Abstract
Epigenetic modifications regulate developmental genes involved in stem cell identity and lineage choice. NFI-A is a posttranscriptional microRNA-223 (miR-223) target directing human hematopoietic progenitor lineage decision: NFI-A induction or silencing boosts erythropoiesis or granulopoiesis, respectively. Here we show that NFI-A promoter silencing, which allows granulopoiesis, is guaranteed by epigenetic events, including the resolution of opposing chromatin “bivalent domains,” hypermethylation, recruitment of polycomb (PcG)–RNAi complexes, and miR-223 promoter targeting activity. During granulopoiesis, miR-223 localizes inside the nucleus and targets the NFI-A promoter region containing PcGs binding sites and miR-223 complementary DNA sequences, evolutionarily conserved in mammalians. Remarkably, both the integrity of the PcGs-RNAi complex and DNA sequences matching the seed region of miR-223 are required to induce NFI-A transcriptional silencing. Moreover, ectopic miR-223 expression in human myeloid progenitors causes heterochromatic repression of NFI-A gene and channels granulopoiesis, whereas its stable knockdown produces the opposite effects. Our findings indicate that, besides the regulation of translation of mRNA targets, endogenous miRs can affect gene expression at the transcriptional level, functioning in a critical interface between chromatin remodeling complexes and the genome to direct fate lineage determination of hematopoietic progenitors.
Introduction
During embryogenesis and postnatal life, development, self-renewal, lineage commitment, and maturation of human hematopoietic stem cells/progenitor cells (HSCs/HPCs) along different lineages are regulated by intimately involved entities: external signals from the BM microenvironment and the dynamic and cooperative interplay between lineage-affiliated transcription factors and posttranscriptional regulators, including microRNAs (miRs).1,2 However, the molecular mechanisms controlling HSC/HPC identity and lineage specification remain poorly understood. Lineage commitment in stem/progenitor cells is accompanied by chromatin modifications occurring at gene promoter “bivalent domains,” with overlapping repressive histone 3 lysine 27 trimethylation (H3K27me3) and activating H3 lysine 4 trimethylation (H3K4me3) marks, which parallel changes in gene expression and transcriptional competence.3-7 This bivalency allows cell commitment to be postponed and, contemporaneously, progenitor cells to be kept primed for an alternate lineage fate.8
Polycomb (PcGs) and trithorax (TrxGs) proteins are responsible for the trimethylation of H3K27 and of H3K4, respectively.9,10 PcGs/TrxGs are evolutionarily conserved transcriptional regulators that preserve the epigenetic memory of each cell type, thus ensuring the correct execution of key developmental programs, including the self-renewal and commitment of hematopoietic cells.11 Several PcGs possess RNA-binding properties, and noncoding RNAs (ncRNAs) can be required for PcG recruitment to DNA.12-14 Small ncRNAs can target homologous promoter DNA sequences and guide chromatin remodeling, thus promoting transcriptional gene silencing (TGS).15-17
The ncRNA family includes miRs, whose expression is tissue specific and is highly regulated according to the cell's developmental lineage and fate.18 miRs mainly mediate posttranscriptional gene silencing through a limited base-pairing in their 5′-end “seed” region and the complementary sequences present in the 3′-untranslated regions of target mRNAs.19 miRs participate in endogenous transcriptional networks that control early development and tissue lineage specification/differentiation in many cell types, including hematopoietic cells.2
Among these networks, we found miR-223 to be functionally involved in a regulatory circuitry that directs human granulopoiesis and includes CCAAT-box binding transcription factors C/EBPα and nuclear factor I-A (NFI-A).20 NFI-A, which belongs to the NFI family of proteins, was initially recognized as a posttranscriptional miR-223 target in hematopoietic cells and subsequently as a transcription factor whose expression levels channel the HSC/HPC erythroid/granulocytic lineage decision: NFI-A induction drives erythropoiesis, whereas its silencing drives granulopoiesis.20-22
Here we show that NFI-A gene silencing required to allow the myeloid progenitor to enter terminal granulocytic commitment results from coordinated epigenetic events triggered by the promoter recognition and transcriptional targeting activity of PcGs and endogenous miR-223.
Methods
Reagents
Reagents included all-trans-retinoic acid, Ara-C (cytosine-1-β-D-arabinofuranoside), cycloheximide, actinomycin-D, and RNase-A and -H (Sigma-Aldrich).
Human samples and cell lines
Normal CD34+ HSCs/HPCs and mononuclear CD34− cell fractions were purified from the BM and peripheral blood (PB) of informed healthy donors as described.20,23 The purity of CD34+ cells (> 90%) was assessed by flow cytometric (FACS) analysis. Leukemia samples were obtained after informed consent from the BM and/or PB of 20 newly diagnosed leukemia patients showing more than 60% leukemic infiltration. Cytogenetic and molecular analyses were performed as reported.23 Cases were classified by the French-American-British (FAB) classification.24 HL60, HL60R, K562, NB4, and NB4-MR4 cell lines and primary acute promyelocytic leukemia (APL) blasts were maintained in a RPMI 1640 GlutaMAX medium supplemented with 50 IU/mL of penicillin, 50 μg/mL streptomycin, and 10% FCS (Invitrogen).
RNA extraction and analysis
Total RNA was extracted from cells using the TRIzol RNA isolation system (Invitrogen). miRs and U6 Northern blot analysis and quantitative RT-PCR using the mir-Vana Detection kit or TaqMan MicroRNA specific assays (Applied Biosystem) were performed as described.20,23 NFI-A, LIM-only-2 (LMO2), and glyceraldehyde phosphate dehydrogenase (GAPDH) mRNAs were measured by quantitative RT-PCR using TaqMan oligonucleotides in the ABI PRISM 7500 (Applied Biosystem). All reactions were performed in triplicate. The relative quantity of miRs and mRNAs was determined by the comparative Ct method using snRNA U6 or GAPDH mRNA levels. Semiquantitative RT-PCRs for the measurement of NFI-A, type II transglutaminase (TGase-II), and GAPDH mRNAs were carried out using the primer sequences listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
ChIP
ChIP assays were performed as described.20,23 Genomic regions on NFI-A and LMO2 genes were amplified using primers designed by the Primer express Version 3.0 software (Applied Biosystems; supplemental Table 2). PCR products obtained from ChIPs performed with anti-Cy5 antibodies were transferred to a Nytran Super Charge membrane (Whatman S&S) and visualized using 32P-labeled specific oligonucleotide probes (supplemental Table 4). RT-PCR quantitation was performed in triplicate samples using the SYBR Green dye detection method and the oligos listed in supplemental Table 2. Chromatin was immunoprecipitated using the antibodies reported in supplemental Table 5. Values obtained for each immunoprecipitated sample were quantified versus the respective input and calculated following the 2−ΔCT method.
Immunoblot, coimmunoprecipitation, and immunophenotypic assays
For immunoblot assays, proteins were fractionated by electrophoresis, electroblotted to nitrocellulose transfer membrane (Protran, Whatman S&S), and probed with antibodies reported in supplemental Table 5. Immunoreactivity was measured using the ECL method (GE Healthcare). Coimmunoprecipitation and immunophenotypic assays were performed by standard procedures reported in supplemental Methods.
RNA immunoprecipitation
miRs were immunoprecipitated from HL60 cell lysates with antibodies against Ying Yang 1 (YY1), Dicer1, Ago1, and rabbit control IgG as nonspecific antibody as described.25 Immunoprecipitated miR-223, Let-7-a3, and miR-126 were detected by quantitative RT-PCR using TaqMan MicroRNA specific assays (Applied Biosystem) and quantified after standardization to reference gene U6 following the 2−ΔCT method. Data were plotted as the ratio of immunoprecipitated miRs (Ipbound) over the respective input and shown as mean ± SD from 3 independent experiments.
Bisulphite sequencing
Bisulphite sequencing assay was performed as described23 on 5 μg of bisulphite-treated genomic DNA using the PCR primers listed in supplemental Table 3.
Plasmid constructs, lentiviral infection, site-directed mutagenesis, and luciferase reporter activity
The lentivirus expressing miR-223 has been previously described.20 The siSuz12 vector was generated by cloning the siSuz12 into the pRRLcPPT.hPGK.EGFP.WPRE (pgk) lentiviral vector. pGIPZ (RHS4346) lentiviral shRNAmir vectors expressing short hairpin RNAs targeting Dicer1 (V3LHS_391373) and Ago1 (V2LHS_15322; target sequences are reported in supplemental Table 6) or the nontargeting control shRNA vector (RHS4346) were purchased from Open Biosystems (supplemental Methods). The NFI-A wild-type promoter sequence from nt −1400 to 24 was cloned in the pGL4.20 luciferase-vector (luc2/puro, Promega; supplemental Methods). All the plasmids were verified by sequencing.
Site-directed mutagenesis was used to mutate 2 putative miR-223 DNA target sequences in the NFI-A promoter region by the Quick Change mutagenesis kit (Stratagene; supplemental Methods). HL60 cells were infected with viral particles carrying the miRZip-223 anti–miR-223 lentiviral construct and the pGreenPuro Scramble Hairpin Control-Construct (System Biosciences). Infected green fluorescent protein-positive cells were sorted and placed in a RPMI-10% FCS medium for further analyses.
Confocal microscopy
Subcellular localization of miR-223, YY1, and Dicer1 was investigated using miRIDIAN miR-223 Mimic, miR-223 inhibitor, Let7-a3 Mimic (Thermo Scientific Dharmacon) and YY1 and Dicer1 antibodies (supplemental Table 5). miRIDIAN Let-7a3 Mimic, miRNA hairpin inhibitor negative control sequence based on Caenorhabditis elegans miR-239b, and miRIDIAN microRNA Mimic negative control 2 were used as specificity controls. AlexaFluor-488 donkey anti–rabbit and AlexaFluor-555 goat anti–mouse (Invitrogen) were used as secondary fluorescent antibodies (supplemental Table 5). miRidian reagents were labeled with Cy5 using label IT siRNA tracker Cy5 labeling reagent (MIR7201-Mirus). Mimic-223, Mimic-Let7-a3, and Mimic negative control 2 Cy5-labeled were transiently transfected in cells following TransIT-TKO transfection reagent instructions. Cy5-labeled–inhibitor-223, –inhibitor negative control, and primary and secondary fluorescent antibodies were added to permeabilized cells (supplemental Methods). Mitotic chromosome spread slides were obtained as described in supplemental Methods. Images were obtained using the Leica TCS-SP5 with a 63× oil objective and LAS-AF software (Leica Microsystems) and CLSM Zeiss 700 confocal laser scanning microscopes with a 100× oil objective and ZEN 2003 software.
Statistical analysis
Data are presented as mean ± SD or SEM, derived from at least 3 independent experiments. Statistical significance between means was assessed by Student t test. P < .05 was considered significant.
Results
Expression levels of NFI-A and miR-223 in myeloid cells
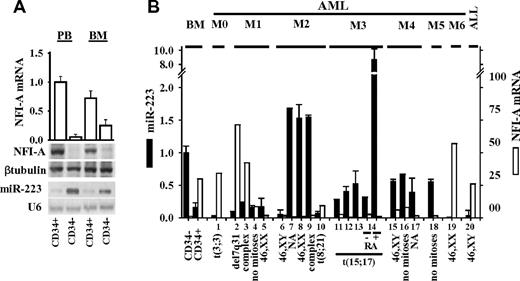
In normal human hematopoietic CD34+ HSCs/HPCs undergoing granulocytic differentiation, we consistently observed an inverse correlation between the expression levels of mature miR-223 and those of NFI-A protein/mRNA.21,22,26 Immature CD34+ HSCs/HPCs isolated from normal donor BM or PB expressed low miR-223 and high NFI-A protein/mRNA levels. High miR-223 and barely detectable NFI-A protein/mRNA levels were detected in CD34− BM and PB cell fractions, respectively, consisting of approximately 60% to 70% granulocytic precursors and mature granulocytes (Figure 1A-B). This inverse correlation was measurable in diagnostic samples obtained from 20 patients with acute leukemia classified according to the FAB system24 (Figure 1B), in the myeloid leukemia HL60 cell line (supplemental Figure 1A), and primary blasts from patients with newly diagnosed FAB-M3 APL undergoing granulocytic differentiation, on treatment with all-trans retinoic acid (RA)20-22 (patient 14 of Figure 1B). No significant changes in miR-223 or NFI-A protein/mRNA levels were measurable after RA treatment of HL60R cells, an RA-resistant subclone of HL6027 (supplemental Figure 1A). Increased miR-223 levels in RA-induced HL60 cells were associated with a decrease in protein, but not mRNA levels of LMO2 (supplemental Figure 1A-B), a posttranscriptional target of miR-223 acting as an essential transcriptional factor in early hematopoiesis and erythroid development.26,28
miR-223 and NFI-A mRNA/protein levels in human hematopoietic cells and leukemias. (A) Quantitative RT-PCR of NFI-A mRNA level (□), Northern blot analysis of miR-223 expression, and immunodetection of NFI-A protein in CD34+ HSC/HPCs and CD34− cells from PB or BM. U6 and β-tubulin were probed as RNA and protein loading controls, respectively. (B) Quantitative RT-PCR of miR-223 (■) and NFI-A (□) mRNA expression levels in leukemia patient samples classified by FAB.24 ALL indicates acute lymphocytic leukemia; and NA, not available. Genetic features of leukemia blasts are indicated. Quantitative RT-PCR data are represented as mean ± SD of 3 independent evaluations.
miR-223 and NFI-A mRNA/protein levels in human hematopoietic cells and leukemias. (A) Quantitative RT-PCR of NFI-A mRNA level (□), Northern blot analysis of miR-223 expression, and immunodetection of NFI-A protein in CD34+ HSC/HPCs and CD34− cells from PB or BM. U6 and β-tubulin were probed as RNA and protein loading controls, respectively. (B) Quantitative RT-PCR of miR-223 (■) and NFI-A (□) mRNA expression levels in leukemia patient samples classified by FAB.24 ALL indicates acute lymphocytic leukemia; and NA, not available. Genetic features of leukemia blasts are indicated. Quantitative RT-PCR data are represented as mean ± SD of 3 independent evaluations.
In CD34+ HSC/HPCs undergoing erythropoiesis and in K562 cells induced to erythroid differentiation by treatment with Ara-C, miR-223 levels decreased while NFI-A mRNA increased (supplemental Figure 1C).21,22,26 Thus, both in primary hematopoietic cells and cell lines, opposite expression patterns of NFI-A mRNA and miR-223 are associated with lineage-specific decisions.
To investigate whether miR-223 affects the stability of NFI-A mRNA, HL60 cells were treated for 5 or 72 hours with RA to attain different levels of miR-223 expression and thereafter with actinomycin D to block transcription. The half-life of NFI-A mRNA was not affected by RA treatment (supplemental Figure 1D), thereby suggesting that the up-modulation of miR-223 did not significantly affect the stability of NFI-A mRNA during granulocytic differentiation.
We tested whether transcriptional inhibitory effects may be required for the correct silencing of the NFI-A gene during granulopoiesis. Treatment with the protein synthesis inhibitor cycloheximide did not prevent the decrease of NFI-A transcripts induced by RA in HL60 cells (supplemental Figure 1E). This indicates that a protein intermediate is not required for RA-induced NFI-A gene silencing.
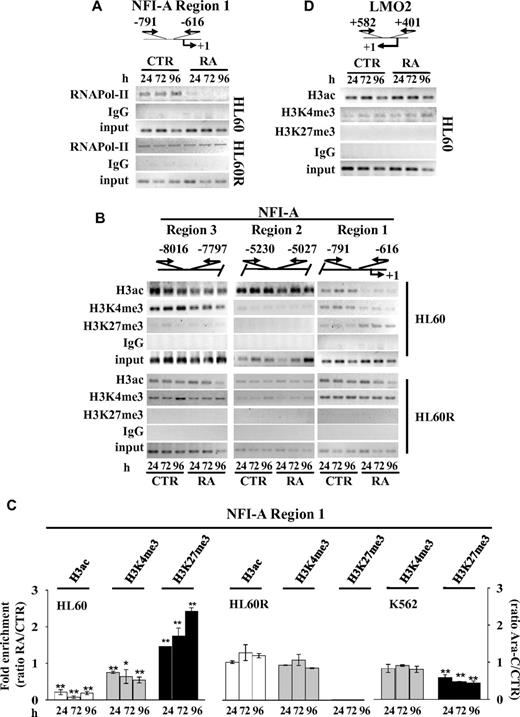
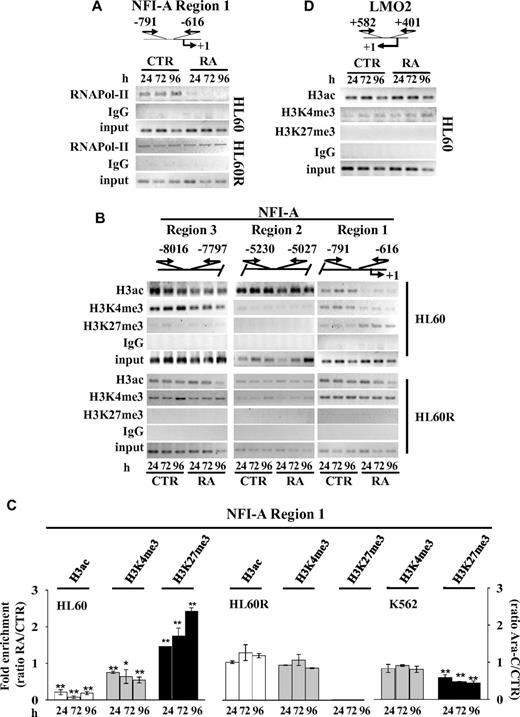
ChIP analyses showed that the in vivo occupancy of the RNA-DNA–dependent polymerase II (RNApol-II) on the NFI-A promoter region 1 (nt −616 to −791) decreases during RA-induced granulocytic differentiation of HL60 cells, is unchanged in RA-resistant HL60R cells (Figure 2A), whereas it increases in Ara-C–treated K562 cells (supplemental Figure 1F). These data suggest that the assemblage of the transcriptional machinery on a regulatory region in close proximity of the NFI-A transcription start site is interrupted during RA-induced granulopoiesis.
Chromatin state at NFI-A gene promoter. Top panels: Schematic representation of the NFI-A regions 1, 2, and 3 and LMO2 gene promoter regions. Numbers are the nucleotides relative to transcriptional start sites (+1) and indicate the location of the PCR primers used in ChIP assay performed at the indicated times in HL60 and HL60R cells in the absence (CTR) or in the presence of 1μM RA (RA) using: (A) anti–RNApol-II; (B) antiacetylated H3 (H3ac), (C) anti-H3K4me3, and (D) anti-H3K27me3 antibodies. Rabbit IgG was used as nonspecific antibody. Samples representing 0.02% of total input chromatin (input) were included in the PCR analysis. (C) Quantitative RT-PCR performed on NFI-A region 1 in ChIP assays using the indicated antibodies in HL60, HL60R treated as described above, and in K562 cells treated or not (CTR) with 0.5μM Ara-C (Ara-C). Data are plotted as the ratio of the values obtained in RA or Ara-C treated versus untreated cell samples. Error bars represents SD of 3 independent evaluations. *P < .05 vs untreated cells. **P < .01 versus untreated cells.
Chromatin state at NFI-A gene promoter. Top panels: Schematic representation of the NFI-A regions 1, 2, and 3 and LMO2 gene promoter regions. Numbers are the nucleotides relative to transcriptional start sites (+1) and indicate the location of the PCR primers used in ChIP assay performed at the indicated times in HL60 and HL60R cells in the absence (CTR) or in the presence of 1μM RA (RA) using: (A) anti–RNApol-II; (B) antiacetylated H3 (H3ac), (C) anti-H3K4me3, and (D) anti-H3K27me3 antibodies. Rabbit IgG was used as nonspecific antibody. Samples representing 0.02% of total input chromatin (input) were included in the PCR analysis. (C) Quantitative RT-PCR performed on NFI-A region 1 in ChIP assays using the indicated antibodies in HL60, HL60R treated as described above, and in K562 cells treated or not (CTR) with 0.5μM Ara-C (Ara-C). Data are plotted as the ratio of the values obtained in RA or Ara-C treated versus untreated cell samples. Error bars represents SD of 3 independent evaluations. *P < .05 vs untreated cells. **P < .01 versus untreated cells.
Generation of a chromatin silent status on the NFI-A gene promoter during granulopoiesis
We next typified the chromatin status in NFI-A gene promoter region 1 in HL60 and HL60R cells before and after RA treatment (Figure 2B-C). ChIP assays revealed that the bivalent epigenetic marks, located in NFI-A region 1,5,6 are resolved by RA treatment. A decrease in transcriptionally active chromatin marks (acetylated H3 and H3K4me3), paralleling an increase in repressive/inactive chromatin mark H3K27me3, occurred at and after 24 hours of RA treatment in HL60 cells. These changes were not detectable in HL60R cells, in NFI-A upstream genomic regions 2 or 3, respectively, approximately −5 kb and −8 kb from the transcription start site or in the context of the main transcription regulation DNA sequence within the LMO2 proximal promoter29 (Figure 2B-D). In the more mature promyelocytic NB4 and NB4-MR4 cell lines, the expression of NFI-A mRNA is lower than in myeloblastic HL60 cells; and the bivalent marks are resolved into high H3K27me3 and low H3K4 me3 levels (supplemental Figure 1H-I). In contrast, the decrease in the repressive/inactive chromatin mark H3K27me3 indicated that an opposite resolution of bivalent epigenetic marks occurred at NFI-A promoter during Ara-C–induced erythroid differentiation of K562 cells (Figure 2C).
Because H3K27me3 is a prerequisite for DNA methylation,30 we analyzed, by genomic bisulphite sequencing, the methylation status of CpG islands 26 and 99, respectively present upstream (∼ −5.5 kb) and downstream (∼ 1 kb) from the NFI-A gene transcriptional start site and clusters of CpGs within the NFI-A genomic region 1 (supplemental Figure 2A). No significant change in the DNA methylation patterns of CpG islands 26 or 99 was detected in RA-treated HL60 cells, whereas the basal methylation status of the CpG clusters in region 1 increased during RA treatment (supplemental Figure 2A). These findings further suggested a lineage specific transcriptional silencing of NFI-A gene in myeloid cells undergoing granulopoiesis.
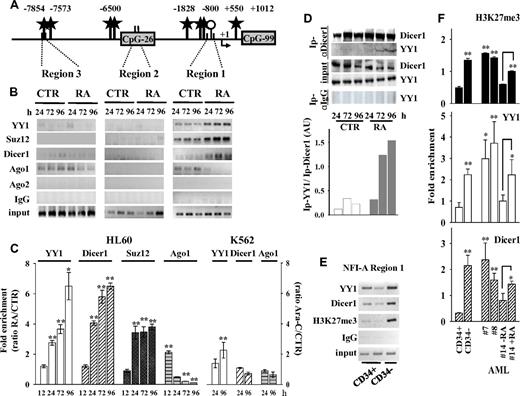
PcG members are recruited on the NFI-A gene promoter during granulopoiesis
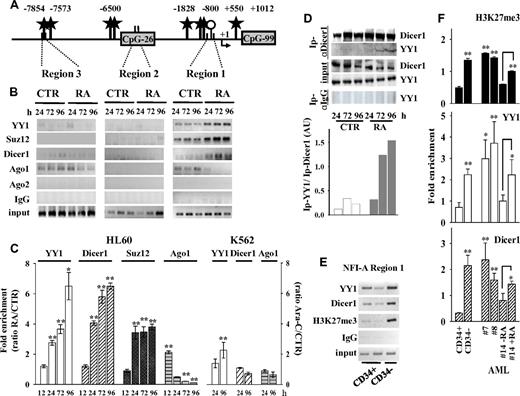
A bioinformatic analysis of the 13.5-kb DNA sequence (from nt −10 067 to 3525), encompassing the NFI-A transcription start site (MatInspector Professional 8.0 software, http://www.genomatix.de), revealed the presence of several CCATnTT binding sites for the PcG repressor complex 1 protein member YY1 (Figure 3A), which has a sequence-specific element for its binding to DNA.31 YY1 acts as a regulator of chromatin compaction and DNA methylation initiated by components of the PRC2, including EZH2-H3K27 histone methyltransferase and the core components Suz12 and EED.30,32,33 ChIP assays showed that both YY1 and Suz12 accumulate on the NFI-A region 1 throughout RA treatment of HL60 cells, although not in HL60R cells or during Ara-C–induced erythroid differentiation of K562 cells (Figure 3B-C; and data not shown). These proteins were depleted in the LMO2 proximal promoter, although YY1 binding sites were present in the transcriptional start site flanking regions (supplemental Figure 1G). Thus, in vivo enrichment of YY1 and Suz12 in the NFI-A regulatory region 1 correlated with epigenetic silencing of the NFI-A gene and granulopoiesis.
Recruitment of polycomb and RNAi machinery components on NFI-A gene. ChIP assays performed in HL60 cells treated (RA) or not (CTR) with RA 1μM, and in K562 cells treated (Ara-C) or not (CTR) with Ara-C 0.5μM for the indicated times. (A) Diagram of the YY1 binding sites (black stars), CpG islands (gray boxes), and CpG clusters (represented by a single ○) in the 5′end regions 1, 2, and 3 of the NFI-A gene promoter region. Numbers indicate nucleotides relative to transcription start sites (+1). ChIP with antibodies anti-YY1, -Suz12, -Dicer1, -Ago1, and -Ago2, and PCR primers as in Figure 2. (B) PCR of NFI-A gene regions 1, 2, and 3. (C) Quantitative RT-PCR of NFI-A region 1 on separate experiments, including the 12-hour time point, and of Ara-C–treated K562 cells. Ratio of the values obtained in RA- or Ara-C–treated versus untreated cell samples. Error bars represent SD of 3 independent evaluations *P < .05. **P < .01. (D) Coimmunoprecipitation/immunoblotting experiments: Ip indicates indicates the antibody used for the coimmunoprecipitation. Rabbit control IgG was used as nonspecific antibody. Coimmunoprecipitates were analyzed by immunoblotting anti-Dicer1 and YY1. Bottom panel: Densitometric analysis by ImageQuant Version 5.2 software of coimmunoprecipitated YY1 in relation to immunoprecipitated Dicer1 after normalization with their respective inputs. (E) ChIP assays carried out on immature human CD34+ HSCs/HPCs and mature CD34− hematopoietic cells isolated from healthy donors, immunoprecipitated with antibodies anti-YY1, anti-Dicer1, and anti-H3K27me3. Recovered DNA was analyzed by PCR using primers described in Figure 2. (F) Quantitative RT-PCRs performed to amplify NFI-A region 1 in immature CD34+ HSCs/HPCs, in CD34− myeloid populations, and in AML patients 7, 8, and 14 treated or not with RA for 72 hours. Error bars represent SD of 3 independent evaluations. In primary human HPCs and AML patient blasts (patients 7 and 8), statistical significance was calculated with respect to immature CD34+ HSCs/HPCs, and for APL patient 14 with respect to untreated APL blasts. *P < .05. **P < .01.
Recruitment of polycomb and RNAi machinery components on NFI-A gene. ChIP assays performed in HL60 cells treated (RA) or not (CTR) with RA 1μM, and in K562 cells treated (Ara-C) or not (CTR) with Ara-C 0.5μM for the indicated times. (A) Diagram of the YY1 binding sites (black stars), CpG islands (gray boxes), and CpG clusters (represented by a single ○) in the 5′end regions 1, 2, and 3 of the NFI-A gene promoter region. Numbers indicate nucleotides relative to transcription start sites (+1). ChIP with antibodies anti-YY1, -Suz12, -Dicer1, -Ago1, and -Ago2, and PCR primers as in Figure 2. (B) PCR of NFI-A gene regions 1, 2, and 3. (C) Quantitative RT-PCR of NFI-A region 1 on separate experiments, including the 12-hour time point, and of Ara-C–treated K562 cells. Ratio of the values obtained in RA- or Ara-C–treated versus untreated cell samples. Error bars represent SD of 3 independent evaluations *P < .05. **P < .01. (D) Coimmunoprecipitation/immunoblotting experiments: Ip indicates indicates the antibody used for the coimmunoprecipitation. Rabbit control IgG was used as nonspecific antibody. Coimmunoprecipitates were analyzed by immunoblotting anti-Dicer1 and YY1. Bottom panel: Densitometric analysis by ImageQuant Version 5.2 software of coimmunoprecipitated YY1 in relation to immunoprecipitated Dicer1 after normalization with their respective inputs. (E) ChIP assays carried out on immature human CD34+ HSCs/HPCs and mature CD34− hematopoietic cells isolated from healthy donors, immunoprecipitated with antibodies anti-YY1, anti-Dicer1, and anti-H3K27me3. Recovered DNA was analyzed by PCR using primers described in Figure 2. (F) Quantitative RT-PCRs performed to amplify NFI-A region 1 in immature CD34+ HSCs/HPCs, in CD34− myeloid populations, and in AML patients 7, 8, and 14 treated or not with RA for 72 hours. Error bars represent SD of 3 independent evaluations. In primary human HPCs and AML patient blasts (patients 7 and 8), statistical significance was calculated with respect to immature CD34+ HSCs/HPCs, and for APL patient 14 with respect to untreated APL blasts. *P < .05. **P < .01.
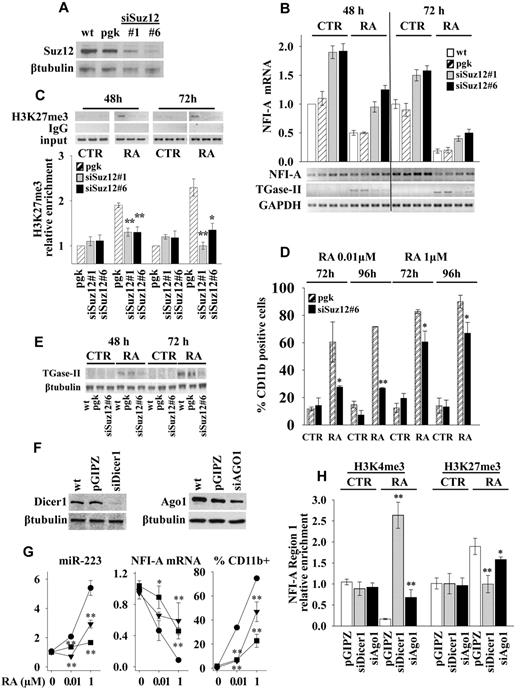
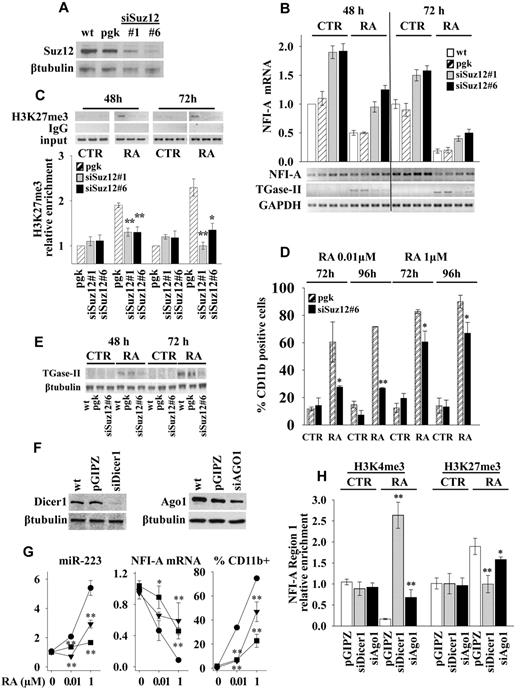
To investigate the functional relevance of PcGs induced heterochromatic silencing of NFI-A gene, we generated a stable Suz12 knockdown in HL60 cells by lentivirus-expressed siRNA and selected 2 clones, siSuz12#1 and siSuz12#6, with efficient inhibition of Suz12 expression (Figure 4A). If compared with wild-type (wt) or vector-infected (pgk) cells, the knockdown of Suz12 increased the expression levels of NFI-A mRNA approximately 2-fold in untreated cells (Figure 4B). In consequence, during RA treatment NFI-A mRNA levels were significantly higher than in control cells. (Figure 4B). ChIP assay showed that the RA-induced specific accumulation of H3K27me3 in NFI-A chromatin region 1 was reduced in both siSuz12#1 and siSuz12#6 cells (Figure 4C), thus confirming the requirement of Suz12 for the accumulation of the H3K27me3 mark in this NFI-A region. Moreover, the Suz12 knockdown decreased RA-induced granulocytic differentiation (at doses of 0.01 and 1μM), as shown by the lower proportion of cells displaying the myeloid differentiation marker CD11b (Figure 4D) and by the decreased expression of mRNA and protein levels of the TGase-II, an RA target gene involved in granulocytic differentiation34 (Figure 4B-E) and by morphologic analysis (supplemental Figure 3).
Suz12, Dicer1, and Ago1 knockdowns. HL60 cells, wild-type (wt), were infected with empty lentiviral vectors (pgk or pGIPZ) or with lentiviral constructs expressing siRNAs against Suz12 (siSuz12#1, siSuz12#6), Dicer1 (siDicer1), and Ago1 (siAgo1). (A) Suz12 product as measured by immunoblot analysis with β-tubulin as loading control. (B) Quantitative RT-PCR of NFI-A mRNA levels in indicated cells treated or not (CTR) with 1μM RA for 48 and 72 hours. Bottom panel: RT-PCR measuring the NFI-A mRNA levels and myeloid differentiation marker TGase-II. GAPDH levels were used as RNA loading control. (C) ChIP assays performed using an anti-H3K27me3 antibody and RT-PCR primers encompassing NFI-A gene region 1 at the indicated times in cells treated or not (CTR) with RA 1μM. Rabbit control IgG was used as nonspecific antibody. Bottom panel: Enrichment of the H3K27me3 on NFI-A promoter region 1 as measured by quantitative RT-PCR. Error bars represent SD of 3 independent evaluations. Statistical significance was calculated with respect to RA-treated pgk cells. *P < .05. **P < .01. (D) Percentage of CD11b-positive cells in cells treated (RA) or not (CTR) at the indicated times and concentrations of RA, as measured by flow cytometry. Statistical significance was calculated with respect to RA-treated pgk cells. *P < .05. **P < .01. (E) Immunoblotting analysis of TGase-II, an RA target gene involved in granulocytic differentiation.34 β-tubulin is a loading control. (F) Immunoblotting analysis of Dicer1 and Ago1 products. β-tubulin as loading control. (G) Quantitative RT-PCRs measuring miR-223 and NFI-A mRNA levels and the percentage of CD11b-positive cells as measured by flow cytometry in pGIPZ (●), siDicer1 (■), and siAgo1 (▾) cell lines, after 72 hours of treatment with the indicated concentrations of RA. Statistical significance was calculated with respect to RA-treated pGIPZ cells. *P < .05. **P < .01. (H) ChIP assay and H3K4me3 and H3K27me3 chromatin mark enrichments on NFI-A promoter region 1 as measured by quantitative RT-PCR. Statistical significance was calculated with respect to RA-treated pGIPZ cells. *P < .05. **P < .01. Results represent the average of 3 independent evaluations ± SD. Bars represent the average of 3 independent evaluations ± SD.
Suz12, Dicer1, and Ago1 knockdowns. HL60 cells, wild-type (wt), were infected with empty lentiviral vectors (pgk or pGIPZ) or with lentiviral constructs expressing siRNAs against Suz12 (siSuz12#1, siSuz12#6), Dicer1 (siDicer1), and Ago1 (siAgo1). (A) Suz12 product as measured by immunoblot analysis with β-tubulin as loading control. (B) Quantitative RT-PCR of NFI-A mRNA levels in indicated cells treated or not (CTR) with 1μM RA for 48 and 72 hours. Bottom panel: RT-PCR measuring the NFI-A mRNA levels and myeloid differentiation marker TGase-II. GAPDH levels were used as RNA loading control. (C) ChIP assays performed using an anti-H3K27me3 antibody and RT-PCR primers encompassing NFI-A gene region 1 at the indicated times in cells treated or not (CTR) with RA 1μM. Rabbit control IgG was used as nonspecific antibody. Bottom panel: Enrichment of the H3K27me3 on NFI-A promoter region 1 as measured by quantitative RT-PCR. Error bars represent SD of 3 independent evaluations. Statistical significance was calculated with respect to RA-treated pgk cells. *P < .05. **P < .01. (D) Percentage of CD11b-positive cells in cells treated (RA) or not (CTR) at the indicated times and concentrations of RA, as measured by flow cytometry. Statistical significance was calculated with respect to RA-treated pgk cells. *P < .05. **P < .01. (E) Immunoblotting analysis of TGase-II, an RA target gene involved in granulocytic differentiation.34 β-tubulin is a loading control. (F) Immunoblotting analysis of Dicer1 and Ago1 products. β-tubulin as loading control. (G) Quantitative RT-PCRs measuring miR-223 and NFI-A mRNA levels and the percentage of CD11b-positive cells as measured by flow cytometry in pGIPZ (●), siDicer1 (■), and siAgo1 (▾) cell lines, after 72 hours of treatment with the indicated concentrations of RA. Statistical significance was calculated with respect to RA-treated pGIPZ cells. *P < .05. **P < .01. (H) ChIP assay and H3K4me3 and H3K27me3 chromatin mark enrichments on NFI-A promoter region 1 as measured by quantitative RT-PCR. Statistical significance was calculated with respect to RA-treated pGIPZ cells. *P < .05. **P < .01. Results represent the average of 3 independent evaluations ± SD. Bars represent the average of 3 independent evaluations ± SD.
Overall, these findings show that PcGs contribute to heterochromatic transcriptional silencing of the NFI-A gene and further link these events to granulopoiesis.
Recruitment of RNAi machinery components and PcGs on the NFI-A promoter
RNAi machinery components can be involved in PcG-dependent TGS.12-14 Dicer1 can maintain heterochromatin domains and full promoter DNA hypermethylation,35,36 whereas Argonaute (Ago) 1 and Ago2 are involved in ncRNA-mediated TGS.37-40 ChIP assays showed that, in untreated HL60 and K562 cells, both Dicer1 and Ago1, but not Ago2, are located on NFI-A regions 1 and 3. On NFI-A region 1, RA treatment gradually increased the accumulation of Dicer1 and, transiently, that of Ago1 (after 12 hours of RA treatment), which was subsequently released from this site (Figure 3B-C; supplemental Figure 2B). Variations in Dicer1 and Ago1 recruitment were not significant on the LMO2 promoter, on the NFI-A region 1 amplified from HL60R cells (data not shown), or from K562 cells induced to erythroid differentiation by Ara-C treatment (Figure 3C; supplemental Figure 1G).
Protein coimmunoprecipitation/immunoblotting experiments revealed that Dicer1 antibodies coimmunoprecipitate Ago1 (not shown) and, more importantly, YY1 (Figure 3D), in a time-dependent manner on RA treatment of HL60 cells, thus supporting the in vivo physical interaction of these proteins.
ChIP assays were also performed on chromatins prepared from primary human HPCs and acute myeloid leukemia (AML) patients' blasts (patients 7, 8, and 14 of Figure 1B), which included an APL (patient 14) undergoing granulopoiesis by RA treatment in vitro. In CD34− HPCs, AMLs, or RA-treated APL, low expression levels of NFI-A mRNA (Figure 1A-B) correlated with the increased occupancy of Dicer1, YY1, and H3K27me3 marks on NFI-A region 1 compared with immature CD34+ HSC/HPCs, which expressed a high NFI-A product (Figure 3E-F). These results in primary cells further linked the accumulation of Dicer1 and YY1 on NFI-A promoter to the constitutive heterochromatin configuration that occurs in NFI-A regulatory region 1 during granulopoiesis.
To further investigate the role of Dicer1 and Ago1 in NFI-A promoter silencing and HL60 cell differentiation, we generated stable Dicer1 and Ago1 knockdown HL60 cells (Figure 4F). Both siDicer1 and siAgo1 silencing significantly affected the induction of miR-223 levels by different concentrations of RA (0.01 and 1μM) compared with RA-treated vector infected cells (Figure 4G). On RA treatment of siDicer1 and siAgo1 cells, the repression of NFI-A mRNA was impaired (Figure 4G), H3K4me3 was increased, and H3K27me3 was decreased in NFI-A gene promoter region 1 (Figure 4H). Furthermore, knocking down Dicer1 and Ago1 weakened RA-induced differentiation, as shown by a reduced CD11b expression (Figure 4G) and by morphologic analysis (supplemental Figure 3B).
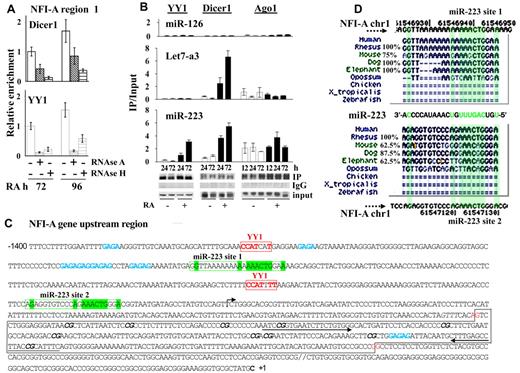
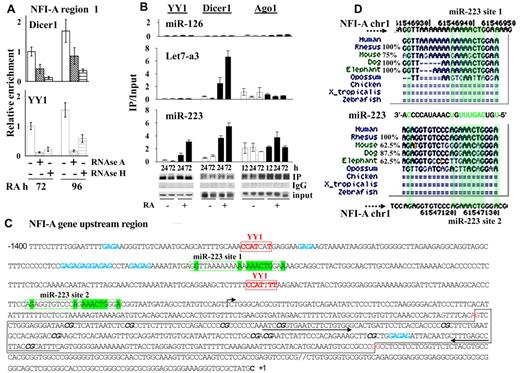
We therefore investigated the involvement of RNA molecules in driving the components of the RNAi machinery and PcGs on the NFI-A region 1, by ChIP assays on chromatins prepared from HL60 cells at different times of RA treatment preceded by the incubation with RNAse A or RNAse H. Of note, RNAse H specifically degrades the RNA present in RNA:DNA hybrids and does not degrade DNA or unhybridize RNA.16 Both these RNAse treatments appreciably reduced the occupancy of Dicer1 and YY1 proteins on the NFI-A region 1 (Figure 5A). Thus, RNA molecules appear to be required for Dicer1 and YY1 presence at NFI-A region 1.
RNA-dependent recruitment of YY1 and Dicer1 at NFI-A regulatory region 1. (A) RNAse A or RNAse H (10 μg/mL) was added for 3 hours to nuclei isolated from HL60 cells treated with RA 1μM for 72 or 96 hours before chromatin cross-linking. ChIP assay was performed using anti-Dicer1, -YY1 antibodies and analyzed by quantitative RT-PCR using the indicated primers. Data are mean ± SD of 2 independent evaluations. (B) RNA immunoprecipitation assay performed on HL60 cells untreated (CTR) and treated 1μM RA for the indicated time points to test endogenous miR-223, Let-7a3, and miR-126 binding to YY1, Dicer1, and Ago1 proteins. Cell lysates were immunoprecipitated with antibodies anti-YY1, -Dicer1, and -Ago1. Rabbit IgGs were used as specificity controls (not shown). Immunoprecipitated miRs were detected by quantitative RT-PCR as described in “RNA immunoprecipitation.” Error bars represent SD of 3 independent evaluations. (C) Nucleotide sequence of the −1.4-kb regulatory region surrounding and including NFI-A region 1. The transcription start site (+1) is marked with a rightward-pointing arrow. Red represents the YY1 binding sites; green, the sequences complementary to that of miR-223; and blue, GAGAG recurrent motifs. A black box represents the CpGs cluster region. CG dinucleotides evaluated by bisulphite sequencing assay are marked in italic and bold. The arrows indicate the position of the primers used to amplify the NFI-A region 1. (D) Genomic site conservation and relative percentage of the miR-223 target sequences (miR-223 site 1 and site 2) in the NFI-A gene promoter. Green represents highly conserved nucleotides.
RNA-dependent recruitment of YY1 and Dicer1 at NFI-A regulatory region 1. (A) RNAse A or RNAse H (10 μg/mL) was added for 3 hours to nuclei isolated from HL60 cells treated with RA 1μM for 72 or 96 hours before chromatin cross-linking. ChIP assay was performed using anti-Dicer1, -YY1 antibodies and analyzed by quantitative RT-PCR using the indicated primers. Data are mean ± SD of 2 independent evaluations. (B) RNA immunoprecipitation assay performed on HL60 cells untreated (CTR) and treated 1μM RA for the indicated time points to test endogenous miR-223, Let-7a3, and miR-126 binding to YY1, Dicer1, and Ago1 proteins. Cell lysates were immunoprecipitated with antibodies anti-YY1, -Dicer1, and -Ago1. Rabbit IgGs were used as specificity controls (not shown). Immunoprecipitated miRs were detected by quantitative RT-PCR as described in “RNA immunoprecipitation.” Error bars represent SD of 3 independent evaluations. (C) Nucleotide sequence of the −1.4-kb regulatory region surrounding and including NFI-A region 1. The transcription start site (+1) is marked with a rightward-pointing arrow. Red represents the YY1 binding sites; green, the sequences complementary to that of miR-223; and blue, GAGAG recurrent motifs. A black box represents the CpGs cluster region. CG dinucleotides evaluated by bisulphite sequencing assay are marked in italic and bold. The arrows indicate the position of the primers used to amplify the NFI-A region 1. (D) Genomic site conservation and relative percentage of the miR-223 target sequences (miR-223 site 1 and site 2) in the NFI-A gene promoter. Green represents highly conserved nucleotides.
miR-223 association with the PcGs-RNAi complex, nuclear localization, and NFI-A targeting
Native RNA immunoprecipitation assays performed in HL60 cell extracts revealed that endogenous mature miR-223 is associated with YY1, Dicer1, and Ago1 over the time of RA treatment (Figure 5B). In contrast, the endogenous Let7-a3, another miR induced by RA treatment in HL60 cells,41 is not retrieved by immunoprecipitation of YY1, whereas its association with Dicer1 is increased in a time-dependent manner after RA treatment (Figure 5B). miR-126, which is not expressed or induced by RA in these cells, remained at undetectable levels (Figure 5B). Thus, miR-223 may have a nuclear role and might take part in NFI-A TGS associated with granulocytic differentiation of myeloid precursors.21,39,42,43
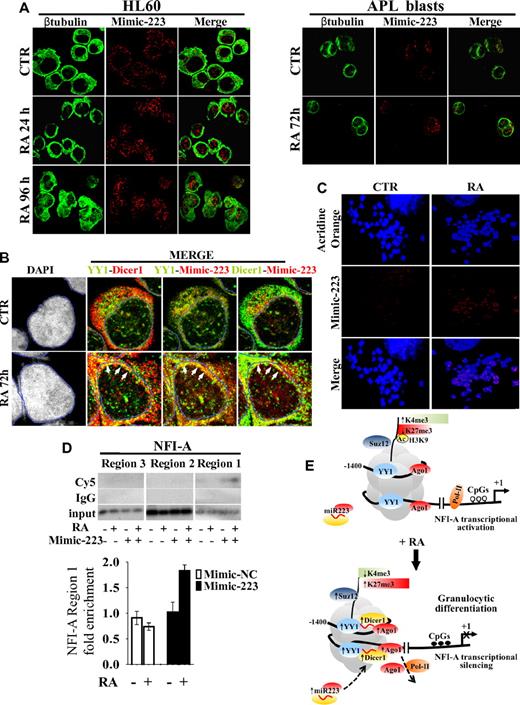
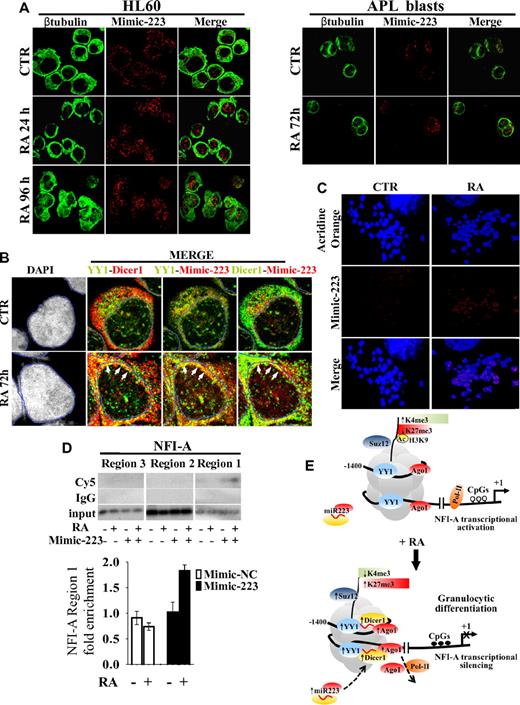
We therefore studied by confocal microscopy the subcellular localization of miR-223 in HL60 cells and in primary blasts from a patient with newly diagnosed APL transfected with Cy5-labeled double-stranded oligonucleotides mimicking endogenous mature miR-223 activity (Mimic-223). A Cy5-labeled miR-223 antisense single-stranded RNA oligonucleotide was also used as a probe to specifically detect endogenous unbound miR-223 (miR-223, Figure 6A; supplemental Figure 4A). In untreated cells, transfected Cy5-labeled Mimic-223 and endogenous miR-223 revealed a punctuated pattern, mainly localized in the cytoplasm. In both HL60 cells and primary APL blasts, the addition of RA increased the nuclear compartmentalization of miR-223 (Figure 6A; supplemental Figure 4A). Cy5-labeled miR-inhibitor negative control and Mimic-Let7-a3 remained in the cytoplasm (supplemental Figure 4B). miR-223 was also present within the nucleus of PB mature granulocytes, whereas only faint miR-223 positivity was detectable in lymphocytes (supplemental Figure 4C). Overall, these data indicate the nuclear import of miR-223 during granulocytic differentiation of myeloid precursors. We also investigated by confocal analysis the subcellular localization of YY1 and Dicer1 and their colocalization with miR-223 in HL60 cells transfected with Cy5-labeled Mimic-223 or Mimic negative control 2 (Mimic-NC, Figure 6B; supplemental Figure 5A). Cell treatment with RA did not affect YY1 localization in cytoplasmic and nuclear regions, although it did induce Dicer1 in the nuclear compartment (supplemental Figure 5B). RA treatment also increased YY1-Dicer1, YY1–Mimic-223, and Dicer1–Mimic-223 colocalization in the nuclear periphery, a site of preferential YY1 and heterochromatin localization44 (Figure 6B; supplemental Figure 5A-C). These changes were not observed when Mimic-NC was used (supplemental Figure 5A-C), thus supporting the formation of a repressive complex composed by YY1, Dicer1, and miR-223.
Cellular localization and colocalization of miR-223, YY1, and Dicer1. HL60 cells and primary APL blasts were treated or not (CTR) with 1μM RA for the indicated times and analyzed by immunofluorescence confocal microscopy. (A) Localization of miR-223–Mimic-Cy5 (Mimic-223) in HL60 cells and in APL blasts, β-tubulin marking cell cytoplasm, and the merge of the 2 channels. (B) Colocalization of Mimic-223, YY1 (AlexaFluor-488), and Dicer1 (AlexaFluor-555). Quadruple labeling (DAPI, YY1, Dicer1, Mimic-223) and double-channel visualization of untreated (CTR) or treated (RA) HL60 cells. Channel colors were palette assigned. A mask landmark (blue line) delineates the nuclear boundaries, and white arrows indicate areas of higher colocalization (yellow dots). (C) Acridine Orange-stained mitotic chromosomes, localization of 223-Mimic-Cy5 (Mimic-223), and the merge of the 2 channels. (D) ChIP assay performed on HL60 cells transiently transfected (+) or not (−) with Cy5-Mimic-223 and treated (+) or not (−) with 1μM RA for 48 hours. Chromatins were immunoprecipitated with an anti-Cy5 antibody. NFI-A regions 1, 2, and 3 were PCR amplified and visualized by 32P-labeled oligonucleotides (supplemental Table 5) as described in supplemental Methods or by quantitative RT-PCR measuring Mimic-223 or Mimic-NC enrichment at NFI-A region 1 (bottom panel). Error bars represent SD of 3 independent evaluations. (E) Schematic model for the heterochromatic silencing of NFI-A gene by PcG-miR-223 complexes in RA-induced granulocytic differentiation. In undifferentiated HL60 cells (top panel), YY1 is present at low levels, with Suz12 and Ago1 at its binding sites on NFI-A promoter, and this correlates with an enrichment (↑) in active chromatin marks (acH3, H3K4me3), decreased (↓) repressive/inactive mark H3K27me3, demethylated CpGs (○), recruitment of RNAPol-II, and NFI-A transcriptional activation. On RA-induced granulocytic differentiation (bottom panel), miR-223 localizes in the nucleus and via the formation of a Dicer1/Ago1-YY1/Suz12 complex, miR-223 targets its complementary DNA sequences flanking YY1 binding sites on NFI-A promoter at a distance of approximately 1 nucleosome. The increased occupancy of the PcG-miR complexes at these sites correlated with a decrease in active H3K4me3 and an enrichment in repressive/inactive mark H3K27me3, CpG methylation (●), transcriptional silencing of NFI-A gene, and granulocytic differentiation of myeloid precursors. Numbers are the nucleotides relative to the start site of the NFI-A gene (+1).
Cellular localization and colocalization of miR-223, YY1, and Dicer1. HL60 cells and primary APL blasts were treated or not (CTR) with 1μM RA for the indicated times and analyzed by immunofluorescence confocal microscopy. (A) Localization of miR-223–Mimic-Cy5 (Mimic-223) in HL60 cells and in APL blasts, β-tubulin marking cell cytoplasm, and the merge of the 2 channels. (B) Colocalization of Mimic-223, YY1 (AlexaFluor-488), and Dicer1 (AlexaFluor-555). Quadruple labeling (DAPI, YY1, Dicer1, Mimic-223) and double-channel visualization of untreated (CTR) or treated (RA) HL60 cells. Channel colors were palette assigned. A mask landmark (blue line) delineates the nuclear boundaries, and white arrows indicate areas of higher colocalization (yellow dots). (C) Acridine Orange-stained mitotic chromosomes, localization of 223-Mimic-Cy5 (Mimic-223), and the merge of the 2 channels. (D) ChIP assay performed on HL60 cells transiently transfected (+) or not (−) with Cy5-Mimic-223 and treated (+) or not (−) with 1μM RA for 48 hours. Chromatins were immunoprecipitated with an anti-Cy5 antibody. NFI-A regions 1, 2, and 3 were PCR amplified and visualized by 32P-labeled oligonucleotides (supplemental Table 5) as described in supplemental Methods or by quantitative RT-PCR measuring Mimic-223 or Mimic-NC enrichment at NFI-A region 1 (bottom panel). Error bars represent SD of 3 independent evaluations. (E) Schematic model for the heterochromatic silencing of NFI-A gene by PcG-miR-223 complexes in RA-induced granulocytic differentiation. In undifferentiated HL60 cells (top panel), YY1 is present at low levels, with Suz12 and Ago1 at its binding sites on NFI-A promoter, and this correlates with an enrichment (↑) in active chromatin marks (acH3, H3K4me3), decreased (↓) repressive/inactive mark H3K27me3, demethylated CpGs (○), recruitment of RNAPol-II, and NFI-A transcriptional activation. On RA-induced granulocytic differentiation (bottom panel), miR-223 localizes in the nucleus and via the formation of a Dicer1/Ago1-YY1/Suz12 complex, miR-223 targets its complementary DNA sequences flanking YY1 binding sites on NFI-A promoter at a distance of approximately 1 nucleosome. The increased occupancy of the PcG-miR complexes at these sites correlated with a decrease in active H3K4me3 and an enrichment in repressive/inactive mark H3K27me3, CpG methylation (●), transcriptional silencing of NFI-A gene, and granulocytic differentiation of myeloid precursors. Numbers are the nucleotides relative to the start site of the NFI-A gene (+1).
We addressed whether miR-223 binds in vivo to native chromatin by investigating the miR-223 location in mitotic chromosome metaphases. Confocal microscopy showed that an RA-induced Mimic-223 punctuated pattern is present under nondenaturing conditions within chromosomes, often showing a symmetric distribution, thus suggesting a site-specific binding of miR-223 on sister chromatids (Figure 6C). Conversely, no signaling is detected in mitotic chromosome metaphases obtained from HL60 cells untransfected or transfected with oligonucleotides inhibiting miR-223 activity (supplemental Figure 6A-B). These results support the possibility that miR-223 binds to native chromatin in vivo.
A search of the 13.5-kb DNA sequence encompassing the NFI-A transcriptional start site (∼ −10 kb to 3.5 kb) revealed the presence of the DNA sequence 5′-AAACTG-3′ complementary to the miR-223 seed sequence (3′-UUUGACUGU-5′), which occurred twice in a region located approximately one or 2 nucleosomes upstream of region 1, where the occupancy of YY1, Dicer1, and transiently of Ago1 and the epigenetic modifications controlling NFI-A expression occurred (Figure 5C). Both the binding sites appear evolutionarily conserved in mammals presenting adult hematopoiesis centered in the BM (Figure 5D). The sequence complementarity between the pre-miR-223 and the NFI-A promoter is clearly reduced outside the 5′ “seed” region of mature miR-223 (Figure 5D; supplemental Figure 7). ChIP assay performed using an anti-Cy5 antibody in HL60 cells transfected with Mimic-223 or Mimic-NC revealed that RA treatment induced an accumulation of miR-223 on NFI-A region 1, although not in regions 2 or 3 (Figure 6D). These findings point to the in vivo presence of miR-223 in the NFI-A promoter at a region that contains its complementary sequences.
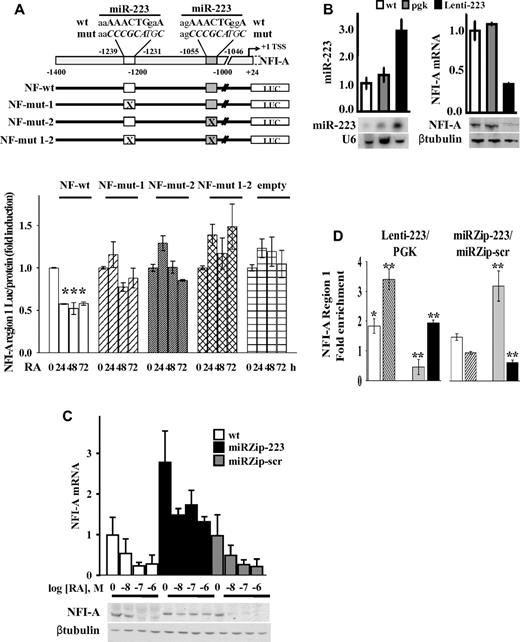
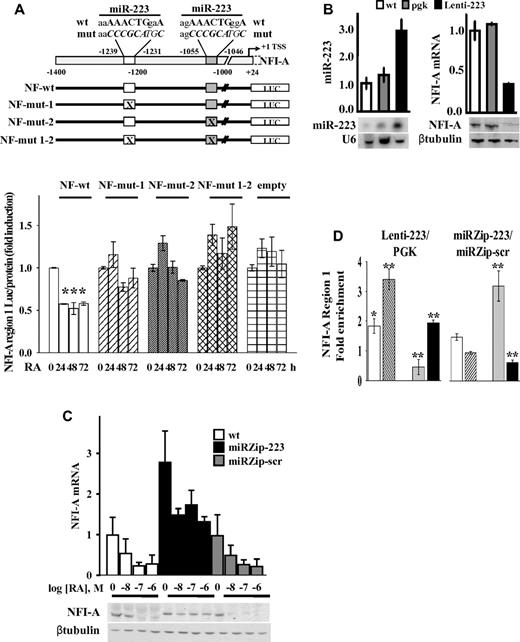
To assess whether miR-223–DNA complementary sequences on the NFI-A promoter region are relevant for NFI-A TGS, we engineered HL60 cells to stably express promoter-luciferase fusion constructs containing the NFI-A promoter (from nt −1.400 to nt 24), presenting the wild-type complementary sequence of the 2 miR-223 sites, site-specific mutants, or double site mutants (Figure 7A). The activity of these constructs was assayed in cells treated with RA for 0, 24, 48, and 72 hours to reach different levels of endogenous miR-223. The luciferase activity was inhibited by approximately 2-fold after 24 hours of RA treatment in HL60 cells stably expressing the wild-type NFI-A reporter vector, (NF-wt), but not in cells expressing vectors mutated in each miR-223 site (NF-mut-1 or NF-mut-2), double mutants (NF-mut 1-2) or an empty vector (Figure 7A). Overall, these results indicate the integrity of both the DNA sequences complementary to miR-223 necessary for the transcriptional inhibition of the NFI-A promoter.
Functional significance of putative miR-223 complementary DNA binding sites and miR-223 levels on NFI-A transcription. (A) Top panel: Schematic representation of the NFI-A promoter region (nt −1400 to 24) containing the complementary putative miR-223 DNA binding sites (nt −1239 to −1231; nt −1055 to −1046). Numbers are relative to the NFI-A transcriptional start site (+1). Bottom panel: HL60 cells stably transfected with pGL4.20 luciferase reporter vectors containing the wild-type NFI-A promoter sequence (NF-wt), the mutagenized forms of the putative miR-223 DNA binding sites (NF-mut-1, NF-mut-2, and NF-mut 1-2), or the “empty” vector were treated (+) or not (−) with 1μM RA for the indicated time points. Data are expressed as the luciferase activity normalized by the total protein amount in each sample. Bars represent the mean of 3 independent experiments performed in duplicate ± SEM. Statistical significance was calculated between RA-treated and untreated samples. *P < .05. (B) HL60 wild-type cells (wt), stably infected with a lentiviral vector expressing miR-223 (Lenti-223) or an empty viral vector (pgk) were tested by quantitative RT-PCR and Northern blot assays to measure mature miR-223 level. U6 detection is shown as RNA loading control. NFI-A mRNA and protein levels were tested by quantitative RT-PCR and immunoblot analysis. β-tubulin is a loading control. Quantitative RT-PCR results represent the average of 3 independent evaluations ± SD. (C) WT HL60 cells (wt) ectopically carrying the miRZip-223 anti–miR-223 (miRZip-223), or the scramble hairpin control (miRZip-scr) constructs, were treated with the indicated concentrations of RA for 72 hours. NFI-A mRNA and protein levels were tested by quantitative RT-PCR and immunoblotting (β-tubulin is a loading control). Quantitative RT-PCR results represent the average of 3 independent evaluations ± SD. (D) ChIP assays performed in HL60 cells ectopically expressing (Lenti-223) or not (pgk) miR-223, or in HL60 cells expressing the miRZip-223 or the miRZip-scr, using the indicated antibodies. Quantitative RT-PCR was performed to amplify NFI-A region 1. Data are plotted as the Lenti-223/pgk and miRZip-223/miRZip-scr infected cell ratio. Error bars represent SD of 3 independent evaluations. *P < .05. **P < .01.
Functional significance of putative miR-223 complementary DNA binding sites and miR-223 levels on NFI-A transcription. (A) Top panel: Schematic representation of the NFI-A promoter region (nt −1400 to 24) containing the complementary putative miR-223 DNA binding sites (nt −1239 to −1231; nt −1055 to −1046). Numbers are relative to the NFI-A transcriptional start site (+1). Bottom panel: HL60 cells stably transfected with pGL4.20 luciferase reporter vectors containing the wild-type NFI-A promoter sequence (NF-wt), the mutagenized forms of the putative miR-223 DNA binding sites (NF-mut-1, NF-mut-2, and NF-mut 1-2), or the “empty” vector were treated (+) or not (−) with 1μM RA for the indicated time points. Data are expressed as the luciferase activity normalized by the total protein amount in each sample. Bars represent the mean of 3 independent experiments performed in duplicate ± SEM. Statistical significance was calculated between RA-treated and untreated samples. *P < .05. (B) HL60 wild-type cells (wt), stably infected with a lentiviral vector expressing miR-223 (Lenti-223) or an empty viral vector (pgk) were tested by quantitative RT-PCR and Northern blot assays to measure mature miR-223 level. U6 detection is shown as RNA loading control. NFI-A mRNA and protein levels were tested by quantitative RT-PCR and immunoblot analysis. β-tubulin is a loading control. Quantitative RT-PCR results represent the average of 3 independent evaluations ± SD. (C) WT HL60 cells (wt) ectopically carrying the miRZip-223 anti–miR-223 (miRZip-223), or the scramble hairpin control (miRZip-scr) constructs, were treated with the indicated concentrations of RA for 72 hours. NFI-A mRNA and protein levels were tested by quantitative RT-PCR and immunoblotting (β-tubulin is a loading control). Quantitative RT-PCR results represent the average of 3 independent evaluations ± SD. (D) ChIP assays performed in HL60 cells ectopically expressing (Lenti-223) or not (pgk) miR-223, or in HL60 cells expressing the miRZip-223 or the miRZip-scr, using the indicated antibodies. Quantitative RT-PCR was performed to amplify NFI-A region 1. Data are plotted as the Lenti-223/pgk and miRZip-223/miRZip-scr infected cell ratio. Error bars represent SD of 3 independent evaluations. *P < .05. **P < .01.
Ectopic expression or knockdown of miR-223 affects RA-induced NFI-A TGS
We investigated whether miR-223 levels directly affect epigenetic NFI-A TGS during granulocytic differentiation in HL60 cells infected with a lentiviral vector to express miR-223 (Lenti-223). miR-223 expression levels increased approximately 3-fold compared with that in control wt or pgk-infected cells (Figure 7B). Lenti-223 cells fully replicated all the events induced by endogenously increased expression of miR-223 levels after RA treatment, including: (1) the enhanced nuclear localization of miR-223 (not shown); (2) the reduction in NFI-A, although not in LMO2 mRNA, at both the protein and mRNA levels (Figure 7B; supplemental Figure 8A); (3) the increased occupancy by YY1 and Dicer1 on NFI-A regulatory region 1 (Figure 7D; supplemental Figure 8B); (4) the reduction in the activating mark H3K4me3 and the increase in the repressive mark H3K27me3 in NFI-A region 1, although not in region 2 or region 3 (Figure 7D; supplemental Figure 8B); and (5) the induction of granulocytic differentiation in HL60 cells (not shown), which extends our previous observations.20,23
We also engineered HL60 cells for stable knockdown of miR-223, using miRZip lentiviral vector technology to sequester endogenous miR-223 and prevent its activity (miRZip-223). A miR-scrambled vector was used as a control (miRZip-scr). Inhibition of miR-223 resulted in approximately a 1.5-fold reduction in the differentiation response to 2 different doses of RA, as shown by immunophenotypic analyses of CD11b expression and morphologic analysis (supplemental Figure 9A-B). In untreated cells, infection with the miRZip-223 vector increased the NFI-A levels both at the mRNA and protein levels compared with uninfected cells or cells infected with a miRZip-scr (Figure 7C). In miRZip-223 cells, NFI-A mRNA/protein levels were only slightly altered by 72 hours treatment with different doses of RA (Figure 7C). Stable miR-223 silencing also affected the chromatin status of NFI-A promoter region 1 with an opposite trend to that measurable in HL60 cells ectopically expressing miR-223 (Lenti-223): increased levels of H3K4me3 activation marks and decreased H3K27me3 repressive marks, corresponding to a reduced recruitment of Dicer1 and to a lesser extent of YY1 on NFI-A promoter (Figure 7D). These direct and specific findings further supported a link between miR-223 activity and epigenetic/transcriptional regulation of NFI-A gene promoter and its relevance in cell fate decisions.
Discussion
Transient developmental signals can impose lineage-specific gene expression patterns through epigenetic modifications, which faithfully transmitted to daughter cells serve as a cellular memory of lineage restriction. The recent discovery of miRs and regulatory mechanisms affecting gene expression and activity is yielding novel clues for the identification of genes and complex regulatory circuits that determine cell and tissue specificity. In this context, we identified NFI-A as a target of miR-223 translational repression while acting as a key transcriptional regulator of miR-223 expression levels during granulopoiesis.20 We also demonstrated that NFI-A is a novel hematopoietic regulatory transcription factor whose expression levels direct the erythropoietic/granulopoietic lineage decisions and differentiation of early HSCs/HPCs.21,22
By analyzing the epigenetic modifications that occur in the NFI-A gene promoter during granulocytic lineage specification of normal and leukemic human hematopoietic progenitors, we disclosed a novel and unexpected view of cooperative layers of transcriptional regulation of site-specific gene expression, which include the resolution of the NFI-A promoter opposing dual chromatin histone marks (H3K4me3/H3K27me3 “bivalent domains”) and heterochromatin formation, triggered by the PcG and miR-223 promoter targeting activities.
In accordance with findings showing that PcGs/TrxGs proteins are implicated in the transcriptional “memory” that maintains lineage restriction over time through cell division, we found an association between the resolution of the “bivalent domains” H3K27me3/H3K4me3 marks on the NFI-A gene5,6 and the presence of PcG proteins YY1 and Suz12. A bioinformatic analysis of the promoter region flanking the NFI-A transcription start site revealed the presence of consensus YY1 binding sites and GAGAG recurrent motifs, validated as polycomb responsive elements in Drosophila and vertebrates.45,46 The knockdown of Suz12 prejudiced the resolution of the NFI-A gene promoter “bivalent domains” and impaired the granulocytic differentiation response to RA in HL60 myeloid progenitors. In line with our findings, down-regulation of Suz12 appears necessary for the induction of erythroid differentiation in K562 cells.47 Suz12 is required for the silencing function of the PRC2 complex32 and participates in transcription repression of the leukemia fusion protein PML/RARα target genes.48 On NFI-A, its repressor effect contributes to gene silencing and granulocytic differentiation.
The RISC component Dicer1, and transiently Ago1, are also present with YY1 on the NFI-A promoter on granulopoiesis, a finding in line with the reported regulatory role of Dicer1 and Ago1 in heterochromatin maintenance.35,49 Remarkably, RNA immunoprecipitation experiments revealed that endogenous miR-223 is associated with both Dicer1 and YY1 in response to RA treatment of HL60 cells. During RA-induced granulopoiesis of HL60 cells and primary APL blasts, miR-223 localizes within the nucleus and in HL60 cells colocalizes with YY1 and Dicer1. Moreover, miR-223 is able to bind to the native chromatin of mitotic chromosomes and is present on the NFI-A chromatin regulatory region where the epigenetic modifications controlling NFI-A expression levels during myelopoiesis occur. In agreement, miR-223 stable ectopic expression induces granulocytic differentiation20,23 and recapitulates the epigenetic events underlying NFI-A TGS, including NFI-A gene silencing through the resolution of the PcG/TrxG “bivalent domains” and the recruitment of Dicer1 on the NFI-A promoter, whereas miR-223 stable knockdown produces opposite effects. In line with recent observations showing that RNAi machinery components and miRs may be involved in PcG-dependent TGS,12-14,39 our results show that miR-223 seed-matching sequences present on the NFI-A promoter nearby YY1 binding sites mediate transcriptional activity of miR-223 on NFI-A gene. miR-223 is indeed detectable at these sites with Dicer1, YY1, and transiently with Ago1 on the NFI-A promoter, which is relevant for granulopoiesis (see model in Figure 6E).
NFI-A transcriptional gene silencing by PcGs–miR-223 appears highly dependent on the integrity of the miR-223 homologous regions on NFI-A promoter. Indeed, mutation of miR-223 binding sites abolished miR-223–mediated repression of NFI-A promoter reporter constructs.
Although the potential chromatin and transcription regulatory scope of ncRNA is only now being appreciated and additional investigation on the mechanism of endogenous miR-induced TGS is necessary, previous work has shown that miR-10a can transcriptionally repress the Hoxd4 gene by binding promoter-associated noncoding RNA and cooperating with Dicer and Ago proteins in modifying chromatin.50 Our observations indicate, for the first time, that the recognition of gene promoters by endogenous miRs can be restricted to the miR “seed” region and contributes to aggregate a Polycomb/RNAi/miRNA repressor complex, which is operative in mammalian cells for lineage determination. Thus, our data point to miR-induced TGS as a natural and general mechanism for regulating chromatin status and gene transcription of developmental regulators of cell identity.
Our findings raise the fascinating scenario whereby, besides the regulation of translation of their mRNA targets, endogenous miRs also affect gene expression at the transcriptional level, functioning as a critical interface between chromatin remodeling complexes and the genome to silence genes, such as hematopoietic lineage-affiliated transcription factors (eg, NFI-A), which require permanent transcriptional inhibition to allow definitive lineage fate decisions. By contrast, fine tuning by posttranscriptional silencing may be reserved to regulators, such as LMO2, acting as master genes in the early stages of hematopoiesis.
Within this context, the inverse differentiation stage-related correlation between the NFI-A and miR-223 levels in normal and leukemic HSCs/HPCs cannot be considered merely as a stochastic biologic event but rather as the cooperation between 2 intimately connected pathways whose aim is to regulate their own gene expression levels because both act as life-long key determinants of HSC/HPC lineage restriction.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Fulvio Florenzano for confocal microscopy analysis and Teresa Mangiacrapa for technical assistance.
This work was supported by the Italian Association for Cancer Research (IG-4082, G.Z.; IG-11586, F.L.-C., IG-9390, F.G.; and IG-11949, C.N.), University of Roma La Sapienza, Ministero dell'Istruzione dell'Università e della Ricerca, and Fondazione Cenci Bolognetti.
Authorship
Contribution: G.Z. designed and performed experiments and wrote the paper; A.C., L.V., F.F., M.B., S.R., M.M., and M.N. generated new reagents and performed experiments; L.M.S. generated new reagents; C.M., N.N., and L.T. performed experiments; G.C. and F.L.-C. provided primary normal and leukemic samples; F.G. contributed to experimental design and writing; and C.N. planned the research strategy and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giuseppe Zardo, Department of Cellular Biotechnologies and Hematology, University of Rome La Sapienza, Viale Regina Elena 324, Rome, 00161, Italy; e-mail: zardo@bce.uniroma1.it; Francesco Grignani, Department of Clinical and Experimental Medicine, General Pathology, University of Perugia, Perugia, 06100, Italy; e-mail: fragrig@unipg.it; and Clara Nervi, Department of Medico-Surgical Sciences and Biotechnologies, University of Rome La Sapienza, Corso della Repubblica, 79, Latina, 04100, Italy; e-mail: clara.nervi@uniroma1.it.
References
Author notes
G.Z., A.C., and L.V. contributed equally to this study.