Abstract
Atypical hemolytic uremic syndrome (aHUS) is a rare renal thrombotic microangiopathy commonly associated with rare genetic variants in complement system genes, unique to each patient/family. Here, we report 14 sporadic aHUS patients carrying the same mutation, R139W, in the complement C3 gene. The clinical presentation was with a rapid progression to end-stage renal disease (6 of 14) and an unusually high frequency of cardiac (8 of 14) and/or neurologic (5 of 14) events. Although resting glomerular endothelial cells (GEnCs) remained unaffected by R139W-C3 sera, the incubation of those sera with GEnC preactivated with pro-inflammatory stimuli led to increased C3 deposition, C5a release, and procoagulant tissue-factor expression. This functional consequence of R139W-C3 resulted from the formation of a hyperactive C3 convertase. Mutant C3 showed an increased affinity for factor B and a reduced binding to membrane cofactor protein (MCP; CD46), but a normal regulation by factor H (FH). In addition, the frequency of at-risk FH and MCP haplotypes was significantly higher in the R139W-aHUS patients, compared with normal donors or to healthy carriers. These genetic background differences could explain the R139W-aHUS incomplete penetrance. These results demonstrate that this C3 mutation, especially when associated with an at-risk FH and/or MCP haplotypes, becomes pathogenic following an inflammatory endothelium-damaging event.
Introduction
The atypical hemolytic uremic syndrome (aHUS) is a rare kidney predominant thrombotic microangiopathy, associated with genetic abnormalities in the regulators and activators of the alternative complement pathway.1 A complement genetic abnormality has been identified in > 60% of patients.2 Most commonly, they correspond to rare distinct point mutation variants unique for each patient/family.3 The small number of patients sharing a particular mutation often precludes investigations on the consequence of each particular mutation and on the influence of additional factors for the disease manifestation. Therefore, it is still unclear why aHUS has incomplete penetrance among the mutation carriers, despite the clear functional consequences of the mutations. Complement dysregulation is linked, by as yet not well-understood mechanisms, to the induction of a procoagulant phenotype on glomerular endothelial cells (GEnCs). Thrombi are formed in the kidney microvasculature, resulting in end-stage renal disease (ESRD) 1 year after the first flare in ∼ 50% of the cases.1
Complement is an innate immune surveillance system, designed to fight infections and to handle damaged or apoptotic cells and debris.4 It may be activated by 3 pathways, all leading to the cleavage of the central component C3 by an enzymatic complex called a C3 convertase. The C3 convertase of the alternative pathway, C3bBb, is composed of the active fragments of C3 and factor B (FB).
To avoid accidental host tissue injury, the complement system is tightly controlled on self-surfaces by regulators such as factor H (FH), factor I (FI) and membrane cofactor protein (MCP; CD46). In aHUS, these regulators are frequently mutated and unable to efficiently protect the endothelium from complement attack.1 C3 mutations were recently discovered in aHUS and only few reports describe the clinical outcome of such patients.5-8 Functional analysis of 9 of these C3 mutations revealed that most of them result in impaired regulation by MCP and hence in indirect, or secondary, gain of function of the C3 convertase.5 It has also become evident that aHUS could be associated with an intrinsically hyperactive C3 convertase. Three such mutations have been reported in FB.9,10 They resulted in the formation of a more potent C3 convertase, resistant to decay by complement regulators and leading to enhanced C3 deposition on resting GEnC. A particular hyperactive C3 convertase, formed with mutated C3, was described in patients with dense deposit disease because of in frame deletion of 2 amino acid residues.11 To date, no C3 mutations in aHUS have been described that are able to directly enhance the function of the C3 convertase.
Here, we report the first large series of aHUS patients carrying the same genetic abnormality in C3, R139W. This is the first description of a direct gain-of-function mutation in C3 that forms a hyperactive C3 convertase as well as being resistant to regulation by MCP, but not by FH. This C3 mutation, especially if associated with at-risk FH and MCP haplotypes, becomes pathogenic following an endothelium-damaging event.
Methods
Patients
Patients were recruited from the French aHUS cohort of patients (n = 343). Diagnosis of HUS was defined by the simultaneous and acute occurrence of at least 3 of the following 4 criteria: acute renal failure, microangiopathic hemolytic anemia, thrombocytopenia, and/or histologic thrombotic microangiopathy. Further definitions and the case reports are given in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The clinical history of patients P7 and P9 has been reported previously.12 This study was approved by the Inserm ethical commission.
Structure analysis
The crystal structures of C3,13 C3b,14 C3b-FH1-4,15 C3bBb,16 C3bB17 and MCP18 are available in Protein Data Bank. Molecular graphic imaging and analysis were produced using the Pymol and UCSF Chimera package19 (http://www.cgl.ucsf.edu/chimera/). Protein numbering throughout this study is according to the sequence of the mature protein, lacking the signal peptide. Wherever appropriate, the numbering will be according to the gene sequence (starting with c.) and to the protein with the 22-amino-acid-long leader peptide (starting with p.).
Endothelial cell assays
Conditionally immortalized GEnCs10,20 (passage 29-35) and third-passage primary HUVECs were used for this study. Detailed of culturing conditions are given in supplemental Methods. Briefly, GEnCs and HUVECs, either resting or stimulated with TNFα/IFNγ, were exposed to normal human sera (NHS) from 50 donors or R139W sera or FH-depleted (FH-dpl) serum. Alternatively, blocking anti-FH (Ox2421 ) mAb was added to these sera or anti-MCP (GB2422 ). In addition, R139W sera were supplemented with different concentrations of purified FH (Comptech). Released C3a, C5a, and sC5b9 were measured in the supernatant using specific ELISAs (Quidel). Cells were incubated with a mouse anti–human C3c mAb (Quidel), followed by a secondary anti–mouse IgG-PE or an anti-tissue factor-PE Ab (BD Pharmingen) and analyzed by flow cytometry as described in supplemental Methods.
Recombinant C3 production
The R139W mutant C3 was produced from the wild-type (WT) C3 plasmid as described.5 The expression level of the plasmids was compared after multiple transient transfections using Lipofectamine. The plasmid was introduced into CHO cells via stable transfection using selection with G418. The constructs were sequenced in their entirety to confirm that no additional mutations had been introduced. The synthesis of C3 was assessed by a sandwich ELISA, using immobilized anti–human C3 Ab for capture and biotinylated anti–human C3, followed by streptavidin-HRP (Amersham) for detection. The expression levels were similar between WT and mutant plasmids. Supernatants derived from stable transfected cells containing recombinant WT and R139W-C3 as well as supernatants of the mock-transformed cells (SN0), were used to purify C3 by ion exchange chromatography using DEAE Sepharose (GE Healthcare) and eluted with 0.2M NaCl.
Surface plasmon resonance
The interaction of wild-type and mutant C3 with FH, MCP, and FB was analyzed using surface plasmon resonance (SPR) technology with Biacore2000 equipment. In the first approach, FH and MCP were coupled to the CM5 biosensor chip (GE Healthcare) using standard amide-coupling technology, according to the manufacturer's instructions. Purified recombinant wild-type and mutant C3 were used as an analyte at concentrations of 1, 0.5, 0.25, 0.125, and 0.06nM. The flow rate was 10 μL/min in a HEPES buffer (10mM HEPES, 25mM NaCl, pH 7.4). An empty activated/deactivated flowcell served as a control.
Alternatively, the anti-C3d Ab (Quidel) was coupled to the 4 flowcells of the chip. Purified WT and R139W C3 and an equivalent purification fraction from the SN0 were loaded on 3 of the flowcells. No binding was detected in the case of SN0. WT and R139W C3 were always loaded to equivalent resonance units. FB was applied as an analyte at 0.06, 0.125, 0.25, 0.5, and 1nM in 1mM MgCl2-containing HEPES buffer (without regeneration). The complex was allowed to decay spontaneously before the next concentration was injected.
Data were analyzed using BIAevaluation software and the RU from the blank flowcell was subtracted. Kinetic parameters were calculated by fitting the obtained sensorgrams into 1:1 interaction with a drifting baseline algorithm to give the lowest χ2.
C3 convertase formation
Alternative pathway C3 convertase was assembled on a CM5 chip by preprogrammed injections of native and recombinant C3, FB, and FD. Native and recombinant C3 were mixed to mimic the heterozygous situation found in patient sera. In each case, 3 different samples were prepared: (1) purified recombinant WT (10 ng) or R139W C3 (10 ng) or equivalent volume of SN0 plus human C3 purified from plasma (Calbiochem; 10 ng), 3 μg of FB (Calbiochem) and 0.2 μg of FD (Calbiochem) were added in 10mM HEPES, 25mM NaCl, 1mM MgCl2 running buffer to a final volume of 100 μL; (2) 7 μg of FB and 0.5 μg of FD in 200 μL of running buffer; and (3) purified recombinant WT (10 ng) or R139W C3 (10 ng) or equivalent volume of SN0 with human C3 purified from plasma (10 ng) in a final volume of 100 μL. Samples were injected as follows: sample 1, followed by sample 2, then sample 3 and, at the end, sample 2 again, followed by 100 s of running buffer only. Alternatively, 1mM NiCl2 was used instead of MgCl2 to stabilize the C3 convertase.
Results
Genetic analysis
Mutation screening.
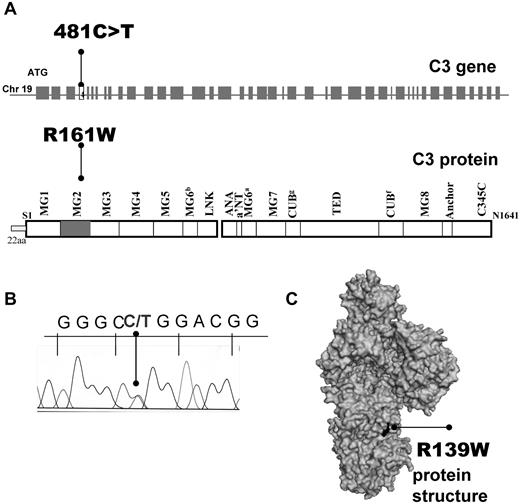
Direct sequencing of the C3 gene allowed identification of 14 patients (4% of the French aHUS cohort and ∼ 50% of patients with C3 mutations) carrying the same heterozygous missense mutation: c.481C>T (Figure 1A), encoding for p.R161W substitution if the first Met was counted as 1 (ie, with a signal peptide of 22 residues, Figure 1B) or R139W of the mature protein (without the signal peptide). The affected R139 residue is partially exposed on the surface of C3 MG2 domain (Figure 1C). This mutation was absent in 550 healthy controls. Most of the patients and 150 of the normal controls came from the North of France. Two of the patients carried a second genetic abnormality in the FH gene (R341H for P9 and F960S for P11, Table 1). No missense or splice site mutations were present in the genes of FH, FI, MCP, C3, FB, and thrombomodulin. No complete deletion of CFHR1/3 was found. Eleven healthy R139W carriers were identified among 19 healthy individuals from the 6 families available for screening.
Localization of R139W C3 mutation. (A) Position within the C3 gene and the protein primary structure. (B) Representative histogram for the sequencing of a patient, carrier of R139W. (C) Mapping of R139W on the surface of C3 using Pymol software.
Localization of R139W C3 mutation. (A) Position within the C3 gene and the protein primary structure. (B) Representative histogram for the sequencing of a patient, carrier of R139W. (C) Mapping of R139W on the surface of C3 using Pymol software.
FH and MCP haplotypes analysis.
The frequency of FH and MCP SNPs and haplotypes23 was evaluated in the R139W-aHUS patients and their relatives and compared with healthy donors (Table 2). FH (FHTGTGT) and MCP (MCPGGAAC) at-risk haplotypes were significantly higher in R139W-aHUS compared with 190 normal donors. These 2 haplotypes were also more frequent in R139W-aHUS, compared with healthy R139W carriers. The presence of FHTGTGT together with R139W gave a 4-fold higher chance for disease development compared with R139W alone. Similarly, the presence of MCPGGAAC with R139W increased 3-fold the risk for development of R139W-aHUS. In addition, 69% of R139W-aHUS were homozygous for the minor TT genotype of the rs3753394 SNP in the promoter region of FH, while it occurred only in 6% of normal donors and was not observed in healthy R139W carriers.
Four of the patients carried homozygous FH risk haplotype TGTGT and 5 patients homozygous MCP risk haplotype GGAAC (Table 1). Two of the patients (P2 and P9) carried both risk haplotypes. If the presence of the FH or MCP at-risk haplotype is considered as 1 risk copy when in a heterozygous form and as 2 risk copies when in a homozygous form, the sum of at-risk haplotype copies in FH and MCP could be estimated in R139W-aHUS and R139W healthy carriers. We were able to assign the individual haplotype alleles for 11 patients and 10 healthy carriers. Eighty-two percent of patients had 2 or more at-risk copies in FH and/or MCP versus 30% of the healthy carriers.
aHUS presentation
The onset of the R139W-aHUS was in the pediatric population (under 18 years) in 5 cases and in adults in 9 cases. A triggering event was suspected in 10 of 14 patients including an infection in 3 of 5 pediatric cases, while the adults < 50 years of age developed the disease postpartum (3 of 7) or in relation to drugs or toxins (4 of 7). No triggering event was identified in the patients > 50 years of age (2 of 2). The pediatric cases had nearly equal distribution of male (2 of 5) and female (3 of 5) patients while in adults 90% of the patients (8 of 9) were female. Shiga toxin–producing Escherichia coli were not present in any patient.
Initial clinical symptoms and laboratory test results are detailed in Table 1. Treatment consisted in plasma therapy in 10 patients: plasma exchange (30-80 mL/kg per session) or exchange transfusions in one case (P1). Cardiac events were reported in 8 of 14 patients. Echocardiography demonstrated a dilated cardiomyopathy with a reduction in left ventricular function in 7 patients. One patient (P13) died in relation to a cardiovascular event but the myocardial process was not defined. Heart failure occurred mostly at the R139W-aHUS onset but 2 patients (P10 and P1) experienced a delayed cardiomyopathy 2 and 6 months, respectively, after the hematologic remission. Cardiac function slowly improved with medical treatment but a left ventricular dysfunction persisted. Neurologic events occurred in 5 of 14 patients, always during the acute phase of the illness. Four patients had seizures and one had brachioplegia. Cranial computed tomographic scans were abnormal in 2 patients, showing diffuse multiple small infarcts. Neurologic manifestations resolved except for one (patient P2), who developed recurrent seizures requiring chronic therapy. Altogether, 9 of 14 patients developed an ESRD, 6 before 1 year of follow-up. Seven kidney transplantations were performed in 4 patients. Four transplanted kidneys were lost after 1 to 23 months because of an aHUS relapse, one of them under preventive plasma therapy.
Complement component assessment.
Low C3 at the acute phase of the disease was detected in 6 of 9 patients, for whom samples were available from the acute phase. In contrast, all screened healthy carriers (samples available for 8 individuals) had normal C3 levels. No patient had anti-factor H autoantibodies. FH and FI antigenic levels and MCP expression (assessed by flow cytometry) were normal in all tested patients.
Functional characterization of the R139W mutation
Using R139W-C3–containing sera.
The R139W mutation led to complement activation on stimulated but not on resting endothelial cells.
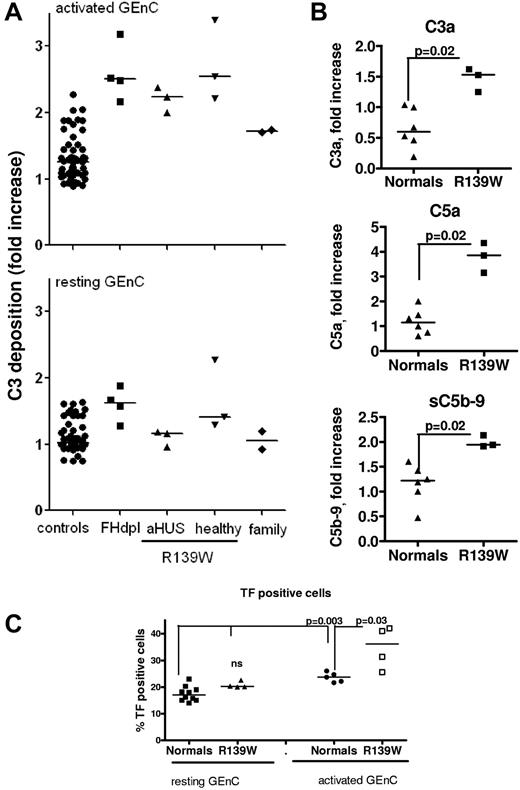
Resting, adherent HUVECs or GEnCs were stimulated with proinflammatory cytokines (TNFα + IFNγ) and then incubated with sera from 3 patients with R139W-aHUS (P2, 5 and 14), 3 healthy carriers of the mutation from patient P5's family, 2 mutation-free members from the same family and compared with 50 different healthy donors (normal controls) and FH-depleted serum (FH-dpl, positive control; Figure 2). Minimal C3 deposition was observed on resting cells incubated with normal sera, aHUS patients' sera and all but 1 healthy carrier's sera (the second sister of patient P5-P5.S2; Figure 2A). FH-dpl led to increased deposition of C3 on the resting cell surface. The high C3 deposition observed P5.S2 serum, with GEnCs as well as with HUVECs from 5 different donors, is mainly alternative pathway dependent (persistent in presence of EGTA-Mg). The screening of FB and FH polymorphisms and CFHR1 copy numbers showed no peculiarity in this subject and, unfortunately, new serum samples were not available for further analysis. On cytokine-stimulated cells, C3 deposition from all tested R139W-HUS patients and healthy R139W carriers was increased, compared with healthy donors and was similar to the FH-dpl (Figure 2A). In the large family tested, the increase of C3 deposition perfectly correlated with the presence of the mutation (as could be seen from the genealogic tree in supplemental Figure 1). The augmented C3 deposition was accompanied by increased C3a and C5a release, sC5b-9 formation, and tissue-factor expression. These effects were observed both using HUVECs (supplemental Figure 1) and GEnCs (Figure 2A).
Complement activation on glomerular endothelial cells, incubated with sera from R139W-aHUS patients and their healthy relatives. (A) C3 deposition on resting or TNFα/IFNγ activated GEnCs in the presence of sera from 50 individual normal donors, FHdpl (4 different lots), R139W-aHUS patients (P2, P5, and P14), healthy relatives of patient P5 bearing the mutation (5.F indicates father; and 5.S1 and 5.S2, sisters) or mutation-free relatives of patient P5, indicated as family (5.M indicates mother; and 5.B, brother). C3 depositions (RFI) obtained with each patient or healthy donor were normalized by the C3 deposition from one normal human serum on resting cells, considered as a standard and run in each experiment to obtain the fold increase. Each point is a mean of 3-5 independent experiments. Statistical significance (***P < .001) was calculated by ANOVA. (B) Levels of C3a, C5a, and soluble C5b-9, released after incubation of the TNFα/IFNγ activated GEnCs with serum from normal donors (n = 6) or R139W sera (n = 3), were measured by ELISA. The level of C3a, C5a, or sC5b-9 in the supernatant (one-third diluted serum) from resting cells was subtracted from the corresponding levels on activated cells, to obtain the specific amount of C5a and sC5b-9 released because of complement activation. Results are expressed as fold increase, compared with a standard normal serum as in panel A. The statistical analysis was a Mann-Whitney test. (C) Tissue-factor expression on TNFα/IFNγ activated or resting GEnCs after overnight incubation with sera from normal donors (n = 6) or R139W-positive sera (n = 4). The percentage of TF-positive cells was measured by flow cytometry. The statistical analysis was the Mann-Whitney test.
Complement activation on glomerular endothelial cells, incubated with sera from R139W-aHUS patients and their healthy relatives. (A) C3 deposition on resting or TNFα/IFNγ activated GEnCs in the presence of sera from 50 individual normal donors, FHdpl (4 different lots), R139W-aHUS patients (P2, P5, and P14), healthy relatives of patient P5 bearing the mutation (5.F indicates father; and 5.S1 and 5.S2, sisters) or mutation-free relatives of patient P5, indicated as family (5.M indicates mother; and 5.B, brother). C3 depositions (RFI) obtained with each patient or healthy donor were normalized by the C3 deposition from one normal human serum on resting cells, considered as a standard and run in each experiment to obtain the fold increase. Each point is a mean of 3-5 independent experiments. Statistical significance (***P < .001) was calculated by ANOVA. (B) Levels of C3a, C5a, and soluble C5b-9, released after incubation of the TNFα/IFNγ activated GEnCs with serum from normal donors (n = 6) or R139W sera (n = 3), were measured by ELISA. The level of C3a, C5a, or sC5b-9 in the supernatant (one-third diluted serum) from resting cells was subtracted from the corresponding levels on activated cells, to obtain the specific amount of C5a and sC5b-9 released because of complement activation. Results are expressed as fold increase, compared with a standard normal serum as in panel A. The statistical analysis was a Mann-Whitney test. (C) Tissue-factor expression on TNFα/IFNγ activated or resting GEnCs after overnight incubation with sera from normal donors (n = 6) or R139W-positive sera (n = 4). The percentage of TF-positive cells was measured by flow cytometry. The statistical analysis was the Mann-Whitney test.
The C3 deposition from R139W serum on stimulated EC was controlled by FH but not by MCP.
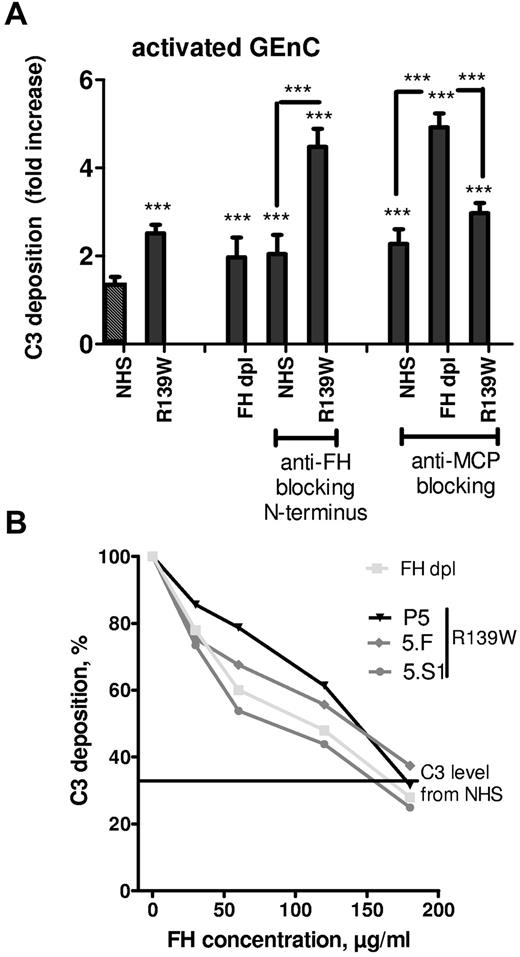
To determine whether the increase in C3 deposition from R139W serum was caused by defective regulation of the R139W C3 by FH or by MCP, function blocking Abs were used (Figure 3A). FH-dpl was used as a control for defective regulation by FH. If the blocking anti-FH OX24 Ab was applied to NHS, C3 deposition on resting cells was increased 2-fold compared with NHS and was equivalent to that of the FH-dpl. The same Ab caused a more than 4-fold increase in C3 deposition when applied to a serum containing R139W-C3. Similar experiments were performed using blocking anti-MCP GB24 Ab. Addition of this Ab to NHS caused a 2.5-fold increase in C3 deposition, similar to FH-dpl. When the anti-MCP Ab was applied to FH-dpl or to R139W serum, the C3 deposition exceeded control levels by 5-fold and 3-fold, respectively. Addition of purified FH to sera of R139W-aHUS patient or healthy relatives but carrying the same mutation resulted in a decreased C3 deposition on activated GEnC (Figure 3B). The C3 deposition reached levels obtained with NHS when the FH concentration was doubled.
Effects of blocking FH and MCP on the regulation of C3 deposition from WT or R139W sera. (A) GEnCs preactivated with TNFα/IFNγ were incubated for 30 minutes with the standard normal human serum, FHdpl, or R139W sera, in the presence or absence of blocking mAbs against FH (Ox24) or MCP (GB24). The C3 deposition was evaluated by flow cytometry as in Figure 2A. ***P < .0001, unpaired t test. Unless specifically mentioned, the comparison was made with the NHS (the first bar with a black and white pattern). (B) GEnCs activated with TNFα/IFNγ were incubated for 30 minutes with normal human sera, FHdpl, or R139W sera in the presence of increasing doses of purified FH. C3 deposition in the absence of FH for the FHdpl or R139W-positive sera (from aHUS patient and healthy carriers) was taken as 100% and each C3 level percentage, in the presence of different doses of FH, was calculated. The starting FH concentration was in the normal range for P5, 5.F, and 5.S1. The level of C3 deposition from a normal serum is given as a straight line.
Effects of blocking FH and MCP on the regulation of C3 deposition from WT or R139W sera. (A) GEnCs preactivated with TNFα/IFNγ were incubated for 30 minutes with the standard normal human serum, FHdpl, or R139W sera, in the presence or absence of blocking mAbs against FH (Ox24) or MCP (GB24). The C3 deposition was evaluated by flow cytometry as in Figure 2A. ***P < .0001, unpaired t test. Unless specifically mentioned, the comparison was made with the NHS (the first bar with a black and white pattern). (B) GEnCs activated with TNFα/IFNγ were incubated for 30 minutes with normal human sera, FHdpl, or R139W sera in the presence of increasing doses of purified FH. C3 deposition in the absence of FH for the FHdpl or R139W-positive sera (from aHUS patient and healthy carriers) was taken as 100% and each C3 level percentage, in the presence of different doses of FH, was calculated. The starting FH concentration was in the normal range for P5, 5.F, and 5.S1. The level of C3 deposition from a normal serum is given as a straight line.
Using recombinant proteins.
The R139W mutation affected C3 interaction with MCP, not with FH.
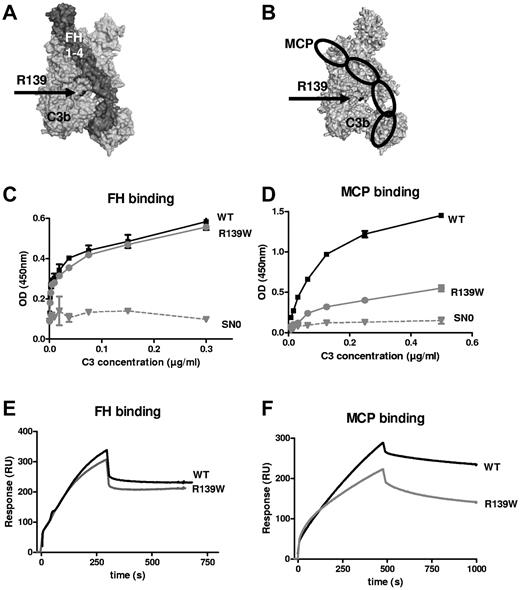
The affected residue R139 is in close proximity to the FH CCP3 binding site15 and to the suggested MCP CCP3 binding site18 (Figure 4A-B). ELISA (Figure 4C-D) and SPR (Figure 4E-F) revealed a normal interaction with FH, but the binding to MCP was decreased. Kinetic analysis indicated a statistically significant 2-fold decrease in the association rate (7.11 × 104 Ms−1 for the WT vs 2.97 × 104 Ms−1 for R139W, P = .016, n = 4, t test) and no difference in the dissociation rate, resulting in a 3-fold reduction in the apparent binding affinity (1.57 × 108 M−1 for the WT vs 5.15 × 107 Ms−1 for R139W, P = .045, n = 4, t test). No difference was observed for the kinetic parameters of the interaction with FH. The decay acceleration activity of FH was identical for the WT and R139W-C3 (supplemental Figure 2A). Both C3 forms were cleaved by factor I in presence of FH as a cofactor, but the cleavage of R139W was slightly delayed compared with the WT (supplemental Figure 2B).
Interaction of R139W with MCP and FH. R139 position (A) on the structure of C3b in a complex with FH CCP1-4 or (B) in a model complex with MCP. R139 is close to CCP3 binding site of both FH and MCP. Direct binding of recombinant WT or mutant C3 to (C) FH or (D) MCP, studied by ELISA. FCS-free supernatant, containing recombinant WT or mutant C3 produced by stably transfected CHO cells, was used as a source of C3. SN0 is the supernatant of cells transfected with a plasmid not containing the C3 gene. The real-time binding of recombinant WT or mutant C3 to (E) FH or (F) MCP was studied by SPR, using recombinant C3 proteins purified from serum-free culture supernatants by DEAE-Sepharose column.
Interaction of R139W with MCP and FH. R139 position (A) on the structure of C3b in a complex with FH CCP1-4 or (B) in a model complex with MCP. R139 is close to CCP3 binding site of both FH and MCP. Direct binding of recombinant WT or mutant C3 to (C) FH or (D) MCP, studied by ELISA. FCS-free supernatant, containing recombinant WT or mutant C3 produced by stably transfected CHO cells, was used as a source of C3. SN0 is the supernatant of cells transfected with a plasmid not containing the C3 gene. The real-time binding of recombinant WT or mutant C3 to (E) FH or (F) MCP was studied by SPR, using recombinant C3 proteins purified from serum-free culture supernatants by DEAE-Sepharose column.
R139W is a gain-of-function mutation leading to a hyperactive C3 convertase.
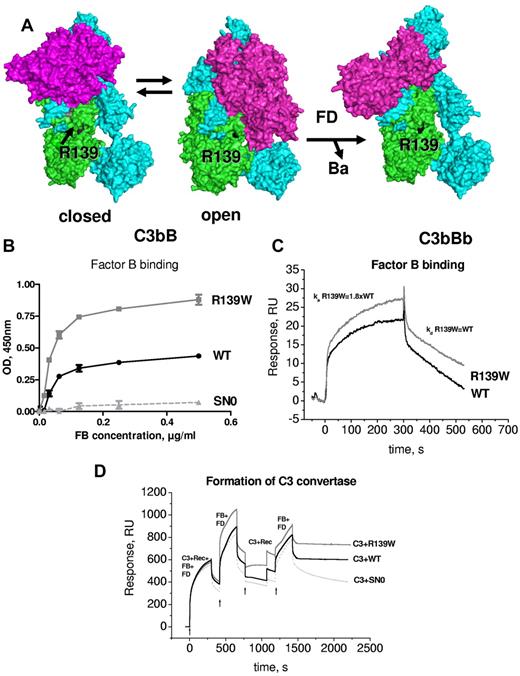
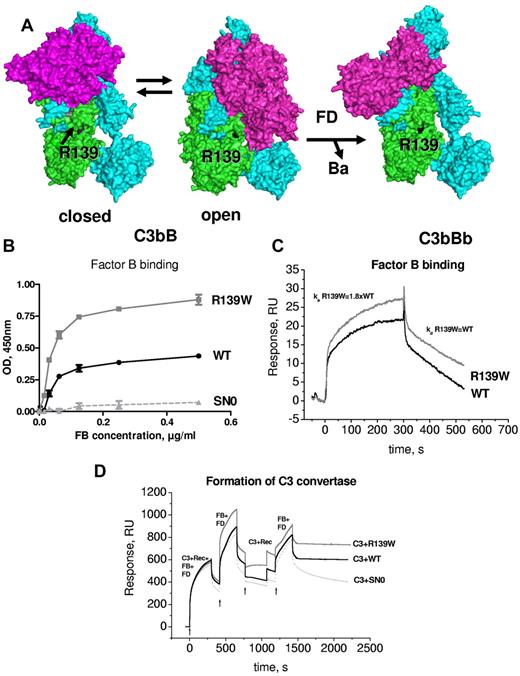
Structural aspects of the formation of the alternative complement pathway C3 convertase C3bBb have been recently described.16,17 R139W mapped far from FB in the closed structure of C3bB and C3bBb. However, it was in close proximity to the SP domain of FB in the so-called open form of C3bB, which is capable of binding FD and allows generation of the active convertase C3bBb (Figure 5A). Therefore, the influence of R139W on the interaction with FB was analyzed. Both ELISA (Figure 5B) and SPR (Figure 5C) demonstrated an increased binding of FB to R139W, specifically, a nearly 2-fold increase in the binding affinity secondary to enhanced association rate. No difference was observed in dissociation parameters. To determine whether this increased binding could lead to hyperfunctional C3 convertase, the formation of the C3 convertase was assessed by SPR. In the presence of R139W the convertase was more efficient, leading to increased C3b deposition on the chip (Figure 5D). This phenomenon was observed both in the presence of Mg (Figure 5D) and of Ni ions (not shown).
Interaction of R139W withfactorB. (A) R139 position on the structures of C3b with FB in closed (refractory to cleavage by FD) and open (prone to cleavage by FD) conformations and on the structure of the C3bBb complex. The α and β chains of C3b are depicted in blue and green and FB is colored in magenta. (B) Binding of FB to WT or mutant recombinant C3, bound to an anti-C3d mAb, coated to the ELISA plate. Serum-free supernatant was used as a source of recombinant C3 molecules. (C) The binding of FB to recombinant WT or R139W C3, bound to an anti-C3d mAb on the CM5 biosensor chip, was studied by SPR using Biacore. Recombinant C3 proteins, purified from serum-free culture supernatants by DEAE-Sepharose column, were used. (D) Formation of a C3 convertase on the biosensor chip by subsequent injection of C3+FB+FD, followed by an injection of FB+FD. Purified WT or R139W recombinant C3 were mixed with native C3, purified from plasma. Proteins deposition on the chip was followed over time. For panels B through D, 1 representative experiment of 3, performed with 3 independent productions of C3, is presented.
Interaction of R139W withfactorB. (A) R139 position on the structures of C3b with FB in closed (refractory to cleavage by FD) and open (prone to cleavage by FD) conformations and on the structure of the C3bBb complex. The α and β chains of C3b are depicted in blue and green and FB is colored in magenta. (B) Binding of FB to WT or mutant recombinant C3, bound to an anti-C3d mAb, coated to the ELISA plate. Serum-free supernatant was used as a source of recombinant C3 molecules. (C) The binding of FB to recombinant WT or R139W C3, bound to an anti-C3d mAb on the CM5 biosensor chip, was studied by SPR using Biacore. Recombinant C3 proteins, purified from serum-free culture supernatants by DEAE-Sepharose column, were used. (D) Formation of a C3 convertase on the biosensor chip by subsequent injection of C3+FB+FD, followed by an injection of FB+FD. Purified WT or R139W recombinant C3 were mixed with native C3, purified from plasma. Proteins deposition on the chip was followed over time. For panels B through D, 1 representative experiment of 3, performed with 3 independent productions of C3, is presented.
Discussion
Atypical HUS is a predominant renal thrombotic microangiopathy strongly associated with complement genetic abnormalities. Nevertheless, the contributions of the complement system mutations to the expression of a procoagulant phenotype of the glomerular endothelium and to the disease manifestation remain elusive. Here, we report a large series of aHUS patients who carry the same genetic abnormality, R139W, in the central complement component C3. This mutation, found in 14 unrelated patients, accounted for half of the C3 mutations associated with aHUS in France. Most of the patients came from the north of France, but did not share a high level of polymorphisms in the vicinity of the C3 gene (data not shown). This suggests that, if there was a founder effect, it was ancient. R139W was not identified in normal controls, confirming it as a mutation and not a rare polymorphism. Given the high frequency of this genetic abnormality, it could be considered as a prototypical aHUS C3 mutation, similar to R1210C in FH.24
The functional consequences of R139W were analyzed using sera containing R139W-C3 and recombinant proteins. Incubation of resting endothelial cells (both HUVECs and GEnCs) with R139W positive sera did not result in increased C3 deposition, except in one case of a healthy carrier. These results are in contrast to what we observed previously for 2 mutations in FB, which caused increased C3 deposition even on resting cells.10 No significant increase in TF expression was observed if resting GEnCs were cultured with R139W serum, compared with healthy controls. The fact that the presence of R139W-C3 is tolerated by healthy endothelium could explain why the disease associated with this mutation occurs at variable ages, in the majority of the cases after a triggering event, and has an incomplete penetrance. Atypical HUS flares are frequently preceded by a triggering event, as in 10 of the 14 patients reported here. Herein, we observed increased complement deposition on glomerular endothelial cells exposed to R139W sera, if cells had been stimulated by proinflammatory cytokines.
All tested R139W containing sera caused increased C3 deposition compared with normal donors and a perfect correlation was observed between this C3 deposition and the presence of the mutation in patient P5's family. This result was similar to our model conditions of complement dysregulation, using FH-depleted serum or blocking FH or MCP with an inhibitory Ab. The enhanced C3 deposition was accompanied by increased release of C3a, C5a, and formation of the soluble terminal complement complex C5b9 (sC5b9), indicating that complement activation on the cell surface proceeded to its final stages. C5a and sC5b9 are known to induce TF expression on HUVECs.25,26 Stimulated GEnCs on culture with R139W sera also expressed higher TF levels, compared with the same cells cultured with normal human sera. Of note, R139W sera from patients and healthy carriers resulted in similar levels of deposited or released complement active fragments and of TF expression. Thus, we provide experimental evidence that complement hyperactivation in aHUS is indeed linked to the expression of a procoagulant phenotype of the GEnCs and therefore participates in the thrombotic process.
The functional consequences of this mutation were studied at the molecular level. Positioning of R139 on the structure of C3b in complex with 3 of its most important ligands revealed that this residue is in proximity to the binding site for CCP3 of FH,15 the putative binding site for CCP3 of MCP18 and the binding site of the FB's serine protease (SP) domain in the open conformations as part of the C3 proconvertase C3bB. Structural aspects of the C3 convertase formation have been extensively studied.16,17,27-29 Binding of FB to C3b induces a dynamic equilibrium between a closed (loading) and open (activation) form. This conformational change is accompanied by a reorientation of FB catalytic SP domain, which comes into contact with the MG2 and the CUB domains of C3b in the open form. The activation form allows factor D binding and formation of the active convertase C3bBb. The R139 residue is far from the FB binding site in the loading form, but the SP domain comes close to this residue in the active form. Experimental results here demonstrated a 2-fold increase in the binding of R139W to FB, because of a faster association rate. Furthermore, mutant C3 formed a hyperactive C3 convertase. These results are in agreement with the structural data and confirm the importance of the MG2 domain for binding of FB.
This is the first rigorously analyzed case of a direct gain of function of a C3 mutation. All mutations previously described5 are indirect gain of function, resulting from ineffective regulation by MCP. Interestingly, the direct binding of R139W C3 to FH and its decay acceleration activity were undistinguishable from wild-type C3, both by ELISA and SPR, notwithstanding the close proximity of the mutated residue to the FH-binding site. The cofactor activity of FH toward R139W cleavage by factor I was only moderately decreased, compared with the wild type. The interaction with MCP was affected by the mutation, causing a strong decrease in binding (measured by ELISA) and a 3-fold decrease in binding affinity (studied by SPR), because of a slower association rate. Once formed, the complex had the same dissociation rate as the WT. These results demonstrate that the binding sites for FH and MCP are not identical and do not involve the same contact residues because R139W differentially influences the interaction with these 2 complement regulators.
The notion that R139W could be regulated by FH but not by MCP was confirmed by a different approach, using function blocking anti-FH and anti-MCP Abs in R139W containing serum. When anti-MCP blocking Ab was applied to R139W sera, C3 deposition was slightly increased, suggesting that in this experimental set-up MCP does not contribute much to the regulation of C3 deposition. In contrast, anti-FH blocking Ab in R139W sera raised C3 deposition to the levels observed in the absence of both MCP and FH. Also, addition of purified FH was able to control the C3 deposition in R139W positive sera in the normal range of healthy donors. These results confirm the capacity of FH to regulate R139W C3 and emphasize FH essential role in complement regulation in sera harboring this mutation.
Although a family history of aHUS was not found in all 14 patients, 11 R139W healthy carriers were identified in 6 families in which genetic analysis was performed. Two patients carried additional mutations in FH outside the hotspot region CCP19-20. To find out whether healthy carriers differed in their genetic background from the R139W-aHUS patients, the frequency of the aHUS at-risk haplotypes in FH (FHTGTGT) and in MCP (MCPGGAAC) were compared. The R139W-aHUS patients had significantly higher frequency of these at-risk haplotypes compared with healthy controls, as previously reported in patients with aHUS.30 In contrast, these frequencies were similar in healthy R139W carriers and in normal donors. The presence of R139W together with FHTGTGT conferred 4-fold higher risk and with MCPGGAAC, 3-fold higher risk for development of aHUS, respectively, compared with R139W alone. Among the R139W-aHUS patients, 82% had 2 or more at-risk alleles in FH and/or MCP genes, compared with 30% of the healthy carriers. This clear distinction between healthy carriers and patients demonstrates the critical role of the FH and MCP genetic background for the development of aHUS in the presence of a mutant C3.
The R139W-aHUS had variable disease manifestations and both severe and milder renal phenotypes were found. Nevertheless, the R139W-aHUS was marked by a high frequency of extrarenal complications (> 60% of the patients). Particularly, 60% of the patients had cardiac events and 35% had neurologic events either during the acute phase or at distance from the aHUS episodes. Cardiac symptoms in HUS and aHUS are rarely mentioned, mostly in isolated case reports. A dilated cardiomyopathy (as in our R139W-aHUS patients) has been the most frequent observation.31-34 In a series of 64 autopsied HUS cases, heart involvement was detected in 19 patients.35 Only one study of aHUS pediatric cases reported a high frequency of cardiomyopathy, affecting 10 children (43% of the cohort). Nevertheless, no mutation screening was performed in these 2 patients' series.36 Cardiac complications have been reported in patients with a FH mutations leading to haploinsufficiency34 ; in 1 of 7 children with autoimmune aHUS37 and in 3 of 45 cases of the French autoimmune aHUS.38 Further studies are needed to determine whether there exists a specific association of cardiac complications with R139W-aHUS. Nevertheless, results suggest that echocardiographic screening of patients with aHUS should be performed at admission and during the follow-up because cardiac complications could be more frequent than reported or associated with particular forms of aHUS, as is the case here.
Starting from the observation that R139W causes a hyperactivity of C3 and reduced binding to MCP, a rational therapeutic strategy could be suggested for these patients. An optimal situation would be an agent capable of controlling the mutant convertase or its consequences. We found that the addition of purified FH to the R139W-C3 of sera was capable of reducing C3 deposition on activated GEnCs in a dose-dependent manner. Therefore, purified FH, or FH regulatory domains containing constructs (as the FH-CR2 hybrid inhibitor TT3038 ), could be considered as a therapeutic option for R139W-aHUS. Experimental assessment for the susceptibility of a mutant convertase to control by FH should be considered as a mean to find potential putative responders to purified FH treatment. Some mutations such as the FB gain of function10 appear to be resistant to regulation by FH. Independent of the localization of the mutation, the generation of adverse products of complement hyperactivation, such as C5a and C5b9, can be efficiency blocked by eculizumab, a mAb blocking the cleavage of the complement protein C5. This therapeutic agent has reduced the magnitude of the thrombotic microangiopathy, restored kidney function, and improved quality of life in a small number of aHUS patients.39,40
In summary, we describe the genotype-phenotype and structure-function relationships for a frequent direct gain-of-function mutation in C3. The number of patients and healthy carriers with the R139W form of C3 on different genetic backgrounds indicates that this mutation, particularly if associated with an at-risk FH and/or MCP haplotypes, becomes pathogenic in association with an endothelium-damaging event.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Stephane Bally, François Bouissou, Eric Boulanger, Michel Foulard, Jacques Fourcade, Jean Pierre Grunfeld, Bruno Hurault de Ligny, Nassim Kamar, Annie Lahoche-Manucci, Christophe Legendre, Evelyne Macnamara, and Lionel Rostaing who provided us clinical data for the disease course of the R139W-aHUS patients. They thank Julie Bloch and Dr François Glowacki for the help with establishing the normal donors' cohort from the north of France. They are grateful to Delphine Beury, Stephanie Ngo, Nelly Poulain, and Tania Rybkine for their excellent technical assistance. They are grateful also to Alain Bacchi for the help with the statistical analysis.
This work was supported by grants from Agence Nationale de la Recherche (ANR Genopath 2009-2012 09geno03101I and ANR Biotheque 2008-2011 R08086DS), Assistance Publique–Hôpitaux de Paris (Program Hospitalier de Recherche Clinique, AOM05130/P051065 and AOM08198), AIRG (Association pour l'information et la recherche sur les maladies rénales génétiques) France, Université Paris Descartes and Université Pierre et Marie Curie, by Inserm, the National Institutes of Health (NIH) GM9111, A1041592 and AR48335, and NIH/National Heart, Lung, and Blood Institute (NHLBI) T32 HL007317.
L.T.R. was a recipient of an European Molecular Biology Organization Long-Term Fellowship ALTF 444-2007.
National Institutes of Health
Authorship
Contribution: L.T.R., M.F., E.C.M., L.H.-M, J.P.A., A.L., and V.F.-B. designed the research; L.T.R., M.F., and E.C.M. performed the research; L.T.R., M.F, E.C.M., M.-A.D.-D., C.S.-F., L.H.-M., J.P.A., A.L., and V.F.-B. analyzed and discussed the data; L.T.R., M.F., E.C.M., L.H.-M., J.P.A., and V.F.-B. wrote the manuscript; F.P., C.M., C.N., and A.L. provided clinical information and were in charge of the patients; P.B., S.B., and C.H. provided technical assistance; and S.C.S. and P.W.M. provided the GEnC cell line.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Veronique Fremeaux-Bacchi, Service d'Immunologie Biologique, Hôpital Européen Georges Pompidou, 20-40 rue Leblanc, 75908 Paris cedex 15, France; e-mail: veronique.fremeaux-bacchi@egp.aphp.fr.
References
Author notes
L.T.R. and M.F. contributed equally to this study.