Abstract
Despite proven benefits, prophylactic treatment for hemophilia A is hampered by the short half-life of factor VIII. A recombinant factor VIII-Fc fusion protein (rFVIIIFc) was constructed to determine the potential for reduced frequency of dosing. rFVIIIFc has an ∼ 2-fold longer half-life than rFVIII in hemophilia A (HemA) mice and dogs. The extension of rFVIIIFc half-life requires interaction of Fc with the neonatal Fc receptor (FcRn). In FcRn knockout mice, the extension of rFVIIIFc half-life is abrogated, and is restored in human FcRn transgenic mice. The Fc fusion has no impact on FVIII-specific activity. rFVIIIFc has comparable acute efficacy as rFVIII in treating tail clip injury in HemA mice, and fully corrects whole blood clotting time (WBCT) in HemA dogs immediately after dosing. Furthermore, consistent with prolonged half-life, rFVIIIFc shows 2-fold longer prophylactic efficacy in protecting HemA mice from tail vein transection bleeding induced 24-48 hours after dosing. In HemA dogs, rFVIIIFc also sustains partial correction of WBCT 1.5- to 2-fold longer than rFVIII. rFVIIIFc was well tolerated in both species. Thus, the rescue of FVIII by Fc fusion to provide prolonged protection presents a novel pathway for FVIII catabolism, and warrants further investigation.
Introduction
Hemophilia A is an X-linked bleeding disorder caused by deficiency of factor VIII (FVIII) activity.1 The disease is characterized by spontaneous hemorrhage and excessive bleeding after trauma. Over time, repeated bleeding into muscles and joints, which begins in early childhood, results in hemophilic arthropathy and irreversible joint damage. This damage is progressive and leads to pronounced musculoskeletal morbidity.1 Prophylaxis significantly reduces joint damage and long-term sequelae, and improves quality of life in comparison to on-demand treatment.2,3 However, the short half-life (10-12 hours) of FVIII necessitates dosing every other day or 3 times per week by IV injection for full prophylaxis.2,4,5 Therefore, a longer-acting FVIII would represent a key advancement in the management of hemophilia A.
We have developed a recombinant factor VIII-Fc (rFVIIIFc) fusion protein to extend the half-life of FVIII by leveraging a naturally occurring biologic pathway. rFVIIIFc is a heterodimeric protein comprising a single B-domain–deleted (BDD) FVIII linked recombinantly to the Fc domain of human IgG1 (IgG1). The Fc domain enables binding to the neonatal Fc receptor (FcRn), which is responsible for protection of IgG from degradation and facilitates its recycling,6,7 resulting in a half-life for IgG of ∼ 3 weeks in humans. The Fc domain of IgG1 has been fused to growth factors, cytokines, enzymes, and ligand-binding regions of receptors8-10 ; several of these fusion proteins have been approved as therapeutics (eg, etanercept, abatacept, belatacept, alefacept, rilonacept, aflibercept, and romiplostim). However, traditional dimeric Fc fusions, created through the fusion of the monomeric effector protein to a monomer of Fc and then coupled through a disulfide bond to create a dimer, were not effective for large coagulation proteins such as FVIII. Thus, we have developed methods to create novel Fc fusion protein constructs in which a single (monomeric) effector molecule is attached to Fc.11-13 We have applied this approach to several proteins, including human rFIX,14 rFVIIa,15 and BDD rFVIII. This report evaluates the pharmacokinetics and efficacy of rFVIIIFc compared with rFVIII in mouse and dog models of hemophilia A, in support of subsequent human studies.
Methods
Recombinant FVIII-Fc fusion protein
The recombinant FVIII-Fc fusion protein (rFVIIIFc) expression plasmid pBUDCE4.1 (Invitrogen) contains 2 expression cassettes. One expresses, under the control of CMV promoter, the native human FVIII signal sequence followed by BDD FVIII (S743 to Q1638 fusion) directly linked to the Fc region of human IgG1 (amino acids D221 to K457, EU numbering) with no intervening sequence. The other uses the EF1α promoter to express the Fc region alone with a heterologous mouse IgκB signal sequence. Human embryonic kidney 293 cells (HEK293H; Invitrogen) were transfected with this plasmid, and a stable clonal suspension cell line was generated that expressed rFVIIIFc. Protein was purified from defined cell-culture harvest media using a 3-column purification process, including a FVIII-specific affinity purification step16 followed by a combination of anion exchange and hydrophobic interaction chromatographic steps.
Recombinant FVIII
Recombinant BDD FVIII (ReFacto and Xyntha) and full-length FVIII (Advate) were purchased from Novis Pharmaceuticals and reconstituted according to the manufacturer's instructions.
Animals
The hemophilia A (HemA) mice bearing a FVIII exon 16 knockout on a 129 × B6 background were obtained from Dr H. Kazazian (University of Pennsylvania)17 and bred at Biogen Idec Hemophilia. Murine FcRn knockout (FcRn KO) and human FcRn transgenic (Tg32B) mice were derived from C57BL/6J mice and were obtained from Dr Derry Roopenian (The Jackson Laboratory). The genotypes for FcRn KO mice are mFcRn (−/−) and mβ2m (−/−), and for Tg32B are mFcRn (−/−), mβ2m (−/−), hFcRn (+/+), and hβ2m (+/+). C57BL/6 mice were purchased from The Jackson Laboratory. All animal activities were approved by the Institutional Animal Care Committees and performed in accordance with the Guide to the Care and Use of Laboratory Animals.18
Pharmacokinetic studies in mice
The pharmacokinetics (PK) of rFVIIIFc and recombinant FVIII (rFVIII; Xyntha) were evaluated in HemA, C57BL/6, FcRn KO, and Tg32B mice after an IV dose of 125 IU/kg. Blood was collected from the vena cava in one-tenth volume of 4% sodium citrate at 5 minutes, and 4, 8, 16, 24, 32, 48, 54, and 72 hours postdosing for rFVIIIFc and at 5 minutes, and 1, 4, 8, 16, 20, 24, 32, and 48 hours after dosing for rFVIII (4 mice/time point/treatment). Plasma was snap-frozen in an ethanol/dry-ice bath and stored at −80°C until analysis for FVIII activity using a human FVIII-specific chromogenic assay. The pharmacokinetic parameters were estimated by noncompartmental modeling using WinNonLin Version 5.2 (Pharsight).
Efficacy studies in HemA mice
All efficacy studies were performed blinded. Acute efficacy was studied in the tail-clip bleeding model. Male HemA mice (8-12 weeks old) were anesthetized with a cocktail of 50 mg/kg ketamine and 0.5 mg/kg dexmedetomidine. The tail was then immersed in 37°C saline for 10 minutes to dilate the lateral vein followed by tail vein injection of rFVIIIFc, rFVIII (Advate), or vehicle. Five minutes later, the distal 1 cm of the tail was clipped and the shed blood was collected into 13 mL of warm saline for 30 minutes. The blood loss was quantified gravimetrically.
The prophylactic efficacy was studied in the tail vein transection (TVT) bleeding model as described previously21 except that HemA mice received a single IV administration of 12 IU/kg rFVIIIFc, rFVIII (Advate), or vehicle at 24 or 48 hours before the transection of a lateral tail vein. The dose of 12 IU/kg was identified from a prior dose-response experiment with rFVIII in which 12 IU/kg achieved 50% protection of HemA mice from a TVT injury inflicted 24 hours after dosing (data not shown).
Hemophilia A dog studies
In a single dose PK/PD study of rFVIIIFc, 2 naive hemophilia A dogs (M10 and M11) received an IV dose of 125 IU/kg. Blood samples were collected before dosing and after dosing at 5 and 30 minutes, and 1, 2, 4, 8, 24, 32, 48, 72, 96, 144, and 168 hours for whole-blood clotting time (WBCT). Blood collections for FVIII activity (activated partial thromboplastin time [aPTT] and chromogenic assay), rFVIIIFc Ag (ELISA), hematology, and blood chemistry included the time points listed for WBCT as well as 15 minutes and 3, 6, and 12 hours after dosing.
In the following sequential design study, rFVIII (ReFacto) was administered IV at 114 IU/kg for dog M12 and 120 IU/kg for dog M38. WBCT was measured until clotting times were ≥ 20 minutes (the time consistent with FVIII:C ≤ 1%), and samples were also collected at the specified time points for FVIII activity (aPTT and chromogenic assay), Ag (ELISA), and hematology tests. Then 125 IU/kg rFVIIIFc was administered IV to the same dogs and blood samples were collected for WBCT, aPTT, ELISA, hematology, and serum chemistry. Time points for WBCT included before dosing, and 5 and 30 minutes and 1, 2, 4, 8, 24, 32, 48, and 72 hours after dosing of rFVIII and rFVIIIFc. Blood was also collected at 96, 120, 144, and 168 hours after dosing with FVIIIFc. Blood collections for FVIII activity and Ags included the time points listed for WBCT as well as 15 minutes and 3, 6, and 12 hours after dosing. The WBCT and aPTT were performed as previously described.22
FVIII chromogenic assays
FVIII activity in hemophilia A dog plasma was tested using an automated chromogenic assay on a Sysmex CA1500 instrument with reagents from Siemans Healthcare Diagnostics. The standard curve was generated with the 7th International Standard Factor VIII Concentrate (NIBSC code 99/678) spiked into human FVIII-depleted plasma (Stago USA) at concentrations ranging from 1.5 to 0.016 IU/mL.
FVIII activity in HemA mouse plasma was measured using the Coatest SP FVIII assay from Chromogenix (DiaPharma), following the manufacturer's instructions. The standard curve was generated using rFVIIIFc or rFVIII serially diluted from 100 mU/mL to 0.78 mU/mL in buffer containing naive HemA mouse plasma. To measure the human FVIII activity in C57BL/6, FcRn KO, and Tg32B mouse plasma, the infused rFVIIIFc or rFVIII in mouse plasma was first captured by human FVIII-specific mAb GMA8016 (Green Mountain Antibodies) followed by the standard Coatest assay.
rFVIII- and rFVIIIFc-specific ELISA
rFVIII and rFVIIIFc Ag levels in hemophilia A dog plasma were measured by ELISA following the standard protocol. The FVIII A1 domain-specific mAb GMA-8002 (Green Mountain Antibodies) was used as the capture Ab. HRP-conjugated polyclonal anti-FVIII Ab F8C-EIA-D (Affinity Biologicals) was used to detect rFVIII. HRP-conjugated donkey anti–human (F(ab)′2) 709-036-098 (Jackson Immunologicals) was used to detect rFVIIIFc.
Surface plasmon resonance (SPR) analysis of rFVIIIFc-FcRn interactions
SPR experiments were performed with a Biacore T100 instrument. Research-grade CM5 sensor chips, buffers, and immobilization reagents were purchased from Biacore (GE Heathcare). Single-chain human, canine, and murine FcRn preparations were immobilized using standard amine coupling on adjacent flow cells of a single chip at a density of ∼ 370 resonance units (RU), followed by blocking with ethanolamine. The steady-state association of Fc-containing analytes (FVIIIFc and IgG) with immobilized FcRn of different species was evaluated by sequential injection of analytes at 16 concentrations (0.0625-2000nM) in pH 6.0 running buffer (50mM MES [4-morpholineethanesulfonic acid], 250mM sodium chloride, 2mM calcium chloride, 0.01% Tween 20 [polyethylene glycol sorbitan monolaurate]). Each cycle was performed in duplicate and comprised a 45-minute association phase and a 15-minute dissociation phase, both at a flow rate of 5 μL/min, followed by regeneration with two 60-second injections of 1M Tris-HCl at 25 μL/min. After double reference subtraction (blank flow cell and running buffer alone), binding responses recorded near the end of the association phase were plotted as a function of analyte concentration, and EC50 values (50% of Rmax) were derived by nonlinear regression analysis.
Statistical analyses
The unpaired t test, 1-way ANOVA, the Mann-Whitney test, the Kruskal-Wallis test with Dunn posttest, survival curves, and the associated log-rank test were performed in GraphPad Prism 5 (Graph-Pad Software Inc). A 2-tailed P value < .05 was considered statistically significant.
Results
Recombinant FVIII-Fc fusion protein
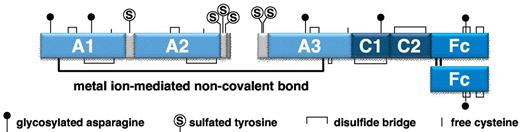
Recombinant FVIII-Fc fusion protein (rFVIIIFc) is a recombinant fusion of human B-domain–deleted FVIII with Fc from human IgG1, with no intervening linker sequence (Figure 1), that was produced in well-characterized HEK 293H cells. The rFVIIIFc is proteolytically cleaved intracellularly to yield an ∼ 90-kDa heavy chain and ∼ 130-kDa light chain-Fc that are bound together noncovalently through a metal bond interaction mediated by the A1 and A3 domains of FVIII.
The average specific activity of rFVIIIFc from 14 separate batches was 8460 ± 699 IU/mg by the 1-stage clotting (aPTT) assay, and 9348 ± 1353 IU/mg by the chromogenic assay, corresponding to 1861 ± 154 and 2057 ± 298 IU/nmol, respectively. The specific activity of rFVIIIFc is comparable with that of wild-type human FVIII in plasma (1429 IU/nmol).23 Thus, the FVIII activity of rFVIIIFc is not affected by fusion of the C-terminus of human FVIII to the N-terminus of human Fc, and the results obtained with the aPTT and chromogenic assays are within ∼ 10% of one another.
Binding of rFVIIIFc to FcRn
The affinity of rFVIIIFc for single-chain mouse, canine, and human FcRn was evaluated using SPR (supplemental Figure 1). The rates of association and dissociation for the complex between rFVIIIFc and mouse FcRn were much slower than those for canine and human FcRn. Half-maximal binding (EC50) of rFVIIIFc to human FcRn was ∼ 4-fold weaker than that to canine FcRn, and > 20-fold weaker than that to mouse FcRn (Table 1). Similarly, human IgG1 also showed the highest affinity to murine FcRn, while binding affinity to canine FcRn was less compared with murine FcRn, but greater compared with human FcRn (Table 1).
FcRn-dependent improvement in pharmacokinetics of rFVIIIFc in mice
Interaction of Fc with FcRn is considered the underlying mechanism for extending half-life for IgG and Fc-fusion proteins. To confirm that this mechanism of action is also responsible for extending half-life of rFVIIIFc, we compared PK profiles of rFVIIIFc with rFVIII in FVIII-deficient (HemA) mice, normal (C57BL/6) mice, FcRn-deficient (FcRn KO) mice, and human FcRn transgenic (Tg32B) mice following a single IV administration of 125 IU/kg (Figure 2).
Half-life extension of rFVIIIFc is dependent on FcRn. PK profiles of rFVIIIFc and rFVIII in (A) HemA mice, (B) C57BL/6 mice, (C) FcRn KO mice, and (D) human FcRn transgenic Tg32B mice following a tail vein injection of 125 IU/kg. Results shown are mean ± SD from 4 mice per treatment at each time point. The PK parameter estimates are summarized in Table 2.
Half-life extension of rFVIIIFc is dependent on FcRn. PK profiles of rFVIIIFc and rFVIII in (A) HemA mice, (B) C57BL/6 mice, (C) FcRn KO mice, and (D) human FcRn transgenic Tg32B mice following a tail vein injection of 125 IU/kg. Results shown are mean ± SD from 4 mice per treatment at each time point. The PK parameter estimates are summarized in Table 2.
The PK parameters (Table 2) were determined by the chromogenic measurement of the human FVIII activity in mouse plasma. The t1/2 of rFVIIIFc was 1.8- to 2.2-fold longer than rFVIII in HemA mice (13.7 vs 7.6 hours) and normal mice (9.6 vs 4.3 hours). The t1/2 extension of rFVIIIFc relative to rFVIII was abolished in FcRn KO mice (6.4 vs 6.9 hours) and restored in human FcRn transgenic Tg32B mice (9.6 vs 4.1 hours). The results thus confirm that the interaction of rFVIIIFc with the FcRn receptor is responsible for its extended t1/2. Furthermore, consistent with the improved t1/2, rFVIIIFc also showed a 1.6- to 2.4-fold longer MRT and a 1.2- to 1.8-fold increased systemic exposure (AUC) compared with rFVIII in FcRn-expressing (HemA, C57Bl/6 and Tg32B) mice but not in FcRn KO mice.
rFVIIIFc is fully active in treating acute bleeds in HemA mice
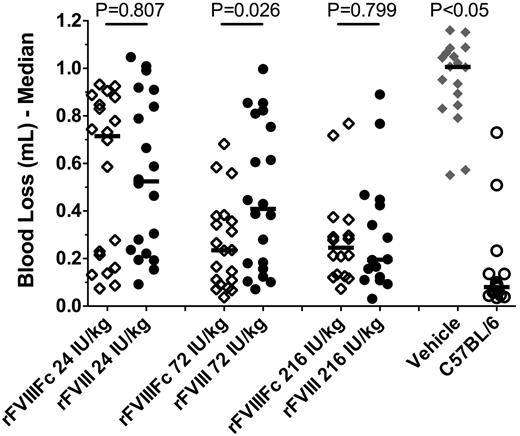
To evaluate the acute efficacy of rFVIIIFc in comparison to rFVIII, HemA mice (16-20 mice/group) were treated with escalating doses (24, 72, and 216 IU/kg) of rFVIIIFc or rFVIII and injured by tail clip 5 minutes after dosing. In comparison to vehicle-treated mice (n = 18) that had a median blood loss of 1 mL, both rFVIIIFc and rFVIII treatments resulted in significantly improved protection (P < .05, Kruskal-Wallis test with Dunn posttest; Figure 3). The median blood loss progressively decreased with increasing doses, reaching maximum reduction to 0.23 mL at 72 IU/kg rFVIIIFc, and 0.20 mL at 216 IU/kg rFVIII. Overall, the blood loss was comparable in animals treated with equal doses of rFVIIIFc or rFVIII, indicating that both therapeutics are comparably active in resolving acute arterial bleeds.
Comparable acute activity of rFVIIIFc and rFVIII in the tail-clip bleeding model. Male HemA mice received a tail vein injection of rFVIIIFc or rFVIII as specified followed by a 10-mm tail clip 5 minutes after dosing. Results presented are individual and median blood loss over 30 minutes following the tail clip from 20 mice in each treatment group. P < .05 for vehicle vs all other treatments, and P > .05 for C57Bl/6 vs HemA mice treated with 72 or 216 IU/kg rFVIIIFc, or 216 IU/kg rFVIII.
Comparable acute activity of rFVIIIFc and rFVIII in the tail-clip bleeding model. Male HemA mice received a tail vein injection of rFVIIIFc or rFVIII as specified followed by a 10-mm tail clip 5 minutes after dosing. Results presented are individual and median blood loss over 30 minutes following the tail clip from 20 mice in each treatment group. P < .05 for vehicle vs all other treatments, and P > .05 for C57Bl/6 vs HemA mice treated with 72 or 216 IU/kg rFVIIIFc, or 216 IU/kg rFVIII.
Prolonged prophylactic efficacy of rFVIIIFc in HemA mice
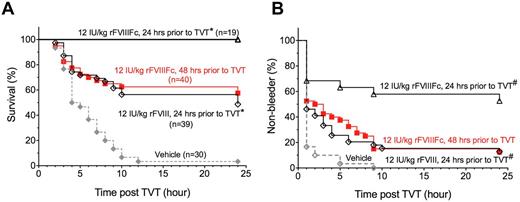
To determine whether prolonged PK leads to prolonged protection from injury, we compared the prophylactic efficacy of rFVIIIFc and rFVIII in HemA mice. Twenty-four hours after an IV dose of 12 IU/kg, 1 lateral tail vein in HemA mice was transected (Figure 4). After injury, 49% of rFVIII-treated mice (n = 39) survived, compared with 100% survival of rFVIIIFc-treated mice (n = 19; P < .001, log-rank test). To further demonstrate that rFVIIIFc sustains a longer duration of efficacy, HemA mice were injured 48 hours after dosing with 12 IU/kg rFVIIIFc. Nevertheless, 58% of rFVIIIFc-treated mice (n = 40) survived, which is similar to that achieved in rFVIII-treated mice (49%) injured at 24 hours after dosing. Both rFVIIIFc and rFVIII treatments are significantly better than the HemA vehicle-control group (n = 30) in which only 3% of mice survived the injury (P < .0001). The improved and prolonged prophylactic efficacy of rFVIIIFc is also evident by the measurement of rebleeding after injury (Figure 4B). Whereas 100% of vehicle-treated HemA mice rebled within 10 hours following tail vein transection, 87% of rFVIII-treated and 47% of rFVIIIFc-treated mice rebled after the injury inflicted at 24 hours after dosing, respectively (P = .002, rFVIIIFc vs rFVIII). The rebleed profile for rFVIIIFc-treated mice injured at 48 hours is largely comparable with that for rFVIII-treated mice injured at 24 hours after dosing. In contrast, both the survival and rebleed profile for rFVIII-treated mice injured at 48 hours are indistinguishable from the profile for the vehicle-control group (data not shown). Therefore, the results indicate that rFVIIIFc protects HemA mice from tail vein injury twice as long as that achieved by the same dose of rFVIII.
Improved prophylactic efficacy of rFVIIIFc relative to rFVIII in the TVT bleeding model. Male HemA mice were injured by TVT either 24 hours following vehicle, or rFVIII, or rFVIIIFc treatment, or 48 hours following rFVIIIFc treatment. The survival and rebleed within 24 hours following TVT are shown in panels A and B, respectively. (A) *P<.001 by log-rank test of the survival curves from animals that received 12 IU/kg rFVIIIFc vs rFVIII 24 hours before TVT. (B) #P = .002 by log-rank test of the non-rebleed curves from animals that received 12 IU/kg rFVIIIFc vs rFVIII 24 hours before TVT.
Improved prophylactic efficacy of rFVIIIFc relative to rFVIII in the TVT bleeding model. Male HemA mice were injured by TVT either 24 hours following vehicle, or rFVIII, or rFVIIIFc treatment, or 48 hours following rFVIIIFc treatment. The survival and rebleed within 24 hours following TVT are shown in panels A and B, respectively. (A) *P<.001 by log-rank test of the survival curves from animals that received 12 IU/kg rFVIIIFc vs rFVIII 24 hours before TVT. (B) #P = .002 by log-rank test of the non-rebleed curves from animals that received 12 IU/kg rFVIIIFc vs rFVIII 24 hours before TVT.
Improved PK/PD of rFVIIIFc in hemophilia A dogs
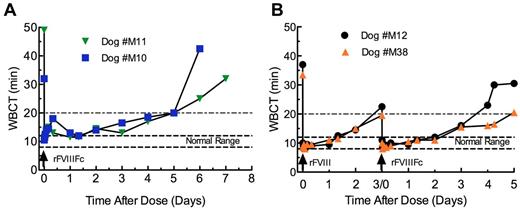
The PK and pharmacodynamics (PD) of rFVIIIFc were also studied in hemophilia A dogs. After an IV dose of 125 IU/kg rFVIIIFc, the WBCT was immediately corrected to normal, which is in the range of 8-12 minutes in normal dogs (Figure 5A-B). The WBCT remained below 20 minutes, indicating FVIII activity > 1%, through ∼ 4 days in 3 of 4 rFVIIIFc-treated dogs and 3 days in the remaining dog (Figure 5A). In dog M12 treated with 114 IU/kg rFVIII and dog M38 with 120 IU/kg rFVIII, the WBCT was also corrected to normal immediately after dosing. However, the WBCT remained below 20 minutes for 2 days in M12 and 3 days in M38, ∼ 1.5- to 2-fold shorter than that achieved by rFVIIIFc (Figure 5B). Furthermore, both rFVIIIFc and rFVIII treatment also improved aPTT clotting time similarly at 5 minutes after dosing (supplemental Table 1).
WBCT of rFVIIIFc and rFVIII in hemophilia A dogs. Normal WBCT range in dogs is shown by the large dashed lines. The area above the small dashed lines (20 minutes) indicates the point at which the plasma FVIII activity is expected to be below 1% of normal. WBCT in hemophilia A dogs of rFVIIIFc (A) and rFVIII followed by rFVIIIFc in a crossover study (B).
WBCT of rFVIIIFc and rFVIII in hemophilia A dogs. Normal WBCT range in dogs is shown by the large dashed lines. The area above the small dashed lines (20 minutes) indicates the point at which the plasma FVIII activity is expected to be below 1% of normal. WBCT in hemophilia A dogs of rFVIIIFc (A) and rFVIII followed by rFVIIIFc in a crossover study (B).
The PK of rFVIIIFc Ag (Figure 6A) was determined by measuring the concentration of rFVIIIFc in plasma with a rFVIIIFc-specific ELISA that detects both the FVIII and Fc portions of the molecule. The t1/2 of rFVIIIFc Ag is 15.7 ± 1.7 hours (Figure 6A), similar to the t1/2 of rFVIIIFc activity (Figure 6B), as measured by the chromogenic assay: 15.4 ± 0.3 hours (Table 3). There is a good correlation between the FVIII activity and the rFVIIIFc Ag data, thereby demonstrating that rFVIIIFc protein was fully active in vivo.
Improved pharmacokinetics of rFVIIIFc compared with rFVIII in hemophilia A dogs after an IV dose. (A) Plasma Ag concentration was measured by ELISA and (B) plasma FVIII activity was measured by chromogenic assay. N = 4 for rFVIIIFc and N = 2 for rFVIII.
Improved pharmacokinetics of rFVIIIFc compared with rFVIII in hemophilia A dogs after an IV dose. (A) Plasma Ag concentration was measured by ELISA and (B) plasma FVIII activity was measured by chromogenic assay. N = 4 for rFVIIIFc and N = 2 for rFVIII.
In 2 of the dogs (M12 and M38) that also received a single dose of rFVIII 72 hours before dosing with rFVIIIFc, the t1/2 of rFVIII Ag was determined to be 6.9 hours and rFVIII activity 7.4 hours. Therefore, the plasma half-life of rFVIIIFc was approximately twice as long compared with that for rFVIII by both Ag and activity measurements.
In addition, platelet count and fibrinogen were assessed to serve as preliminary tests for thrombogenicity. After dosing with either rFVIIIFc or rFVIII, platelet numbers and plasma fibrinogen concentration did not change from predose values (data not shown).
Discussion
In this report, rFVIIIFc has been shown to be fully active in treating acute bleeds in HemA mice, in addition to retaining normal specific activity. Other studies, not reported here, have shown that rFVIIIFc is also fully functional in interacting with FIXa, FX, and phospholipids in forming the Xase complex (R. Peters, rFVIIFc biochemical characterization, manuscript in preparation). Furthermore, the binding affinity to von Willebrand factor (VWF) is comparable between rFVIIIFc and rFVIII, with a Kd of ∼ 1.4 and 0.8nM for rFVIIIFc and rFVIII, respectively (R. Peters, rFVIIIFc biochemical characterization, manuscript in preparation). It is somewhat surprising that the activity of rFVIIIFc is not affected by fusion of the C-terminus of FVIII with the N-terminus of Fc because the C1 and C2 domains of FVIII are involved in phospholipid binding which is essential for the formation of prothrombinase complex on activated platelet surfaces.24 However, the finding is consistent with the observation that residues thought to bind phospholipids, for example, K2092/F2093 in C1, M2199/F2200 and L2251/L2252 in C2, all appear to form a surface that is distant from the C-terminal residues of FVIII.25,26
In our studies, the half-life of rFVIIIFc was doubled only in mice expressing either endogenous murine or transgenic human FcRn, but not in FcRn KO mice (Figure 2, Table 2), demonstrating that the mechanism of prolonging half-life of rFVIIIFc is clearly mediated by FcRn. While it is known that both endothelial and hematopoietic cells contribute equally in recycling internalized IgG to the cell surface to facilitate the longevity of IgG and protection from degradation,27,28 it is not known specifically which FcRn-expressing cell type(s) are responsible for the uptake and recycling of rFVIIIFc. FcRn is broadly expressed in the vascular endothelium, epithelium of kidney, liver, spleen, as well as in bone marrow–derived APCs including macrophages.27-29 Because FVIII circulates largely (∼ 98%) in complex with VWF,30 and both proteins were colocalized to the macrophages in liver and spleen when recombinant FVIII and VWF were coinjected into VWF-deficient mice,31 it is possible that macrophages may play a role in rescue of rFVIIIFc from degradation and prolongation of half-life. However, the results may also suggest a previously unrecognized pathway for FVIII catabolism, and rescue of the protein permitted by Fc fusion.
Approaches in development to extend the half-life of clotting factors include pegylation,32,33 glycopegylation,34,35 and conjugation with albumin.36,37 Interestingly, regardless of the protein engineering used, the half-life of modified rFVIII variants appears to be maximally twice as long as wild-type FVIII in a variety of preclinical animal models.38,39 Consistent results have been demonstrated in humans, for example, rFVIIIFc was reported to improve half-life ∼ 1.7-fold compared with Advate in hemophilia A patients.40 This limitation of extending FVIII half-life appears to be related to VWF. In FVIII and VWF knockout mice, preliminary experiments observed a 5-fold increase in the half-life of rFVIIIFc compared with rFVIII (T.L., unpublished results, October 2011). Similar findings were reported previously in VWF knockout mice using Pegylated-FVIII.33 Taken together, these results suggest that VWF may be a limiting factor for further extending FVIII half-life.
Beyond extending half-life, rFVIIIFc may provide additional benefits. One major challenge with FVIII replacement therapy is the development of neutralizing anti-FVIII Abs (inhibitors). This occurs in 15% to 30% of previously untreated patients. rFVIIIFc has the potential to induce immune tolerance and thus prevent the development of neutralizing Abs. It has been reported that retroviral vector-transduced B cells, presenting FVIII domains as Ig fusion proteins, specifically prevent or decrease existing FVIII Abs in HemA mice.41 It was also found that Fc contains regulatory T-cell epitopes capable of inducing Treg expansion and suppression of Ag-specific immune responses in vitro.42 In addition, the FcRn-mediated transfer of maternal IgG and Fc-fusion proteins across placenta to fetal circulation43,44 could induce neonatal tolerance to rFVIIIFc while also providing needed protection in the newborn from bleeding during delivery.
In conclusion, we have demonstrated that rFVIIIFc provides ∼ 2-fold longer efficacy duration relative to rFVIII in protecting HemA mice from tail-vein transection injury and improving WBCT in HemA dogs. The prolonged efficacy correlates well with a 2-fold extended t1/2 of rFVIIIFc, a result of recycling of the Fc fusion protein via a specific and well-established intracellular pathway. Future studies in humans will evaluate the potential benefit of rFVIIIFc in patients with hemophilia A for decreasing dosing frequency for prophylaxis, treatment of episodic bleeds, and possible protection from the generation of neutralizing Abs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Bob Pape, Amy Correia, and Sandy Walker for their cell culture work; Mark Slein for support on aPTT and chromogenic assays; Joe Salas and Elena Kistanova for cloning of the single-chain FcRn constructs; Kobe Dufu for purification of single-chain FcRn protein; Tamera Ashworth for help with the ELISA assay on HemA dog plasma; Robert Peters for the contribution of Figure 1; John Kulman for advice on the SPR experiment design and data analysis; David Light and Judy Berlfein for review and editing of the manuscript; and the Biogen Idec Hemophilia group for early protein production, characterization, and research support.
Authorship
Contribution: J.A.D., S.C.L., G.F.P., and H.J. designed research, analyzed data, and wrote the manuscript; T.L. designed research, conducted research, and analyzed data; X.Z., G.K., P.S., C.F., and T.R. conducted research and analyzed data; D.D., H.W.G.F., E.P.M., and T.C.N. conducted research; J.M. provided vital reagents; and A.J.B. designed research and analyzed data.
Conflict-of-interest disclosure: J.A.D., T.L., S.C.L., X.Z., G.K., P.S., C.F., D.D., T.R., J.M., A.J.B., G.F.P. and H.J. were employees of Biogen Idec and Biogen Idec Hemophilia (formerly known as Syntonix Pharmaceuticals) when the research was conducted. The remaining authors declare no competing financial interests.
Correspondence: Haiyan Jiang, Biogen Idec Hemophilia, 9 4th Ave, Waltham, MA 02451; e-mail: haiyan.jiang@biogenidec.com.