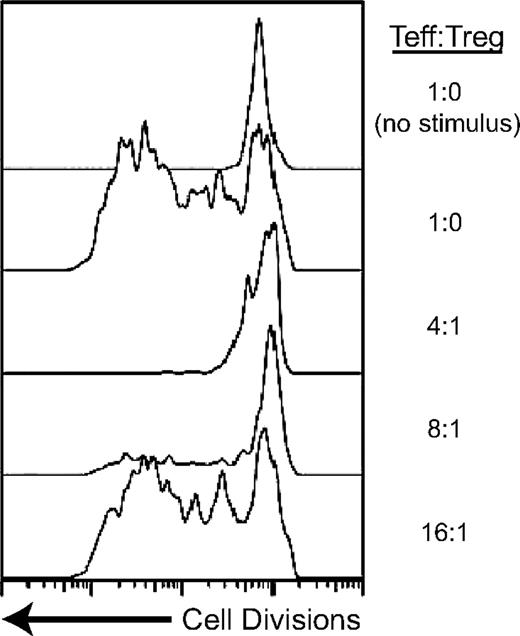
Retargeted class I MHC-restricted Tregs suppress antigen-specific T-cell proliferation. Tregs were transduced with an HLA-A2–restricted TCR specific for NY-ESO-1 and effector T cells (Teffs) with an A2-restricted TCR specific for HIV-1gag. The Teff cells were labeled with CFSE, a dye that intercalates into the cell membrane and is diluted with cell division. The top 2 tracings demonstrate the induction of cell division when Teffs are stimulated with antigen. The bottom tracings demonstrate that in the presence of the NY-ESO-1–specific Tregs, Teff proliferation is inhibited. The Tregs must be stimulated with specific antigen to be able to suppress the Teffs.
Retargeted class I MHC-restricted Tregs suppress antigen-specific T-cell proliferation. Tregs were transduced with an HLA-A2–restricted TCR specific for NY-ESO-1 and effector T cells (Teffs) with an A2-restricted TCR specific for HIV-1gag. The Teff cells were labeled with CFSE, a dye that intercalates into the cell membrane and is diluted with cell division. The top 2 tracings demonstrate the induction of cell division when Teffs are stimulated with antigen. The bottom tracings demonstrate that in the presence of the NY-ESO-1–specific Tregs, Teff proliferation is inhibited. The Tregs must be stimulated with specific antigen to be able to suppress the Teffs.
Foxp3+ regulatory T lymphocytes (Tregs) preserve immune homeostasis and protect against overexuberant immune responses.2 Their absence, either because of FOXP3 mutations impairing their development or their experimental ablation, leads to spontaneous, fulminant multiorgan autoimmunity. Despite their unique role as immune regulators, Tregs are very much T cells. At their core is their T-cell receptor (TCR), a heterodimer expressed on all T cells that recognizes peptide antigens associated with MHC molecules. The antigen specificities of Treg TCRs, however, are distinct from those of other effector T cells. Whereas conventional T cells are generated in the thymus after very low avidity TCR recognition of self-antigens, Tregs are selected after higher avidity recognition.
Clinical and preclinical studies transferring Tregs for the treatment of auto- and alloimmune conditions have shown promise.3 Tregs must be activated to suppress immune responses, and their function is therefore antigen specific. Indeed, Tregs present at sites of autoimmunity are found to recognize tissue-specific self-antigens, often the same autoantigens recognized by effector T cells.
The Treg TCR repertoire is extraordinarily diverse. Few circulating Tregs will therefore possess a desired therapeutic specificity. Isolating and expanding these populations to acquire large doses of targeted human Tregs for immunotherapy remains a significant challenge. One possibility is to engineer specificity into Tregs by giving them a new TCR. TCR-transduced CTLs have shown clinical benefit in cancer immunotherapy, and TCR-modified Tregs have shown therapeutic potency in animal models.4
Tregs are virtually exclusively CD4+, and recognize class II MHC-restricted antigens. Retargeted Tregs may benefit from the acquisition of specificity for class I MHC-restricted antigens. Class I MHC is more broadly expressed than class II, and class I–restricted antigens are produced endogenously rather than exogenously acquired, potentially allowing a more refined targeting to sites of antigen expression. Plesa and colleagues take on the challenge of generating large quantities of functional, class I–restricted human Tregs by the genetic introduction of new TCRs (see figure).
Class I MHC-restricted TCRs would be expected to perform poorly when expressed on Tregs. These TCRs use CD8 as a co-receptor. Tregs possess the CD4 co-receptor engaged by class II MHC, but lack CD8. Loss of co-receptor signaling would be anticipated to decrease ligand sensitivity of retargeted Tregs by an estimated 2log10.5 Plesa et al evaluated the impact of this sensitivity loss, studying TCRs with various affinities and 2 different antigen specificities to identify signaling thresholds for Treg function. Not surprisingly, class I–restricted TCRs with affinities high enough to obviate the requirement for co-receptor signaling successfully retarget Tregs, which are then able to suppress the proliferation of conventional T cells. More interestingly, a class I–restricted TCR with much lower antigen affinity, so low that it is unable to induce cytokine secretion when expressed in conventional CD4+ T cells, is equally capable of inducing Treg suppressive activity.
Signaling thresholds for Treg and conventional T-cell function therefore seem to be quite different. One potential explanation for this is the existence of different TCR signaling requirements for different T-cell activities. For example, induction of cytotoxicity in CD8+ T cells requires a lower signal strength than that needed for cytokine secretion, which in turn occurs with weaker signals than those required to induce proliferation.6 In an analogous manner, Treg-mediated suppression may not need the same signal intensity that cytokine release in effector T cells does. Alternatively, new data on Treg TCR signal transduction indicates that Treg TCRs form a distinct synapse with antigen-presenting cells compared with conventional T cells. The molecular pathways for TCR signaling further differ between the T-cell types, suggesting that Tregs may be biochemically tuned to a different signaling threshold than conventional T cells.7
More refined analyses of how retargeted class I–restricted TCRs stimulate Tregs is needed and will help better define their therapeutic potential. The in vitro assays performed by Plesa et al monitor Treg activity by measuring their ability to suppress conventional T-cell proliferation. Additional mechanisms may be operational in vivo. Establishing how Tregs with dual TCRs, one class I and the other class II–MHC restricted, migrate, survive, and behave in animal models will be necessary to guide future clinical application. Importantly, much as specificity is genetically manipulated here by introducing new TCRs, it may be possible to further manipulate and optimize Tregs by modifying additional pathways. Plesa and colleagues therefore provide us with a significant first step in delineating how human Tregs can be functionally modified, and a start in engineering the next generation of Treg-based cellular immunotherapeutics.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■