Abstract
The systemic capillary leak syndrome (SCLS) is a rare disorder characterized by transient episodes of hypotensive shock and anasarca thought to arise from reversible microvascular barrier dysfunction. Although the high prevalence of a monoclonal gammopathy of unknown significance in SCLS suggests a pathogenic contribution of endogenous immunoglobulins, the mechanisms of vascular hyperpermeability remain obscure. Herein, we report clinical and molecular findings on 23 patients, the largest SCLS case series to date. Application of episodic SCLS sera, but neither the purified immunoglobulin fraction nor sera obtained from patients during remission, to human microvascular endothelial cells caused vascular endothelial cadherin internalization, disruption of interendothelial junctions, actin stress fiber formation, and increased permeability in complementary functional assays without inducing endothelial apoptosis. Intravenous immunoglobulin, one promising therapy for SCLS, mitigated the permeability effects of episodic sera. Consistent with the presence of endogenous, nonimmunoglobulin, circulating permeability factor(s) constrained to SCLS episodes, we found that vascular endothelial growth factor (VEGF) and angiopoietin 2 (Ang2), were elevated in episodic SCLS sera but not in remission sera. Ab-based inhibition of Ang2 counteracted permeability induced by episodic SCLS sera. Comparable experiments with anti-VEGF Ab (bevacizumab) yielded less interpretable results, probably because of endothelial toxicity of VEGF withdrawal. Our results support a model of SCLS pathogenesis in which nonimmunoglobulin humoral factors such as VEGF and Ang2 contribute to transient endothelial contraction, suggesting a molecular mechanism for this highly lethal disorder.
Introduction
In 1960, Dr Bayard Clarkson described a patient who experienced sporadic bouts of hypovolemia, hypotension, and edema.1 The systemic capillary leak syndrome (SCLS), also called Clarkson syndrome, is now known as a disorder of unknown cause characterized by transient but severe hypotension that results in vascular collapse and shock, hemoconcentration, and ultimately anasarca because of accumulation of fluids and macromolecules (≤ 900 kDa) in tissues.2,3 The most typical presenting signs are the triad of hypotension, elevated Hgb and hematocrit, and hypoalbuminemia. The symptoms reverse almost as quickly as they arise, with massive fluid remobilization from tissues into circulation, resulting in diuresis.
The most common treatment modality during episodes is judicious use of intravenous fluids and vasopressors to maintain perfusion to the brain and other vital organs. Although no more than 100 cases of SCLS were reported in the literature from 1960 to 2006, the nonspecific nature of the presenting signs and symptoms and high mortality rate during episodes may have resulted in considerable underdiagnosis. Fifty new cases of SCLS were reported from 2006 to 2011, suggesting that there may be increased awareness of this disorder.4,5 The 5-year survival rate is ∼ 75%, and deaths are most commonly related to acute SCLS events.4,6
A monoclonal gammopathy of unknown significance, typically of the IgG class, is present in most of the SCLS cases.7,8 Although paraprotein levels in SCLS are uniformly < 1 g/dL, recent case reports of symptom resolution after treatment of the underlying plasma cell dyscrasia and a small cohort study that reported efficacy of intravenous immunoglobulin administration for prevention of SCLS episodes have suggested a pathogenic role for the monoclonal IgG in the recurrent episodes of vascular leakage.5,9 Although early studies that used serial measurements of infused radiolabeled albumin established the link between marked, but transient, vascular hyperpermeability and the clinical manifestations of SCLS episodes,1,10 little is known about the molecular events leading to the episodic hyperpermeability of SCLS. The only molecular clues come from the original description by Clarkson,1 who reported that plasma drawn during an episode from an index case induced a shock-like syndrome when injected into rats and contained heparin-precipitable protein.
One such heparin-precipitable protein, vascular endothelial growth factor (VEGF), was reported in 1983, and at that time this protein was named “vascular permeability factor” for its ability to induce rapid leakage from blood vessels.11 VEGF is secreted by a variety of cells, including fibroblasts, keratinocytes, and mast cells, and binds receptor tyrosine kinases expressed on the surface of vascular endothelial cells. An analogous endothelial pathway regulating vascular barrier function, the angiopoietin–TEK tyrosine kinase-2 (Ang/Tie2) signaling axis, was first described in 1996.12 Although studies in rodent and cell culture models have clarified the mechanisms by which VEGF and Angs regulate permeability, the importance of these molecules in human disorders of vascular leakage has only been appreciated with the introduction of neutralizing biotherapeutic agents.13
Previous mechanistic studies on SCLS have been limited for 2 reasons: (1) the rarity of the condition, resulting in experiments performed on only 1-2 patients, and (2) limited prior efforts to adapt cellular models of endothelial barrier function for use with SCLS biologic material. Here, we assembled and studied blood samples from 20 patients who met the criteria for “classic acute” SCLS and 3 patients classified as “chronic” SCLS.5 In a subset of patients, we were also able to capture blood samples at or near the onset of their episode, including serial samples collected daily over a 1-week period in one patient. Using these materials, we performed studies on the functional and structural effects SCLS sera exert on human microvascular endothelial cells (HMVECs) and measured levels of candidate permeability mediators. We studied the barrier-defending effect of a standard SCLS treatment, intravenous immunoglobulin (IVIG), and evaluated the potential benefits of inhibiting specific factors (VEGF and Ang2).
Methods
Patients
Patients were classified according to established criteria by ≥ 1 episode of reversible hypotension, hemoconcentration, and hypoalbuminemia or chronic edema and hypoalbuminemia in the absence of secondary causes.4,5 Although a total of 23 patients were evaluated (Tables 1 and 2), experimental studies of sera from the 3 patients with chronic SCLS were not included for the sake of uniformity. Experiments on paired basal and episodic sera (Figures 1 and 6) are labeled with the patient number designated in Tables 1 and 2. All patients were seen at the Clinical Center of the National Institutes of Health during an asymptomatic period. Written informed consent was obtained from each patient, and the study protocol conformed to the ethical guidelines of the 2008 Declaration of Helsinki as reflected in a priori approval from the Institutional Review Board of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH). Age-, sex-, and race-matched serum and plasma samples were obtained from the NIH Blood Bank.
Reagents and cells
Adult dermal HMVECs were purchased from Lonza (catalog no. CC-2811) or obtained from the Center for Vascular Biology Research Core Facility at Beth Israel Deaconess Medical Center and cultured in endothelial growth medium (EGM-2) that contained growth factors per the manufacturer's instructions. Caspase-Glo 3/7 assay system was purchased from Promega. Recombinant human TNFα was purchased from PeproTech, and staurosporine was purchased from Sigma-Aldrich. The Lab-TekII Chamber Slide System coated with CC2 was obtained from Nalge Nunc International. Annexin V–FITC fluorescence microscopy kit was purchased from BD Biosciences. Bevacizumab (Avastin) was obtained from Genentech/Roche, IVIG (Gamunex) was from Talecris, and recombinant human VEGF-165 and Tie2-Fc were from R&D Systems.
Caspase-Glo 3/7 apoptosis assay
HMVECs were seeded in 96-well plates overnight in EGM-2. Cells were starved for 6 hours in basal EBM-2 without growth factors, followed by incubation with 10% serum or apoptosis-inducing reagents in basal medium overnight. In some experiments, cells were incubated with medium containing both test sera and TNFα (1.25 ng/mL). Caspase 3/7 activity was assayed with the use of the Caspase-Glo 3/7 luciferase system, according to the manufacturer's guidelines. Luciferase activity was measured with the use of the POLARstar Optima luminescence plate reader (BMG LabTech).
Annexin V staining
HMVECs were seeded in Chamberwell slides for 24 hours in EGM-2, followed by incubation with basal EBM-2 containing 10% test serum or reagents (staurosporine) overnight. Cells were then stained with annexin V–FITC, according to the manufacturer's protocol, fixed in annexin V buffer containing 1% paraformaldehyde, and mounted with glass coverslips with the use of ProLong Gold antifade reagent with 4′-6′-diamidine-2-phenylindole (DAPI; Invitrogen). Images were collected on a Leica SP5 inverted confocal microscope using Leica Application Suite AF software (Leica Microsystems).
ELISA
Serum or plasma cytokine levels were determined with the use of Quantikine ELISA kits (R&D Systems), according to the manufacturer's protocols. Absorbance was determined with a GENios plate reader at 450 nm with the use of a wavelength of 550 nm as reference.
IgG purification
Total IgG was purified from individual patient serum samples with the use of the Melon Gel IgG Purification Kit (Thermo Scientific). Purity was evaluated by SDS-PAGE and Coomassie blue staining, and concentration was calculated on the basis of measurement of OD260 and comparison of values obtained with a standard curve generated with the use of BSA of known concentrations.
Transwell permeability assay
Confluent HMVEC monolayers were grown on collagen-1–coated Costar Transwell membranes (polyester 0.4-μm filter; Corning), and permeability was determined by measurement of fluorometric signal in the luminal and abluminal chambers at the indicated time points after luminal addition of 1 mg/mL FITC-labeled HSA (Sigma-Aldrich) as described previously.14 Relative fluorescence units were used in the following equation to determine the permeability coefficient of albumin (Pa): Pa/hour = [A]/[L] × V/tA, where [A] is abluminal concentration, [L] is luminal concentration, V is volume of abluminal chamber, t is time in hours, and A is area of membrane in cm2.
TEER assays
Raw resistance (transendothelial electrical resistance, TEER) values across HMVEC monolayers were recorded at an amplitude of 4000 Hz with the use of an electrical cell-substrate impedance sensing system (Applied BioPhysics Inc). A cutoff resistance of 1500 ohms, indicative of confluence, was required before commencing an experiment. Human patient serum (5% final vol/vol concentration) was added at time zero to confluent cells in basal medium containing 1% FBS. Serial changes in TEER were recorded thereafter over a period of several hours. To enable comparisons across experiments, raw resistance values were divided by the mean time-zero raw resistance for the given experimental run. Data were generated from 3 to 6 replicates per condition. In experiments with IVIG (1.25 mg/mL), bevacizumab (10 μg/mL), or Tie2-Fc (1 μg/mL), these reagents or equivalent concentrations of BSA or control IgG were applied to HMVECs 1 hour before adding episodic SCLS sera (5% final vol/vol concentration). The raw resistance value at the time of SCLS serum addition was again taken as the time-zero data point for normalizing subsequent readings. We used the normalized resistance at 2.5 hours after the addition of sera for statistical analyses.
Immunofluorescence
HMVECs were grown to confluence on glass coverslips coated with collagen. Cells were serum starved with 1% serum-containing growth medium for 3 hours before adding patient serum at a 5% (vol/vol) final concentration for 2.5 hours. Cells were fixed for 10 minutes in 2.5% paraformaldehyde and permeabilized for 5 minutes in PBS containing 0.2% Triton X-100. Cells were incubated overnight at 4°C in blocking buffer (PBS containing 1% BSA, 0.2% Triton X-100, sodium azide) followed by incubation with anti–VE-cadherin Ab (BD Biosciences) for 12 hours. Cells were washed several times with PBS, followed by incubation with DyLight 488–conjugated AffiniPure goat anti–mouse IgG (Jackson ImmunoResearch Laboratories Inc) and Alexa Fluor 594–conjugated phalloidin (Invitrogen). In experiments that used purified IgG, cells were incubated with purified IgG (final concentration of 300 μg/mL) in growth medium followed by fixation and immunostaining with Alexa Fluor 594–conjugated anti–ZO-1 Ab (Invitrogen). After a second round of washing, cells were mounted onto coverslips with ProLong Gold anti-fade/DAPI. Cells were visualized with the use of a Zeiss LSM510 META confocal system at 63× magnification. All images were obtained with the use of identical laser power, gain, and offset instrument settings.
Statistical analysis
Data were analyzed with the GraphPad Prism 5 software package. For transwell and TEER assays, Student t tests for 2 groups and 1-way ANOVA for multiple groups were used to analyze functional replicates from individual patients. Mann-Whitney or Wilcoxon tests (for pairwise comparisons) or Kruskal-Wallis and ANOVA (for multiple groups) were used for grouped cytokine analyses because nonparametric distributions were assumed because of small sample size. P values < .05 were considered significant.
Results
Clinical and laboratory characteristics of study patients
Patient demographics and disease characteristics are reported in Table 1, and laboratory evaluations obtained at the time of study enrollment are reported in Table 2. Twenty of 23 patients had bona fide classic acute SCLS as defined by ≥ 1 episode meeting ≥ 3 of the diagnostic criteria recently established by Gousseff et al4 (edema with acute weight gain of > 1 kg in < 1 week, systolic blood pressure < 100 mm Hg or mean blood pressure < 70 mm Hg, Hgb elevation, hypoalbuminemia) in the absence of secondary causes. Sixty-one percent of the patients were male; 22 patients were white, and 1 patient was African American. The median age at disease onset was 52 years (range, 41-67 years). Approximately one-fourth (26%) of patients were classified as having moderately frequent attacks (> 2/year), whereas 26% of the total cohort had frequent attacks (defined as > 6/year, ranging from every other month to biweekly). All (100%) of the patients with classic acute SCLS experienced ≥ 1 “severe” episode according to the Gousseff criteria.4 Well-defined events that occurred before attacks could be identified in only 22% of patients, which included seropositive influenza in one patient (2 separate instances) and seropositive West Nile virus infection in another. Three patients met the criteria for chronic SCLS in that they experienced noncyclical peripheral edema and hypoalbuminemia in the absence of secondary causes of edema. A majority (90%) of the patients were receiving prophylactic treatment at the time of evaluation, including theophylline plus terbutaline or IVIG. One patient died of a severe SCLS attack during the study. No patient reported a family history of SCLS.
A majority (97%) of study patients had a monoclonal gammopathy, typically IgG, with the exception of an IgA M-spike in one patient. The mean serum monoclonal IgG level was 0.36 ± 0.05 g/dL, and M-spike isotypes were characterized by κ light chains in 61% of patients. Approximately one-third (32%) of patients had a skewed serum free light chain ratio, indicative of excess circulating free light chains. In most patients, routine laboratory evaluations, including Hgb and hematocrit, albumin, C-reactive protein, erythrocyte sedimentation rate, C1 esterase inhibitor level and function, tryptase, and complement components, were within the normal ranges at the time of their initial evaluation during remission.
SCLS serum induces endothelial permeability in vitro
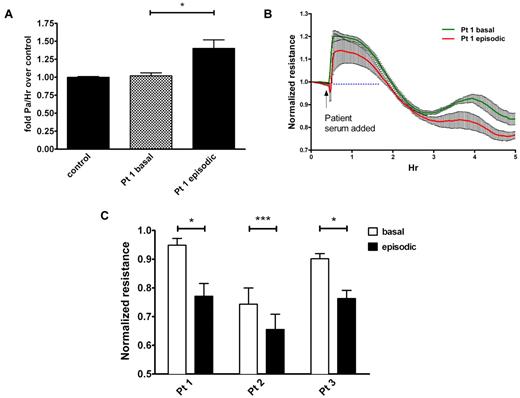
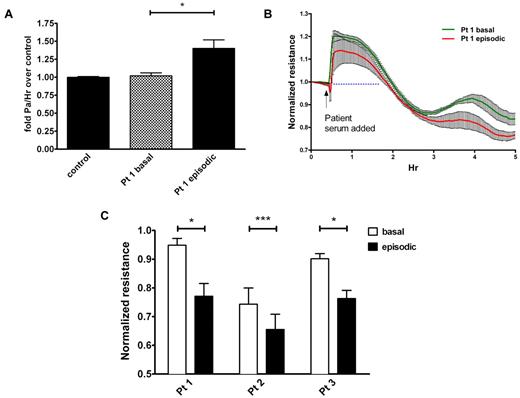
Although major assumptions have been made about the cause of SCLS, no prior studies have reported that this disease actually results directly from endothelial hyperpermeability. We hypothesized that SCLS serum would increase microvascular endothelial barrier dysfunction and permeability. To test this, we applied serum obtained from a patient during either an acute attack (episodic) or a quiescent period (basal) to confluent primary HMVECs and measured macromolecule flux and electrical resistance across the cell monolayer. These measurements have been previously shown to provide a highly sensitive biophysical assay that indicates the state of endothelial cell shape and focal adhesion as described.14 FITC-labeled albumin migrated at an equivalent rate across confluent HMVECs treated with basal SCLS serum and serum pooled from age- and sex-matched healthy controls (Figure 1A). In contrast, FITC-albumin migrated at a significantly faster rate across monolayers treated with episodic SCLS serum, mimicking the signs and symptoms of vascular leakage observed clinically. In agreement with this finding, application of the same episodic serum progressively decreased the electrical resistance of confluent HMVECs compared with matched basal serum, indicating increased endothelial permeability (Figure 1B). As resistance increased transiently (most probably because of mechanical disruption of the monolayer induced by pipetting) followed by divergence under the 2 conditions at 2-3 hours, we used resistance values at 2.5 hours after application of episodic and basal sera from several patients to perform the comparison. In each pair, serum obtained during an episode decreased endothelial resistance significantly more than its basal counterpart (Figure 1C). Although prior heat inactivation of serum affected absolute resistance values, the pattern of reduction in TEER by episodic serum relative to basal serum was similar to that observed with untreated serum, suggesting that a major contribution of serum complement is unlikely (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Together, these data strongly suggest that circulating factor(s) present in SCLS serum during an acute crisis provoke vascular leak symptoms by eliciting endothelial hyperpermeability.
Sera from patients with SCLS elicit permeability of endothelial monolayers. (A) Migration of FITC-albumin across HMVEC monolayers was determined as outlined in “Methods” after adding medium that contained 5% serum from a patient with SCLS during an asymptomatic interval (“basal”) or during an acute attack (“episodic”) or sera pooled from healthy controls without SCLS (n = 8); *P = .02, 1-way ANOVA. (B-C) TEER of HMVEC monolayers after application of matched basal and episodic SCLS sera from 3 patients with SCLS. (C) Values are end point resistance values at 2.5 hours (mean ± SEM; *P < .03 and ***P = .0003, paired t test).
Sera from patients with SCLS elicit permeability of endothelial monolayers. (A) Migration of FITC-albumin across HMVEC monolayers was determined as outlined in “Methods” after adding medium that contained 5% serum from a patient with SCLS during an asymptomatic interval (“basal”) or during an acute attack (“episodic”) or sera pooled from healthy controls without SCLS (n = 8); *P = .02, 1-way ANOVA. (B-C) TEER of HMVEC monolayers after application of matched basal and episodic SCLS sera from 3 patients with SCLS. (C) Values are end point resistance values at 2.5 hours (mean ± SEM; *P < .03 and ***P = .0003, paired t test).
SCLS serum does not induce endothelial apoptosis
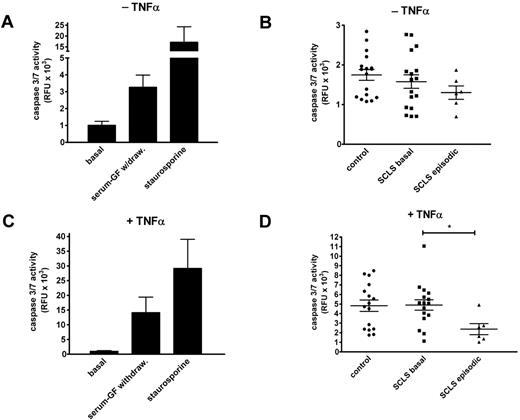
Prior work showed apoptosis of endothelial cells treated with serum from 2 patients experiencing an acute SCLS attack,15 and endothelial apoptosis could be one of several explanations for the barrier dysfunction we observed in Figure 1. Therefore, we tested whether treatment of endothelial cells with serum from patients or healthy controls affected cell viability. We measured the activity of caspases 3 and 7 in growth factor–deprived HMVECs, which reflects activation of both the intrinsic and extrinsic apoptosis pathways.
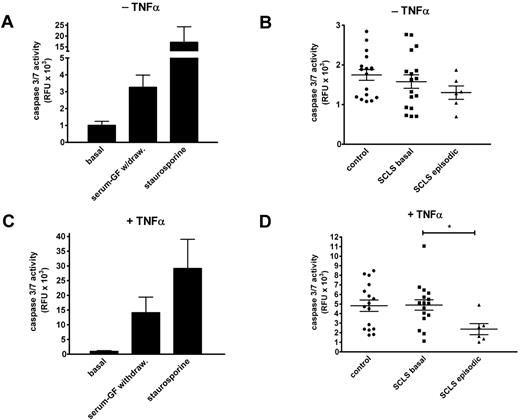
Growth factor withdrawal induced a 4-fold increase in caspase activity compared with untreated cells, whereas exposure to staurosporine, a known proapoptotic agent,16 elicited a 20-fold increase in caspase 3/7 activity (Figure 2A). By contrast, neither serum from patients with SCLS (basal or episodic) nor serum from healthy donors elicited any increased caspase activity compared with growth factor deprivation alone (Figure 2B). To sensitize cells further to proapoptotic stress, we tested the effect of study sera on growth factor–deprived cells in the presence of TNFα. Overnight incubation of HMVECs with TNFα in the presence or absence of staurosporine induced robust caspase 3/7 activity (Figure 2C). The addition of basal SCLS sera elicited caspase activity comparable with that induced by TNFα alone (Figure 2D). Interestingly, HMVECs treated with both TNFα and episodic SCLS sera had significantly reduced caspase activity compared with cells exposed to serum from asymptomatic patients with SCLS or healthy controls (Figure 2D). These results suggest that serum factor(s) present during acute attack phase of SCLS may have an antiapoptotic effect on endothelial cells in vitro.
Sera from patients with SCLS fail to induce endothelial apoptosis. (A) HMVEC monolayers were cultured in the presence (“basal”) or absence of growth factors or after adding staurosporine (1μM) overnight, followed by measurement of caspase 3/7 activities by luciferase assay. Data are mean ± SEM of ≥ 2 experiments measured in duplicate. (B) Growth factor–deprived HMVECs were incubated overnight with sera from healthy controls or patients with SCLS (basal or episodic) at 10% (vol/vol) concentrations. Data are mean ± SEM. (C-D) Same experiments as in panels A and B, except that growth factor–deprived cells were cultured with TNFα (1.25 ng/mL). Data are mean ± SEM; *P = .03, Mann-Whitney test.
Sera from patients with SCLS fail to induce endothelial apoptosis. (A) HMVEC monolayers were cultured in the presence (“basal”) or absence of growth factors or after adding staurosporine (1μM) overnight, followed by measurement of caspase 3/7 activities by luciferase assay. Data are mean ± SEM of ≥ 2 experiments measured in duplicate. (B) Growth factor–deprived HMVECs were incubated overnight with sera from healthy controls or patients with SCLS (basal or episodic) at 10% (vol/vol) concentrations. Data are mean ± SEM. (C-D) Same experiments as in panels A and B, except that growth factor–deprived cells were cultured with TNFα (1.25 ng/mL). Data are mean ± SEM; *P = .03, Mann-Whitney test.
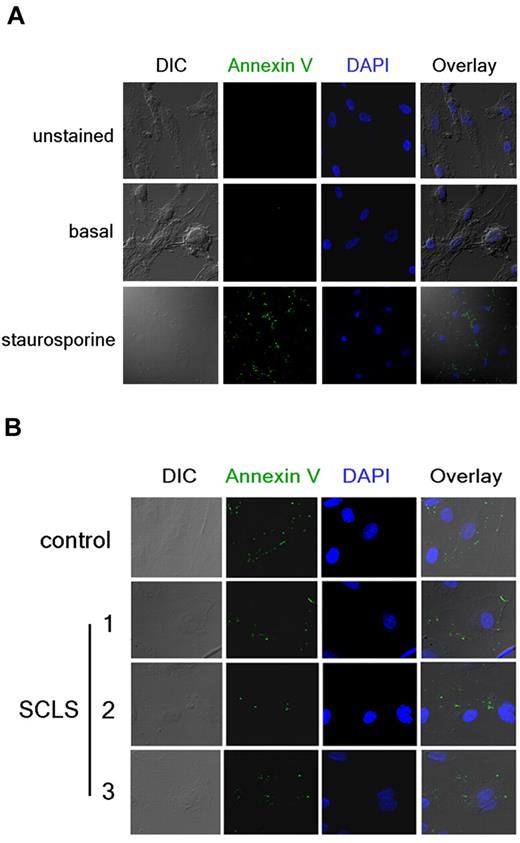
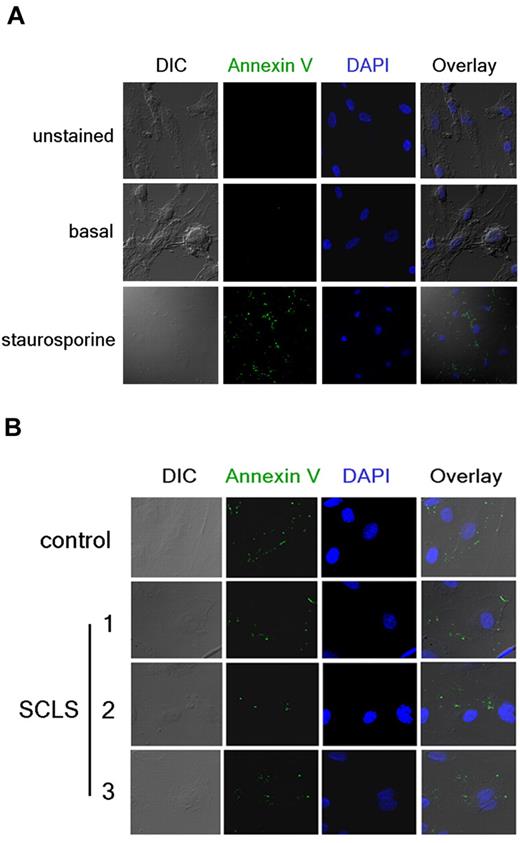
In accordance with the prior study, we also evaluated apoptosis independently with the use of annexin V staining.15 Annexin V binds to translocated phosphatidylserine on the plasma membrane outer surface of cells undergoing apoptosis. We visualized annexin V–FITC staining and cell structure by immunofluorescence and confocal microscopy after overnight treatment with proapoptotic compounds or patient sera in growth factor–deprived cells. Staurosporine elicited prominent annexin V–FITC staining in most cells, which was accompanied by visible changes in structure, including membrane blebbing and fragmentation (Figure 3A). We detected comparable (minimal) annexin V membrane staining in growth factor–deprived HMVECs after application of control or episodic SCLS sera (Figure 3B). Furthermore, we did not observe gross structural changes indicative of necrotic death, such as cell detachment, even after prolonged incubation with either sera. These results do not support the hypothesis that a circulating factor elicits permeability in SCLS by inducing endothelial apoptosis.
Detection of endothelial apoptosis by annexin V staining. (A) HMVECs were incubated overnight in the presence (bottom) or absence (top and middle) of staurosporine. Cells were left untreated (top) or stained with FITC-labeled annexin V Ab (green) followed by fixation and visualization by confocal microscopy. DAPI (blue) was used to identify nuclei. (B) Annexin V staining in growth factor–deprived HMVECs incubated overnight with sera pooled from healthy donors (“control”) or obtained from individual patients with SCLS during acute episodes (10% vol/vol concentrations). Images in panel A were taken at 40× and enlarged and or at 160× in panel B.
Detection of endothelial apoptosis by annexin V staining. (A) HMVECs were incubated overnight in the presence (bottom) or absence (top and middle) of staurosporine. Cells were left untreated (top) or stained with FITC-labeled annexin V Ab (green) followed by fixation and visualization by confocal microscopy. DAPI (blue) was used to identify nuclei. (B) Annexin V staining in growth factor–deprived HMVECs incubated overnight with sera pooled from healthy donors (“control”) or obtained from individual patients with SCLS during acute episodes (10% vol/vol concentrations). Images in panel A were taken at 40× and enlarged and or at 160× in panel B.
SCLS serum elicits disruption of endothelial adherens junctions and cell retraction
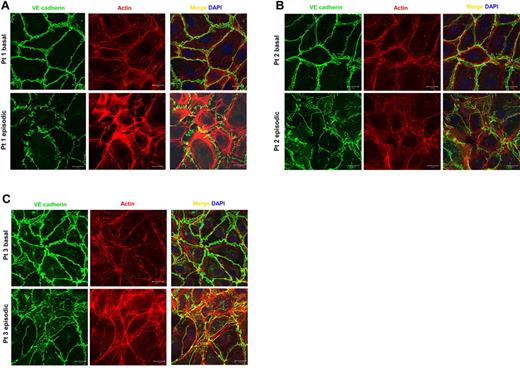
Having observed that endothelial apoptosis could not account for barrier dysfunction induced by episodic sera, we asked whether episodic serum promoted structural changes that would favor paracellular movement of water and solutes. To test this, we applied 5% study patient serum or purified IgG to confluent HMVECs for 2.5 hours, fixed them, and stained for F-actin and VE-cadherin or the tight junction–associated protein ZO-1. F-actin is a critical component of the cytoskeleton, known to rearrange into stress fibers that enable retraction of cell boundaries after cellular exposure to diverse permeability mediators.17 VE-cadherin and ZO-1 are vascular endothelial-specific transmembrane proteins, whose calcium-mediated homotypic interactions between adjacent cells are indispensable for endothelial barrier function.18,19 Application of episodic serum, but not its basal counterpart, induced prominent actin stress fibers and attenuated the junctional localization of VE-cadherin (Figure 4A). Disrupted paracellular junctions and cell retraction were also observed when sera pairs from other study patients were used (Figure 4B-C). In contrast, incubation of HMVECs with the purified IgG fraction of SCLS sera alone failed to induce structural changes or cell–cell junctional disruption (supplemental Figure 2). Together with the albumin flux, electrical resistance, and apoptosis assays, these data promote the interpretation that episodic SCLS serum, but not basal serum, contains factors other than IgG or complement that augment permeability and disrupt quiescent endothelial cell structure without inducing cell death.
SCLS sera disrupt endothelial adhesive junctions and elicit retraction. (A-C) Matched basal or episodic serum from 3 patients with SCLS was applied to HMVEC monolayers followed by immunostaining with VE-cadherin Ab (green) and phalloidin (red) to identify F-actin. Bar scale = 20 μm.
SCLS sera disrupt endothelial adhesive junctions and elicit retraction. (A-C) Matched basal or episodic serum from 3 patients with SCLS was applied to HMVEC monolayers followed by immunostaining with VE-cadherin Ab (green) and phalloidin (red) to identify F-actin. Bar scale = 20 μm.
Elevated VEGF and Ang2 levels in SCLS
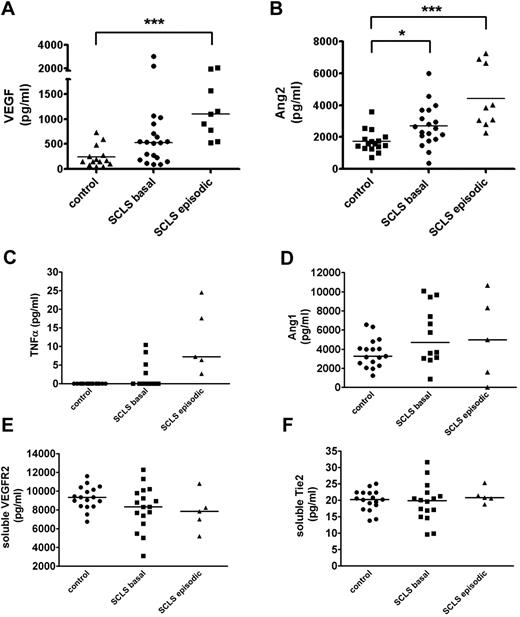
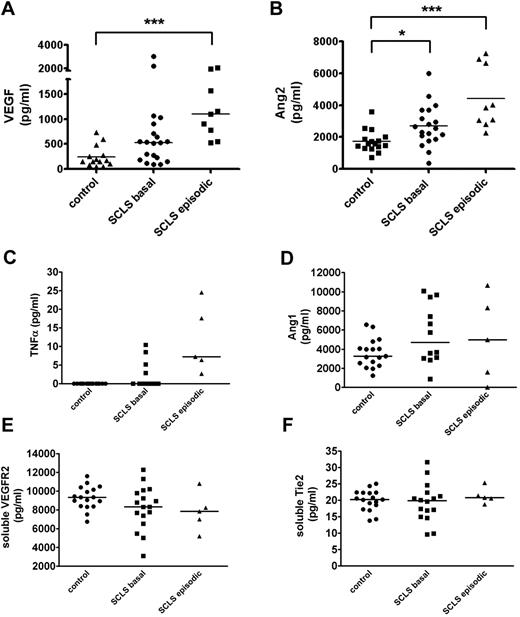
We evaluated levels of soluble factor(s) that could potentially contribute to acute SCLS symptoms by eliciting transient endothelial permeability. We measured levels of VEGF and Ang2 as abnormalities in these cytokines which have been described in disorders associated with vascular leakage, including sepsis, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes), and hemorrhagic fevers caused by infections with hantaviruses.20,21 A recent case report found acutely elevated serum VEGF in one patient with SCLS experiencing active symptoms, which correlated with the clinical course.22 In our series of 20 patients with classic acute SCLS, serum VEGF and Ang2 levels were significantly elevated in the SCLS group during acute episodes compared with asymptomatic SCLS patients and healthy controls (Figure 5A-B). Notably, serum Ang2 was also elevated in patients with SCLS during asymptomatic periods relative to the control group (Figure 5B), which may render them more susceptible to vascular leakage. On the basis of a case report of SCLS episodes in 1-2 patients being associated with elevated markers of inflammation,23 we analyzed prototypical “inflammatory” cytokines associated with vascular hyperpermeability. We found modestly increased TNFα levels in acute SCLS serum samples relative to the basal and control groups (Figure 5C). However, although direct comparisons with previously reported values are difficult because of methodologic differences in cytokine analysis, the values we measured were considerably lower than those described.23,24 Neither IL-2 nor IL-8 was elevated in patients with SCLS compared with controls (supplemental Figure 3; data not shown). Given the distinct clinical profile of patients who experienced chronic, continuous symptoms rather than transient, reversible episodes (n = 3), we excluded them from this cytokine analysis. Although VEGF and Ang2 levels were also significantly higher in the chronic subset of patients with SCLS than in healthy controls, inclusion or exclusion of these values did not affect statistical differences between groups one way or another (data not shown).
Serum VEGF and Ang2 are increased in acute SCLS. (A-F) Serum VEGF (A), Ang2 (B), TNFα (C), plasma Ang1 (D), serum soluble VEGFR2 (E), or serum soluble Tie2 (F) in patients with SCLS without symptoms (“basal”), during acute attacks (“episodic”), or healthy controls without SCLS were determined by ELISA. Horizontal bars depict the median value. (A-B) P < .0003, Kruskal-Wallis test for all across-group comparisons; *P < .05; **P < .005; and ***P < .0005, Dunn posttest.
Serum VEGF and Ang2 are increased in acute SCLS. (A-F) Serum VEGF (A), Ang2 (B), TNFα (C), plasma Ang1 (D), serum soluble VEGFR2 (E), or serum soluble Tie2 (F) in patients with SCLS without symptoms (“basal”), during acute attacks (“episodic”), or healthy controls without SCLS were determined by ELISA. Horizontal bars depict the median value. (A-B) P < .0003, Kruskal-Wallis test for all across-group comparisons; *P < .05; **P < .005; and ***P < .0005, Dunn posttest.
We also examined factors known to modify VEGF and Ang2 activity. Although Ang1 shares a common receptor with Ang2 (Tie2), Ang1 occupation of Tie2 receptors is thought to promote endothelial barrier function, vascular development, and angiogenesis.17 Ang1 levels were similar in asymptomatic or symptomatic patients with SCLS and healthy controls without SCLS (Figure 5D). Soluble Tie2 and VEGF receptors (VEGFR2) may be shed from activated or damaged endothelial cells and inhibit the activity of Ang1-2 or VEGF, respectively, by acting as decoys for the circulating pool.25,26 However, soluble Tie2 and VEGFR2 levels were similar in sera of patients with SCLS and healthy controls (Figure 5E-F).
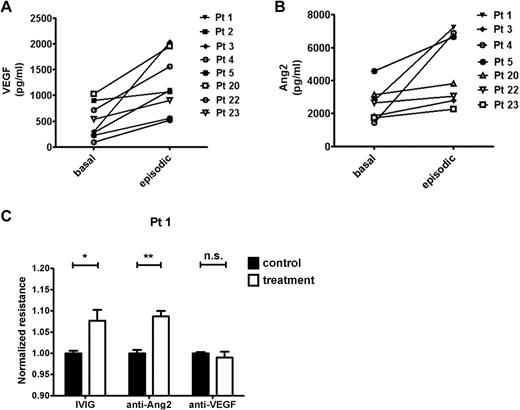
Evaluation of AG and experimental inhibition of VEGF and Ang2 on permeability
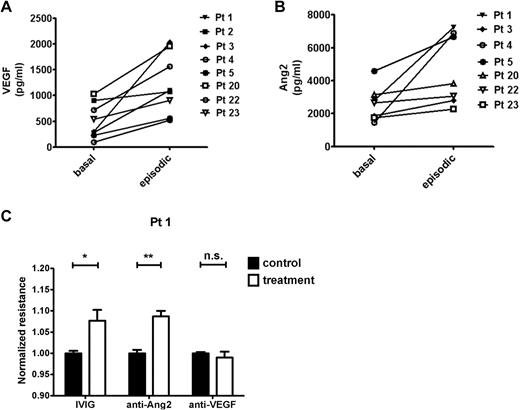
We compared samples obtained during an SCLS crisis with samples from the same patients during a well-demarcated, asymptomatic period. In these matched specimens, serum VEGF and Ang2 increased significantly during the acute SCLS attack compared with the baseline value (Figure 6A-B). On the basis of the permeability induced by episodic, but not basal, serum in the TEER assay (Figure 1C), we determined the contribution of VEGF or Ang2 in these sera to the phenotype with the use of Ab-based neutralization of these factors. As a positive control for these experiments, we evaluated the effect of a promising SCLS treatment, IVIG. Using the episodic samples of 4-6 patients, we found that IVIG pretreatment mitigated serum-induced permeability significantly as measured by electrical resistance (P = .002, 2-way ANOVA). Similarly, anti-Ang2 pretreatment counteracted the hyperpermeability elicited by these episodic sera (P = .0192). Surprisingly, anti-VEGF Ab (bevacizumab) showed no protective effect (P = .4387). Representative data are shown in Figure 6C. However, application of bevacizumab alone to endothelial monolayers in the presence of culture medium containing VEGF progressively weakened electrical resistance over a period of several hours (supplemental Figure 4). Dead and floating cells observed at the end of these experiments suggested that the toxicity of anti-VEGF in this assay might have confounded our ability to evaluate its barrier-fortifying effect against episodic sera.
Permeability-modifying factors in SCLS. (A-B) Comparison of basal and episodic VEGF or Ang2 in individual patients with SCLS. (VEGF: P = .015; Ang2: P = .008, Wilcoxon signed rank test). (C) The barrier-defending roles of IVIG, anti-Ang2 (Tie2-Fc), and anti-VEGF (bevacizumab) or equivalent concentrations of control IgG (for anti-Ang2 and bevacizumab) or BSA (for IVIG) against episodic sera from 4 to 6 patients was evaluated in the TEER assay. Shown is the response to the episodic sera of patient 1 (n = 3-5 replicates per condition). *P < .05 and **P < .01.
Permeability-modifying factors in SCLS. (A-B) Comparison of basal and episodic VEGF or Ang2 in individual patients with SCLS. (VEGF: P = .015; Ang2: P = .008, Wilcoxon signed rank test). (C) The barrier-defending roles of IVIG, anti-Ang2 (Tie2-Fc), and anti-VEGF (bevacizumab) or equivalent concentrations of control IgG (for anti-Ang2 and bevacizumab) or BSA (for IVIG) against episodic sera from 4 to 6 patients was evaluated in the TEER assay. Shown is the response to the episodic sera of patient 1 (n = 3-5 replicates per condition). *P < .05 and **P < .01.
Convergent findings in a single patient with SCLS
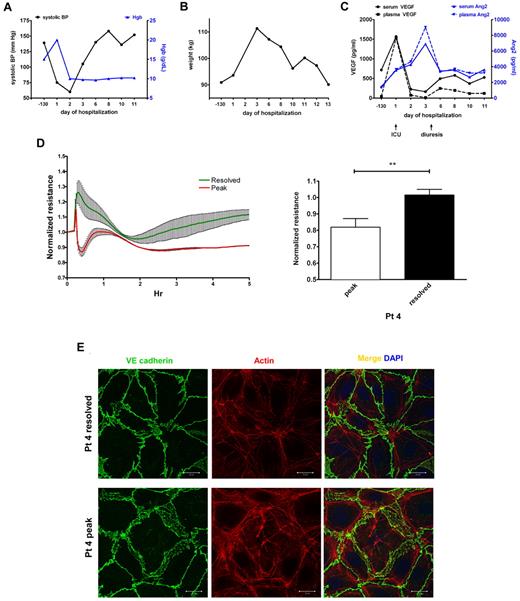
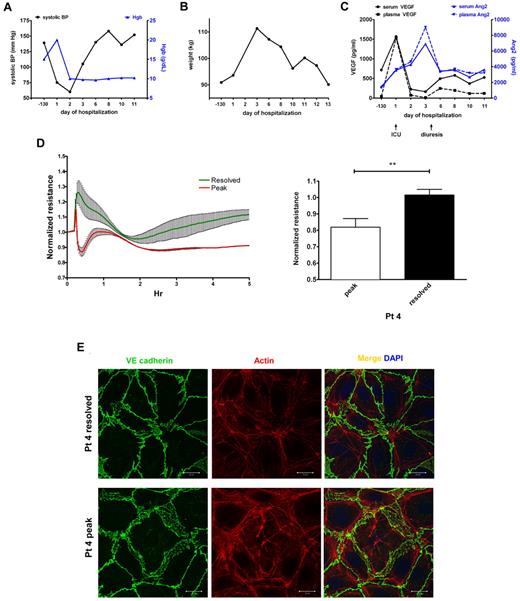
We collected serial samples from a single patient during a severe SCLS episode characterized by profound hypotension, hemoconcentration as evidenced by a rapid rise in Hgb level to > 20 g/dL (Figure 7A), and anasarca, with a nearly 20-kg weight gain in a period of 3 days (Figure 7B). This patient required massive fluid resuscitation, hemodynamic support by vasopressors, prolonged mechanical ventilation, and fasciotomy in all 4 extremities. Compared with the patient's baseline values 3 months before the episode during an asymptomatic period, serum and plasma VEGF levels were markedly elevated at the start of the episode but declined rapidly on admission to intensive care (Figure 7C). At the beginning of the “postleak” phase, which is characterized by massive fluid remobilization from tissues into the intravascular space and diuresis, VEGF levels had already returned to baseline. Circulating Ang2 was only slightly higher than basal values at the beginning of the leak phase, increasing at a slower rate than VEGF. Ang2 levels peaked several days into the hospitalization after VEGF levels had normalized and remained elevated during the postleak phase relative to pre-episode values (Figure 7C). We applied serum from the peak of this patient's clinical illness (day 1) and from the postleak resolution phase (day 3) on confluent HMVECs. Consistent with the results obtained with other SCLS sera (Figure 1B-C), endothelial resistance was substantially depressed by peak illness serum and restored to a normal level as the flare resolved (Figure 7D). Furthermore, peak serum from this patient disrupted cytoskeletal and adherens junctions, whereas day 3 serum did not (Figure 7E).
Elevations in circulating VEGF and Ang2 correlate with progression of an acute SCLS episode and induction of endothelial permeability. (A-B) Clinical course of a patient with a severe SCLS crisis characterized by hypotension, hemoconcentration (elevated Hgb; A) and a 20-kg weight gain in 3 days (B). (C) Serum and plasma VEGF and Ang2 in serial samples taken during the course of the illness were measured by ELISA. Baseline values were obtained 130 days before the episode during an asymptomatic period and at the beginning of the leak (admission to intensive care) and postleak (diuresis) phases as indicated by arrows. (D-E) Serum obtained at the peak of symptoms (“peak”) but not serum from the postleak resolution phase (“resolved”) induces endothelial permeability as assessed by decreased TEER (D) and reorganization of adhesive junctions and cell retraction (E); **P = .003, paired t test.
Elevations in circulating VEGF and Ang2 correlate with progression of an acute SCLS episode and induction of endothelial permeability. (A-B) Clinical course of a patient with a severe SCLS crisis characterized by hypotension, hemoconcentration (elevated Hgb; A) and a 20-kg weight gain in 3 days (B). (C) Serum and plasma VEGF and Ang2 in serial samples taken during the course of the illness were measured by ELISA. Baseline values were obtained 130 days before the episode during an asymptomatic period and at the beginning of the leak (admission to intensive care) and postleak (diuresis) phases as indicated by arrows. (D-E) Serum obtained at the peak of symptoms (“peak”) but not serum from the postleak resolution phase (“resolved”) induces endothelial permeability as assessed by decreased TEER (D) and reorganization of adhesive junctions and cell retraction (E); **P = .003, paired t test.
Discussion
Although it has long been speculated that the transient episodes of hypotension, intravascular volume depletion, and anasarca characteristic of SCLS are caused, at least in part, by a massive, reversible endothelial barrier breach, this hypothesis has never been formally tested. We show for the first time that episodic SCLS sera directly induce endothelial hyperpermeability and barrier breakdown, whereas matched sera from asymptomatic periods or the IgG fraction of sera has no such effect. Furthermore, we identified 2 mediators of permeability, VEGF and Ang2, whose induction in the circulation is associated with SCLS episodes across our study population and whose deflection tracks the clinical course in an index patient.
One feature that distinguishes SCLS from more common clinical scenarios associated with vascular leak, such as sepsis and chemotherapeutic toxicity, is the lack of inflammation. For example, perivascular mononuclear infiltrates have been detected in only a few cases of SCLS, whereas most skin biopsies done in these patients have been reported as normal at the light microscopic level.27-33 These studies are consistent with our finding that soluble serum factors present in SCLS sera induce endothelial hyperpermeability without a requirement for accessory immune cells (eg, leukocytes) to elicit an inflammatory vasculitis. The absence of substantial acute inflammation also makes involvement of traditional inflammatory mediators of vascular leakage such as TNF-α, platelet activating factor, thrombin, histamine, and IL-8 unlikely.
Limited ultrastructural studies of skeletal muscle endothelial cells from one patients with SCLS suggested apoptosis without intercellular gap formation.34 In contrast to these findings and other prior studies suggestive of endothelial apoptosis in SCLS,15 we found no evidence that serum or plasma from patients with SCLS induced apoptosis of HMVECs under any conditions, including in the absence of growth factors or in the presence of TNFα. The reason or reasons for this discrepancy are unclear. Notably, both patients reported in the previous study had experienced a flulike illness before their hypotensive SCLS crisis, and serum from patients with sepsis and pancreatitis induced a similar degree of apoptosis as did the SCLS serum (as detected by annexin V staining).15 These results suggest that systemic inflammation, perhaps because of viral infection, rather than factor(s) unique to SCLS, might account for these results.
The most significant aspect of our study is the finding that sera from patients experiencing an acute SCLS episode induce endothelial permeability through remodeling of endothelial cell–cell junctions. Although all study patients were seen initially during asymptomatic periods (Table 2), we were successful in obtaining several samples taken at the onset of an acute capillary leak episode, which were mailed to the NIH clinical center for evaluation. All episodic samples tested increased endothelium monolayer permeability (Figures 1, 6, and 7) and induced disruption of endothelial adherens junctions (Figures 4 and 7), suggesting a common mechanism underlying the vascular hyperpermeability characteristic of acute SCLS. Endothelial shape change, rather than apoptosis of endothelial cells or pericytes,35,36 may mediate the transient symptoms of SCLS. Vascular endothelial barrier function is maintained by intercellular contacts primarily through VE-cadherin, whose transcellular interactions fortify adherens junctions.37 Notably, the only treatments shown to reduce the frequency and severity of SCLS attacks, aside from IVIG, is a regimen of theophylline (phosphodiesterase inhibitor) and terbutaline (β-adrenergic agonist),6,38 which are known to promote endothelial barrier function by stabilizing VE-cadherin–mediated adhesive junctions.39-41 Thus, our results could account for the effectiveness of these therapies for SCLS.
The permeability mediators in SCLS we identified, VEGF and Ang2, also regulate endothelial barrier function through VE-cadherin. VEGF promotes internalization of VE-cadherin by inducing its tyrosine phosphorylation,42 whereas Ang2 disrupts adherens junctions through myosin light chain phosphorylation.43 In our cohort of 20 patients with classic acute SCLS, VEGF and Ang2 levels were significantly higher than healthy controls without SCLS, which could account for the disrupted adherens junctions observed. In agreement with individual case reports, our patients had elevated serum VEGF levels at the onset of an acute SCLS episode compared with their baseline, and in one patient VEGF returned to baseline rapidly on presentation with a severe hypotensive crisis.10,22 Our results show that a short interval of elevated VEGF (and possibly other permeability factors) may exist in SCLS.
Ang2 promotes vascular permeability by inhibiting its receptor, Tie2, whose tonic activation otherwise stabilizes VE-cadherin at adherens junctions.43,44 Although Ang2 levels were significantly increased in patients experiencing an acute SCLS attack, levels of the Tie2 agonist ligand, Ang1, were similar in symptomatic and asymptomatic patients. Elevated Ang2 in SCLS basal sera relative to healthy controls may increase the susceptibility of these patients to subsequent vascular leakage. Further increases in Ang2 during acute episodes, together with VEGF, could worsen or prolong the leak state. High levels of circulating Ang2 have been reported in several conditions associated with endothelial hyperpermeability such as sepsis, adult respiratory distress syndrome, and toxicity induced by therapeutic IL-2 administration.45,46
Several questions are suggested by the current findings. First, what physiologic and molecular processes trigger the induction of leak-promoting mediators such as VEGF and Ang2 in SCLS? Although clinical risk factors may include viral infections and psychological stressors, genetic predisposition could also be important. Hemodynamic and metabolic factors, including tissue hypoxia,47 cardiac function,48 and shear stress,49 also influence VEGF and Ang2 levels. Second, our results suggest that the TEER assay is a useful tool for mediator discovery and evaluation of therapies in SCLS. To assess the contribution of Ang2, we used Tie2-Fc, a commercial reagent previously shown to prevent Ang2-mediated destabilization of confluent endothelium.50 In the future, Ang2-targeted Abs currently under pharmaceutical development may become available for specific testing in this assay.51 Our results suggest additional therapeutic avenues for future exploration such as c-Src inhibition,52,53 which would be expected to stabilize VE-cadherin.
Third, future refinements to the presented methodology should improve the ability to discriminate between basal and episodic specimens and between control and candidate treatments. In this regard, our evaluation of anti-VEGF Ab may have been confounded by the endothelial toxicity of VEGF withdrawal (supplemental Figure 4). Endothelial hyperpermeability in vitro can arise from regulated mechanisms (eg, thrombin, lipopolysaccharide exposure), which are typically transient and reversible on stimulus removal, or endothelial cell injury (eg, after hydrogen peroxide treatment), which is irreversible and develops over a longer period of time. Patients receiving anti-VEGF therapy may develop endothelial injury, which manifests as hypertension, proteinuria, and thrombotic microangiopathy.54 Although it has long been established that VEGF induces endothelial hyperpermeability in vitro42 and in vivo,11 it also stimulates cellular proliferation over time. As confluent monolayers proliferate, cells become more tightly packed, leading to an increase in electrical resistance that is not related to barrier function per se but is an artifact of cellular proliferation. Because this situation may not mirror the in vivo setting and because of VEGF's unique dichotomous effects on electrical resistance (based on whether one is looking at the first few minutes or over several hours), our interpretation of TEER assays in the presence of anti-VEGF is thereby limited at this point.
Although most of our study cohort has monoclonal gammopathy of unknown significance, further studies are needed to determine whether the paraprotein contributes to disease pathogenesis. On the basis of the limited experience with IVIG and reports of SCLS resolution after treatment of the underlying plasma cell dyscrasia, we speculate that the monoclonal gammopathy is related to the clinical manifestations of SCLS. However, our results suggest that the paraprotein may function indirectly or upstream of vascular permeability mediators because the direct application of IgG isolated from patient sera could not recapitulate the effects of episodic sera on the HMVEC structure. This result is also consistent with the observation that serum paraprotein levels do not substantially fluctuate between episodes and remissions.
Notably, although application of IVIG to endothelial monolayers lessened the permeability-inducing effects of acute episodic sera, it is unknown whether this mechanism accounts for the clinical efficacy of IVIG for the treatment of SCLS. IVIG is also thought to possess numerous immunomodulatory properties, including regulation of cell receptor and adhesion molecules, suppression or neutralization of cytokines (by specific Abs present in the IVIG preparation), activation of regulatory macrophages and/or dendritic cells, and accelerated clearance and/or blockade of autoantibodies.55,56 We are actively investigating whether the monoclonal IgG present in SCLS sera contains specific permeability-enhancing activity. For example, might the paraprotein activate a permeability-promoting receptor or neutralize a permeability-fortifying substance? It is entirely possible that Abs present in IVIG preparations could bind and inhibit permeability-promoting factor(s) present in acute SCLS serum, including the monoclonal IgG itself.
In summary, our results provide the first demonstration that episodes of SCLS, but not asymptomatic periods, are associated with a circulating activity that promotes microvascular endothelial barrier breakdown. VEGF and Ang2 appear to be contributors to this process. Further study of the mechanism of endothelial barrier dysfunction in SCLS may not only result in novel targeted therapeutic approaches to this underdiagnosed disease but may also inform our understanding of more common clinical disorders of vascular leak, including diabetic retinopathy, lupus nephritis,57 and infection with malaria and Ebola/Marburg viruses,58 among others. Our results offer an explicit framework for the development of drug targets for SCLS and may eventually lead to the identification of new therapeutic strategies for other diseases associated with aberrant vascular function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases grant AI001083 LAD (K.M.D.) and by NIH grants R01HL093234, R01HL093234-01S1, and K08DK06916 (S.M.P.).
National Institutes of Health
Authorship
Contribution: Z.X., C.C.G., and R.P. designed and performed experiments; S.I. processed research samples; D.G., C.N., N.J., and P.R.G. recruited and cared for patients; and S.M.P. and K.M.D. conceived and directed the project and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kirk M. Druey, 10 Center Dr, Rm 11N242, Bethesda, MD 20982; e-mail: kdruey@niaid.nih.gov; and Samir M. Parikh, 330 Brookline Ave, RN 280C, Boston, MA 02215; e-mail: sparikh1@bidmc.harvard.edu.
References
Author notes
Z.X. and C.C.G. contributed equally to this study.