Abstract
Tyrosine kinase inhibitors (TKIs) and reduced intensity conditioning (RIC)/nonmyeloablative (NMA) conditioning hematopoietic cell transplants (HCTs) have changed the therapeutic strategy for chronic myelogenous leukemia (CML) patients. We analyzed post-HCT outcomes of 306 CML patients reported to the Center for International Blood and Marrow Transplant Research aged 40 years and older undergoing RIC/NMA HCT from 2001 to 2007: 117 (38%) aged 40 to 49 years, 119 (39%) 50 to 59 years, and 70 (23%) 60 years or older. The majority (74%) had treatment with imatinib before HCT. At HCT, most patients aged 40 to 49 years were in chronic phase (CP) 1 (74%), compared with 31% aged 60 years or older. Siblings were donors for 56% aged 40 to 49 years; older cohorts had more unrelated donors. The majority received peripheral blood grafts and RIC across all age groups. 3 year overall survival (54%, 52%, and 41%), day + 100 grade II-IV acute GVHD (26%, 32%, and 32%), chronic GVHD (58%, 51%, and 43%), and 1-year treatment-related mortality (18%, 20%, and 13%) were similar across ages. The 3-year relapse incidence (36%, 43%, and 66%) and disease-free survival (35%, 32%, and 16%) were inferior in the oldest cohort. Importantly, for CP1 patients, relapse and disease-free survival were similar across age cohorts. Allogeneic RIC HCT for older patients with CML can control relapse with acceptable toxicity and survival in TKI-exposed CML, especially if still in CP1.
Introduction
Chronic myelogenous leukemia (CML) has become the paradigm hematologic malignancy for which effective targeted drug therapy with tyrosine kinase inhibitors (TKIs) has changed the therapeutic landscape. As the only potential curative therapy for CML, allogeneic stem cell transplantation had historically been the standard of care for patients in chronic phase CML with long-term disease-free survival (DFS) ranging from 30% to 70% depending on age, disease status at transplant, time from diagnosis to transplant, and donor source.1 However, favorable outcome and side effect profiles for imatinib compared with interferon therapy,2 long-term outcomes from the International Randomized Study of Interferon and imatinib,3 and newer data with second generation TKIs dasatinib4 and nilotinib5 have shifted the use of hematopoietic cell transplant (HCT) in the CML treatment paradigm. The high tolerability and success of CML disease control using the TKIs expands the clinical options for patients and clinicians. However, the curative potential of HCT remains, and the transplant community is challenged to determine the most effective time to use this curative strategy, including for older patients, the most common age group with CML.
We report the HCT outcomes of CML patients aged 40 years or older reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) from 2001 to 2007, focusing on this time period because it captures the entry of imatinib into clinical practice. Assessing the impact of age on after transplantation outcomes in the tyrosine kinase era, this analysis can help guide clinical decision making regarding timing of HCT for older patients with CML.
Methods
Data source
The CIBMTR includes a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous HCT to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; patients are followed longitudinally, and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians' review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR's capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act privacy rule.
Patient selection
This study was reviewed and approved by the Medical College of Wisconsin Institutional Review Board. All patients reported to the CIBMTR aged 40 years or older who received a reduced intensity conditioning (RIC) or nonmyeloablative (NMA) conditioning HCT for CML from either an HLA-identical sibling or unrelated donor (URD) from 2001 to 2007 were included in this analysis. Patients receiving cord blood transplants were excluded, but patients receiving prior autologous transplants were not.
In total, 306 patients were identified from 125 centers. Patients were divided into 3 cohorts for analysis: age 40 to 49 years, 50 to 59 years, and 60 years or older. Pre-HCT therapy information was available in the majority of patients. CIBMTR classifications of URD matching were used to define well-matched, partially matched, or mismatched categories.6 Preparative regimens were classified as either RIC or NMA. RIC regimens were classified by established CIBMTR functional definitions and included any regimen with either (1) 500 cGy or less of total body irradiation (TBI) as a single fraction or 800 cGy or less if fractionated; (2) less than 9 mg/kg busulfan oral (or intravenous equivalent); (3) less than 140 mg/m2 melphalan; (4) less than 10 mg/kg thiotepa; or (5) BEAM regimen (carmustine, etoposide, cytarabine, and melphalan).7,8 T-cell depletion for GVHD prophylaxis was included and captured both in vivo and ex vivo T-cell depletion. All other regimens were classified as NMA according to Champlin et al9 where prompt hematopoietic recovery could reasonably be expected without a transplant and would produce mixed chimerism after engraftment after transplantation with the majority including regimens such as (1) TBI 200 cGy, (2) fludarabine + TBI 200 cGy, and (3) fludarabine + cytarabine.
Study end points
End points of hematopoietic recovery, occurrence of acute graft-versus-host disease (aGVHD) and chronic graft-versus-host disease (cGVHD), treatment-related mortality (TRM), incidence of relapse, DFS, and overall survival (OS) were assessed. Hematopoietic recovery was defined as time to absolute neutrophil count greater than or equal to 500 neutrophils/mcL sustained for 3 consecutive measures. Criteria for aGVHD and cGVHD were based on consensus criteria as defined previously.10,11 TRM was defined as any death in the first 28 days after transplant or any death after day 28 without recurrent or progressive leukemia. Relapse was defined as hematologic or cytogenetic evidence of disease recurrence with those surviving without relapse censored at the date of last contact and using death in remission as the competing hazard. DFS was defined as survival without death or relapse with those who survived without recurrence or persistent disease censored at the date of last contact. OS was defined as time to death from any cause with surviving patients censored at time of last contact.
Statistical analysis
Patient, disease, and transplant-related factors were compared between age cohorts using a χ2 test for categorical variables and the Wilcoxon 2-sample test for continuous variables. The product-limit estimator proposed by Kaplan-Meier12 was used to estimate the median and range of the follow-up time.
Univariate probabilities of DFS and OS were calculated using the Kaplan-Meier estimator12 with the variance estimated by Greenwood's formula. Probabilities of aGVHD and cGVHD, TRM and relapse were calculated using cumulative incidence curves to accommodate competing risks. We derived 95% confidence intervals (CI) for all probabilities and P values of pairwise comparisons from pointwise estimates, and calculated values using standard techniques.
The age groups were compared using proportional hazards regression models. The proportional hazards assumptions for all variables were examined by adding a time-dependent covariate. Time-dependent covariate with piecewise constant of regression coefficients were used to model time-varying effect when the proportionality assumption did not hold. The proportionality assumption was further examined for the piecewise constant regression coefficient Cox model. A stepwise forward method was used to build the regression model for the outcomes of TRM, relapse, DFS, and OS. Because recipient age was the main interest of this study, the risk factor of age was included in all steps of model building. Patient-related variables including sex and Karnofsky performance status of less than 80 versus greater than or equal to 80, and pre-HCT therapy with imatinib (yes vs no) were considered in the analysis. Disease-related variables included chronic phase (CP) 1 versus accelerated phase (AP)/CP2+ versus blast crisis; and time from diagnosis to transplantation for patients in CP1 (< 1 year vs 1-2 years vs ≥ 2 years). Transplant-related variables considered in the analysis included year of transplant 2001 to 2004 versus 2005 to 2007, donor-recipient CMV serostatus (negative/negative vs negative/positive vs positive/negative vs positive/positive), donor-recipient HLA match (HLA-matched sibling vs well-matched URD vs partially matched URD vs mismatched URD), stem cell source (bone marrow vs peripheral blood), GVHD prophylaxis (cyclosporine/tacrolimus ± methotrexate ± other vs T-cell depletion), donor-recipient sex match (male/male vs male/female vs female/male vs female/female), and conditioning regimen intensity (RIC vs NMA). The risk factors with significant level of P less than .05 were included in the final model. The potential interactions between the main effect of age and all significant covariates were examined. SAS Version 9.1 (SAS Institute) was used for all analyses.
Results
Patient characteristics
Patient demographic, disease, and transplant-related factors are included in Table 1. Karnofsky score, peripheral blood graft source, conditioning regimen intensity (RIC vs NMA), donor recipient sex match, donor recipient CMV status, time period transplanted, and GVHD prophylaxis were similar across age groups. Patients receiving transplant in CP1 were more common in the 40- to 49-year age group with increasing numbers of CP2/AP in the 50- to 59-year and 60-year or older age cohorts. Sibling donors were slightly more common in the 40- to 49-year age group, and URD more frequent in the older groups
Data regarding pre-HCT TKI use was available in the majority of patients (n = 299); however, the rationale for timing of HCT and the reason for RIC/NMA conditioning were unavailable. The majority of patients with known pre-HCT treatment history available had some exposure to imatinib therapy pre transplant (220, n = 74%) either alone (n = 29) or in combination with either cytotoxic chemotherapy (n = 123), interferon (n = 48) or with second line TKI therapy (n = 20). By time period 64% (n = 134) of patients during 2001 to 2004 were treated with pre-HCT imatinib, and this increased to 89% (n = 86) for those transplanted in 2005 to 2007. There was similar exposure to pre-HCT imatinib, ranging from 69% to 79% across age groups. Data regarding pre-HCT imatinib resistance, BCR-abl mutational status, or post-HCT TKI use were not available.
Neutrophil engraftment
Table 2 describes univariate probabilities of outcome. Neutrophil engraftment at day +28 was frequent across all age cohorts ranging between 91% and 95%. At day +60, between 96% and 97% of patients in all age groups had engrafted. In multivariate analysis, age did not impact engraftment, but peripheral blood stem cell source was associated with quicker engraftment (odds ratio, 5.83 [95% CI, 2.22%-15.28%]; P = .0003) compared with bone marrow.
Survival
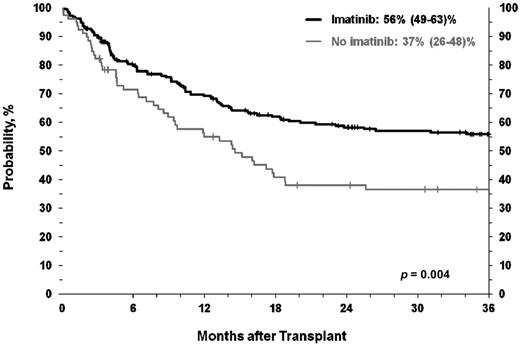
At the time of analysis, 150 patients are alive between 2 and 105 months (median, 48 months) after HCT. Survival was similar across age groups (Figure 1). OS at 3 years for the age 40- to 49-year, 50- to 59-year, and 60-year or older age cohorts were 54% (95% CI, 44%-64%), 52% (42%-61%), and 41% (30%-54%; P = .26), respectively. As seen in Figure 2, OS was associated with pre-HCT imatinib therapy with 3-year OS for those treated with imatinib of 56% (49%-63%) compared with 37% (26%-48%) for those not receiving imatinib (P = .004).
In multivariate analysis, OS was affected primarily by disease stage at transplant and pre-HCT imatinib therapy (Table 3). Risk of death was increased in both the AP/CP2+ and blast phase (BP) groups. Survival was better in those treated with imatinib before HCT.
Causes of death differed slightly between age groups. In the youngest age cohort (age 40-49 years) the most common causes of death were relapse (21%), organ failure (21%), and infection (13%). The older cohort (age 60+ years) had a higher incidence of CML-related death (44%), with GVHD (19%) and organ failure (14%) making up most of the remainder.
Transplant-related mortality
TRM was low and similar across all age cohorts. One-year TRM ranged from 18% in the youngest to 13% in the oldest cohort. At 3 years, there was a slight increase in TRM that was similar across age groups, ranging from 29% (95% CI, 20%-38%) to 19% (10%-30%) in the youngest and oldest cohorts, respectively (P = .33). Multivariate analysis confirmed a similar TRM across age and highlighted the significant negative impact of advanced disease status at HCT and donor-recipient HLA disparity (Table 3).
Graft-versus-host disease
Incidence of aGVHD grades II to IV at day +100 were similar across all age groups, ranging from 26% to 32% (P = .53). Multivariate analysis revealed increased aGVHD with partially matched and mismatched URD.
Rates of cGVHD at 3 years were similar in all age groups, ranging from 43% to 58% (P = .19). cGVHD was significantly influenced by GVHD prophylaxis and conditioning intensity. In multivariate analysis compared with cyclosporine ± methotrexate–based regimens, there was decreased cGVHD in those receiving prophylaxis with either tacrolimus ± methotrexate (relatative risk [RR], 0.60 [95% CI, 0.36%-0.98%]) or T-cell depletion (RR, 0.48 (95% CI, 0.32%-0.72%]; P = .002). Compared with NMA, RIC was associated with increased risk of cGVHD (RR, 1.81 [95% CI, 1.13%-2.9%]; P = .0134). Surprisingly, there was increased risk of cGVHD in those who had been treated with pre-HCT imatinib.
Relapse
The incidence of relapse increased with age. At 3 years, the incidence of relapse in the 60+-year age group was high at 66% (95% CI, 53%-77%) compared with 36% (95% CI, 27%-46%) in the group aged 40 to 49 years (P = .001). Given the higher percentage of patients with advanced disease in older patients, we performed a subset analysis comparing CP1 versus CP2/AP/BP and noted the difference in relapse to be less prominent and no longer statistically significant in the older groups. CP1 patients 60 years or older had an incidence of relapse of 51% at 1 year (that remained stable out to 3 years) compared with an incidence of relapse of 34% (95% CI, 23%-46%) and 42% (95% CI, 28%-56%) in those aged 40 to 49 years and 50 to 59 years, respectively (P = .40; Figure 3). Most relapses occurred by 1 year, with very few later relapses. Relapse was not altered by pre-HCT imatinib with a cumulative incidence of relapse at 3 years of 44% and 47% for those CP1 recipients with and without pretransplant imatinib, respectively.
In multivariate analysis older age, CML disease status at HCT, sex, and conditioning intensity all significantly influenced relapse (Table 3). The RR of relapse was significantly higher in those patients 60 years and older and in those in BP at HCT, but it was lower in females. The RR of relapse also was significantly lower in those receiving RIC compared with NMA conditioning.
Disease-free survival
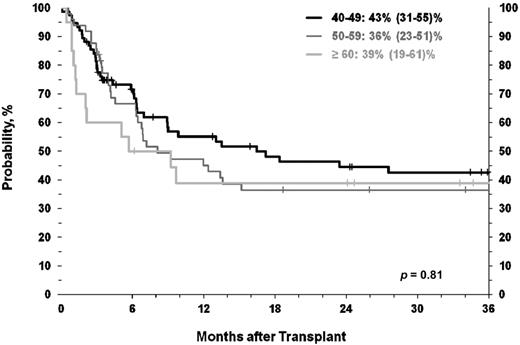
DFS differed based on age. 3-year DFS for those age 40 to 49 years was 35% (95% CI, 26%-45%) compared with a disappointing 16% (95% CI, 7%-27%) in those aged 60 years and older (P = .01). To account for disease stage at HCT, in a subset analysis of CP1 patients the difference in DFS by age was no longer apparent and ranged from 43% (95% CI, 31%-55%) in the youngest age cohort (age 40-49 years) to 39% (95% CI, 19%-61%) in the 60+-year cohort (P = .81; Figure 4).
Multivariate analysis (Table 3) on DFS showed that older patients, those with more advanced disease, males, and those receiving NMA regimens had inferior outcomes.
Discussion
Our study presents the largest series of CML patients in the imatinib era undergoing RIC or NMA conditioning before HCT, with specific assessment of age on outcome. Our data indicate that HCT is safe in older patients with CML, with similar incidence of neutrophil engraftment, aGVHD, cGVHD, TRM, and OS across age cohorts. The outcomes highlight specific populations who benefit from HCT and can thus direct clinical decision making regarding patient selection and transplant timing for older patients with CML.
In comparison with historical outcomes of CML patients undergoing myeloablative conditioning before sibling or URD transplant published by Weisdorf et al,1 our survival was similar though observed in a much older age population (age range, 40-75 years). DFS for CP1 patients in our series (3-year DFS range, 36%-43%), however, was slightly reduced compared with historical myeloablative outcomes for younger patients in the pre-imatinib era (5-year DFS range, 46%-57%).1
Recent outcomes from a randomized German CML Study IV reporting on a smaller series of 84 younger CML patients (median age, 37 years) undergoing predominantly myeloablative allogeneic HCT after imatinib therapy reveal very high 3-year OS rates of more than 90%. They identified no difference for those transplanted during CP1 regardless of HCT either early after diagnosis versus after imatinib failure. OS was lower for those with more advanced disease (59%).13 In these much younger patients receiving predominantly myeloablative conditioning, the observed outcomes were somewhat better than the current and uncontrolled registry report.
Our series does reveal an increased risk of relapse and inferior DFS with older age, most of whom had advanced disease at HCT. Analysis of relapse for those in CP1 revealed a less striking and not significant impact of age, whereas DFS was equivalent regardless of age, suggesting that disease status played the more important role yielding the observed inferior outcomes.
Treatment related mortality and overall survival were not impacted by age, indicating that advanced age should not be a contraindication to transplant. Our oldest cohort (≥ 60 years) did have a higher percentage of patients with more advanced disease at the time of HCT suggesting that either they presented at a later stage of disease at diagnosis or that reluctance to transplant in the older group led to delay until disease progression.
Conditioning intensity significantly impacted relapse and DFS with multivariate analysis, highlighting inferior outcomes in those receiving NMA compared with RIC conditioning. Limited application of NMA conditioning in advanced patients precluded meaningful comparison of NMA versus RIC in CP1 versus AP/CP2/BP. The observed data support the use of RIC over NMA conditioning.
Interestingly, our series revealed a significantly lower risk of relapse and improved DFS in female patients undergoing HCT. Although a slightly higher percentage of females were in CP1 at HCT, the improved outcome was confirmed in multivariate analysis. This finding cannot be explained by differences in donor or recipient sex or HLA matching, and its etiology is unclear.
A majority of our patients were treated with imatinib before HCT; however, the rationale for timing of transplant or information regarding the development of imatinib resistance or mutational analysis was not available. Imatinib was associated with improved post-transplantation survival, but interestingly it was not associated with a lower risk of post-HCT relapse. The lesser toxicity of imatinib over alternative chemotherapy agents could potentially influence the observed improved OS in that group of patients, or associated cytogenetic or molecular monitoring could have highlighted the need to proceed promptly to HCT in TKI failures. The rationale underlying little impact of pre-HCT imatinib on post-transplantation relapse is less clear. TKI therapy might reduce the bulk of CML disease burden, yet it might not eradicate the CML stem cell and thus not limit the risk of post HCT relapse. However, further analysis would require details of the molecular and cytogenetic changes underlying the decision to proceed to HCT.
The entry of second line generation TKI inhibitors into clinical practice again challenges the blood and marrow transplant community to determine the optimal timing of HCT for CML patients resistant or intolerant to imatinib. Using second generation TKI in this patient population is promising because both dasatinib14 and nilotinib15,16 yield high rates of cytogenetic and molecular responses and OS at 2 years ranging from 70% to 90%.14-17 Additional study is needed to guide the approach to management of imatinib resistant patients and to highlight the safest and most effective timing for HCT in the treatment of CML. Using newer data highlighting the impact of 3-month molecular response kinetics on survival also may help to identify those patients at risk for poor long-term outcomes.18 This strategy could result in a higher percentage of patients proceeding to transplant during the preferred disease status, still CP1, even if TKI-resistant.
In summary, our data show that allogeneic HCT using RIC conditioning for patients aged 40 to 75 years with CP1 CML can control relapse with acceptable toxicity and survival in line with numerous reports showing HCT efficacy in imatinib-resistant CML.19-22 The favorable findings of this analysis indicate prompt application of HCT for older patients with TKI-resistant CML, especially if still in CR1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the contributions of these additional authors: Camille Abboud, Mojtaba Akhtari, Joseph Antin, Ulrike Bacher, Brian Bolwell, Chris Bredeson, Claudio Brunstein, Nelson Chao, Edward Copelan, Jack Hsu, Rammurti Kamble, Mohamed Kharfan-Dabaja, José Leis, ZiYi Lim, Selina Luger, David Marks, Brian McClune, Joseph McGuirk, Alan Miller, Betul Oran, Muthalagu Ramanathan, Harry Schouten, Shimon Slavin, Gerard Socié, Robert Soiffer, Keith Stockerl-Goldstein, Jeff Szer, Marcie Tomblyn, Roy Weiner, and Ann Woolfrey.
The Center for Informational Blood and Marrow Transplant Research is supported by US Public Health Service grant/cooperative agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases; grant/cooperative agreement 5U01HL069294 from NHLBI and NCI; contract HHSH234200637015C with Health Resources and Services Administration; grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; grants from AABB (National Blood Foundation); Allos Inc; Amgen Inc; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US Inc; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical Inc; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix GmbH; Children's Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai Inc; Genentech Inc; Genzyme Corporation; Histogenetics Inc; HKS Medical Information Systems; Hospira Inc; Kirin Brewery Co Ltd; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals Inc; Miller Pharmacal Group; Milliman USA Inc; Miltenyi Biotec Inc; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics Inc; Otsuka America Pharmaceutical Inc; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix Inc; StemCyte Inc; StemSoft Software Inc; Sysmex America Inc; THERAKOS Inc; Vidacare Corporation; ViraCor Laboratories; ViroPharma Inc; and Wellpoint Inc.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US government.
National Institutes of Health
Authorship
Contribution: E.W., D.W., and K.W.A. designed the research; E.W., D.W., T.L.P., K.W.A., A.A., M.d.L., M.P., G.A., M.A., J.-Y.C., M.C., Y.-B.C., B.C., A.D., S.G., V.G., H.J.K., H.K., H.M.L., I.L., R.O., J.P., B.S., M.S., G.S., M.T., C.U., R.V., and L.V. analyzed and interpreted the data; T.L.P. and K.W.A. performed the statistical analysis; and E.W., D.W., T.L.P., and K.W.A. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Erica Warlick, Division of Hematology, Oncology, and Transplant, University of Minnesota, 420 Delaware St SE, Box 480, Mayo Bldg, Minneapolis, MN 55455; e-mail: ewarlick@umn.edu.