectively. In a multivariate analysis adjusted for differences between the 2 groups (ie, year of transplantation, diagnosis, presence of pretransplantation infections, and the use of myeloablative conditioning), there was no significant difference in OS between the 2 groups (P = .58; Table 3). Other prognostic factors associated with decreased survival are given in Table 3. A total of 97 patients died: 67 in the MMRDT group and 30 in the UCBT group. The causes of death are shown in Table 4. It is noteworthy that infections represented the most frequent cause of death in both groups.
Discussion
The objective of the present study was to compare the outcomes of transplantation with 2 different stem cell sources retrospectively to help transplantation centers choose the best treatment option. There were notable differences in the number of transplantations performed by each transplantation center. The fact that few centers performed MMRDT is almost certainly explained by the need for specific ex vivo graft processing technology to eliminate mature donor T cells.4 In contrast, many transplantation centers were able to perform UCBT because of the absence of graft manipulation in this latter technique. A center effect on outcomes has been reported for MMRDT21 and UCBT (Eurocord unpublished data) procedures in children with acute lymphoblastic leukemia. Unfortunately, only 4 centers in our study performed both techniques, and this prevented us from testing for a center effect that could have introduced bias into this retrospective study. Nevertheless, we showed that the UCBT and MMRDT groups did not differ significantly in terms of 5-year survival despite a higher incidence of chronic GVHD in UCBT recipients.
The 2 groups were found to differ significantly in terms of several patient-, disease-, and transplantation-related factors. It is well known that a B-negative SCID phenotype influences the outcome whether assessed as survival or immune reconstitution.4,5,14,22,23 In the present study, more UCBT recipients with B-negative SCID phenotype were enrolled. However, in a multivariate analysis, only patients with Omenn syndrome had a relatively low survival rate. Conversely, SCID patients who underwent MMRDT were more likely to have suffered from a viral infection before transplantation, a setting known to be associated with an adverse prognosis.4,5
Another donor-related factor was the significantly different number of HLA mismatches in the 2 groups. The majority of MMRDT patients had 3 of 6 HLA mismatches, whereas UCBT patients received grafts with 0 HLA mismatches (ie, 6 matches; n = 21) or 1 of 6 HLA mismatches (ie, 5 matches; n = 29). It is interesting to speculate on how to build an algorithm for UCBT versus MMRDT donor choice. It is well known that the outcome for UCBT patients with nonmalignant diseases is correlated with the cell dose (the number of cells infused per kilogram of bodyweight)24 and the degree of HLA mismatch. Cell dose is not an issue in this particular pediatric population. The OS was higher in UCBT patients with 6 of 6 matches (76% ± 9%, n = 21) or 5 of 6 matches (62% ± 10%, n = 29) than in those with 4 of 6 HLA matches (35% ± 10%, n = 24; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In fact, because only 24 UCBT recipients were given a HLA 4 of 6 or 3 of 6 CB graft, we were not able to compare this group with the MMRDT group because of the impossibility of adjusting for the risk factors. Therefore, any definitive conclusion based on this small population should be taken with caution because we do not know if the worse outcomes observed were independently associated with a 4 or 3 of 6 CB graft or other, patient-, disease-, or transplantation-related factors. However, based on a larger series of patients with nonmalignant diseases (including aplastic anemia, metabolic diseases, and other genetic diseases), Eurocord has recommended avoiding grafts with 2 or more HLA mismatches because of worse outcomes.25
The MMRDT and UCBT groups had similar engraftment rates. However, a higher proportion of patients in the MMRD group had to undergo a repeated transplantation as a result of poor graft function compared with UCB recipients. This finding could be related to the higher frequency of complete donor chimerism in myeloid cells in UCBT recipients, as reported previously.5,26 However, the higher proportion of myeloid donor chimerism may also be related to the fact that most UCBT recipients underwent myeloablative conditioning. In a multivariate analysis, we found that use of a myeloablative preparative regimen was associated with better OS. In both groups of patients, the major cause of death was infection; this emphasizes the importance of the time course of immune reconstitution. Despite the observation that UCB recipients had higher total lymphocyte counts during the first year after transplantation, CD3+ and CD4+ T-cell numbers did not significantly differ at any time point. Therefore, the higher total lymphocyte counts recorded soon after transplantation in UCB recipients probably correspond to B and/or natural killer cells, as has already been reported by the Eurocord group and others.11,15,17 Accordingly, the UCBT recipients studied here were able to discontinue IVIg replacement therapy sooner and more frequently compared with MMRDT recipients. However, this parameter did not appear to influence the total number of deaths from infections in the 2 groups.
It would be interesting to look at long-term outcomes, because this analysis gather patients with long term follow up. However, because of the retrospective and multicenter nature of our study, these outcomes were not defined previously and might reflect only a tendency for long-term follow-up of these patients. In addition, we were able to collect information on only a subset of this group of patients. Neurologic development, growth, and school attendance were normal in 88%, 55%, and 89% of patients surviving more than 2 years in the MMRDT group and in 90%, 66%, and 74% in the UCBT group, respectively. Secondary malignancies were observed in 3 patients after MMRDT, one with a posttransplantation lymphoproliferative disease and 2 with myelodysplastic syndrome (MDS). These 2 patients with MDS were SCID patients with reticular dysgenesis, and the development of MDS was associated with a genetic defect rather than with a transplantation modality.27 None of the patients developed secondary malignancies after UCBT.
In conclusion, MMRDT and UCBT are both valid options in SCID patients lacking an HLA-identical sibling donor. Therefore, each transplantation center should choose the strategy as a function of their own experience and skills, while considering the rapid availability of suitable UCB or the availability of techniques for the efficient removal of mature T cells from MMRD grafts. Strategies capable of speeding up immune reconstitution in these severely affected patients could produce significant improvements in outcome for both transplantation approaches.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chaim Roifman (Hospital for Sick Children, University of Toronto, ON) for his helpful contribution in the critical revision of the paper; the families of the patients for their continuous support of the study; the medical and nursing staff of all of the contributing centers; and the European Group for Blood and Marrow Transplantation (EBMT), Stem Cell Transplant for Immunodeficiencies in Europe (SCETIDE), and Eurocord statistical staff.
The following centers and people contributed to the acquisition of data: Barcelona: C. Diaz de Heredia and J. Ortega; Berlin: W. Ebell; Brescia: F. Porta; Brussels, C. Vermylen; Curitiba: C. Bonfim; Dusseldorf: A. Borkhardt; Göteborg, A. Fasth; Halle: D. Koerholz; Kobe: K. Kosaka; Leiden: R. Bredius; Lisbon: M. Abecassis; London: P. Veys and H. B. Gaspar; Lyon: Y. Bertrand; Madrid: M. A. Diaz; Melbourne: K. Tiedemann; Missouri: D. Pietryga; Newcastle: A. R. Gennery, M.Slatter, and A. J. Cant; Paris: A. Fischer, B. Neven, D. Moshous, M. Cavazzana-Calvo, P. Taupin, and P. Landais; Pavia: F. Locatelli; Prague: P. Sedlacek; Randwick, T. O'Brien; Rhyiad: M. Ayas; Rio de Janeiro: L. F. Bouzas; Saint Louis: D. Wall and A. P. Knutsen; San Antonio: D. Wall, K. W. Chan, and M. Grimley; Singapore: T. A. Moy; Stockolm: J. Winiarski; Sydney: P. Shaw; Torino: F. Fagioli; Ulm: A. Schultz and W. Friedrich; Utrecht: J. J. Boelens; Vienna: C. Peters; Wroclaw: K. Kalwak; and Zürich: T. Güngör and R. Seger.
J.F.F. was provided an unrestricted grant by Eurocod, a nonprofit organization that is working in close collaboration with the EBMT and the French agence de la Biomedecine. Eurocord has established a worldwide registry of patients treated with cord blood transplantations and provides data to the cord blood banks for quality control. For this article, apart from giving financial support to J.F.F., Eurocord provided registry data on patient outcome with the approval of the transplantation centers, extracted data, verified the quality of the data entry, and helped in the analysis.
Authorship
Contribution: J.F.F., V.R., E.G., and M.C.-C. designed and supervised the study; B.N., D.M., A.R.G., W.F., F.P., C.D.d.H., D.W., Y.B., P.V., M.S., A.S., K.W.C., M.G., M.A., T.G., W.E., C.B., K.K., and H.B.G. contributed to the acquisition of data; J.F.F., V.R, M.L, P.T., P.L, A.F., E.G., and M.C.-C. analyzed and interpreted the data; J.F.F., V.R., E.G., and M.C.-C. wrote the manuscript; V.R., A.R.G., M.S., K.W.C., M.A., S.B., A.F., E.G., and M.C.-C. contributed to the critical revision of the manuscript; and J.F.F., V.R., M.L., and P.L. performed the statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of Inborn Errors Working Party members, please see the supplemental Appendix.
Correspondence: Marina Cavazzana-Calvo, Dept of Biotherapy, Hôpital Necker Enfants-Malades, 149 rue de Sèvres, 75015 Paris, France; e-mail: m.cavazzana@nck.aphp.fr.