Members of the Rac/Rho family of small GTPases play an essential role in phagocytic cells in organization of the actin cytoskeleton and production of toxic oxygen compounds. GTPase-activating proteins (GAPs) decrease the amount of the GTP-bound active form of small GTPases, and contribute to the control of biologic signals. The number of potential Rac/RhoGAPs largely exceeds the number of Rac/Rho GTPases and the expression profile, and their specific role in different cell types is largely unknown. In this study, we report for the first time the properties of full-length ARHGAP25 protein, and show that it is specifically expressed in hematopoietic cells, and acts as a RacGAP both in vitro and in vivo. By silencing and overexpressing the protein in neutrophil model cell lines (PLB-985 and CosPhoxFcγR, respectively) and in primary macrophages, we demonstrate that ARHGAP25 is a negative regulator of phagocytosis acting probably via modulation of the actin cytoskeleton.
Introduction
Neutrophilic granulocytes carry out oriented migration directed by soluble chemotactic agents or adhesion molecules on the cell surface, phagocytosis initiated by opsonin receptors, and production of superoxide (O2·−) ions. All of these processes require fine temporal and spatial regulation of the small GTPases of the Rho family (Rac, Rho, and Cdc42).1,–3 As molecular switches, G proteins alternate between a GTP-bound active form, and an inactive GDP-bound state. Operation of the GTPases is modulated by 3 types of proteins. Guanine nucleotide dissociation inhibitors (GDIs) keep small G proteins in an inactive GDP-bound state. Guanine nucleotide exchange factors (GEFs) facilitate the GDP dissociation, and allow binding of intracellularly abundant GTP. GTPase-activating proteins (GAPs) inactivate the small G proteins by enhancing their GTP hydrolysis, which leads to signal down-regulation or termination.4
On the basis of the homologous catalytic domain, approximately 70 proteins are predicted to function as Rac/RhoGAPs in different human tissues.5,6 A couple of the GAP-domain–containing proteins have no catalytic activity,7,8 and many of the potential Rho-family GAPs have never been expressed as proteins, thus their catalytic activity, substrate specificity, and physiologic functions are presently unknown. GAPs are characterized by diverse domain structures, which suggest that these proteins are components of well-defined molecular complexes.9 However, the GAP repertoire expressed by individual cells is unknown, and the question whether GAP proteins have overlapping roles in signal transduction or whether each GAP regulates a specific function is still open.
In human peripheral blood neutrophils, 3 GAPs of the Rac/Rho family GTPases—p50RhoGAP, Bcr, and p190RhoGAP—have been detected by immunoblotting,10 but their functions have been only partially characterized. In vivo data, obtained in genetically modified mice, indicate that p50RhoGAP (also called Cdc42GAP) is involved in regulation of cell motility,11 the Rac-specific Bcr functions in down-regulation of superoxide production,12 whereas the recently described ArhGAP15 seems to affect all Rac-dependent functions.13 Surprisingly, p190RhoGAP that was suggested to be involved in the regulation of RhoA through β2-integrins in human neutrophils,14 in a recent study proved not to be a major regulator of integrin-mediated neutrophil functions in mice.15 These observations raise the question of species-specific regulatory roles of Rac/RhoGAPs. In a cell-free system, using human or bovine neutrophils, p190RhoGAP, p50RhoGAP, and Bcr were shown to regulate the phagocyte NADPH oxidase through Rac under in vitro condtions.10,16 However, data on participation of Rho-family GAPs in regulation of human neutrophils at the cellular level are still missing.
The ARHGAP25 gene is located on human chromosome 2p13 and encodes a protein of 639 amino acids with a potential Rho/RacGAP domain (151-340 aa).17 A homologous protein, ARHGAP24 (also called FilGAP) has been expressed and functions in fibroblasts as a GAP specific for Cdc42 and Rac.18 According to the EST database ARHGAP25 is significantly expressed in the spleen and in peripheral leukocytes. However, ARHGAP25 has never been expressed as a full-length protein and its potential functions have never been reported.
The aim of the present study was to investigate ARHGAP25 as a potential Rho-family GAP in human neutrophils. In this paper we show that: (1) ARHGAP25 protein is present in leukocytes, including neutrophilic granulocytes, and is expressed in the PLB-985 cell line; (2) it acts as a GAP for Rac both in vitro and in vivo; and (3) functions as a negative regulator of phagocytosis of neutrophils.
Methods
Materials
Saponin was from Calbiochem; cytochrome c, lucigenin, phorbol myristate acetate (PMA), paraformaldehyde were from Sigma-Aldrich. [γ-32P]GTP was purchased from the Izotóp Intézet. The extracellular medium (called H-medium) contained 145mM NaCl, 5mM KCl, 1mM MgCl2, 0.8mM CaCl2, 10mM HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid), and 5mM glucose; pH 7.4.
Northern blot analysis
Full-length cDNA of ARHGAP25 was labeled with the Prime-It RmT random primer labeling kit (Stratagene). Human 12-lane multiple tissue Northern blot (Clontech) containing poly(A)+-selected RNA was hybridized with the radiolabeled cDNA at 65°C using standard hybridization protocols (Amersham Biosciences). The membrane was washed twice for 15 minutes in 2× SSC (1× SSC = 0.15M NaCl, 0.015M sodium citrate) with 0.1% SDS at room temperature, and then a 30 minutes wash in 0.1× SSC with 0.1% (w/v) SDS at 60°C.19
Proteins and plasmids
Plasmids of glutathione S-transferase fusion protein of Rac1, RhoA, and Cdc42 were a kind gift from Dr Alan Hall (London, United Kingdom). Fragments encoding the PH domain, the GAP domain, the PH+GAP domain, the coiled coil domain and the full-length ARHGAP25 were generated from leukocyte cDNA using polymerase chain reactions and were inserted into the pGEX4T-1 vector. (The applied primers are summarized in supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article.) Thereafter, glutathione S-transferase (GST) fusion proteins were produced in Escherichia coli. For transfection, ARHGAP25 constructs were cloned into pCFP-C1 vector and p50RhoGAP was cloned into the pCFP-N1 vector. Mutation of the critical arginine of the GAP domain (GST-ARHGAP25R192A and CFP-ARHGAP25R192A) was designed on the basis of the consensus sequence of Rac/RhoGAPs,20 and was performed using QuickChange site-directed mutagenesis kit (Stratagene) following the manufacturer's instructions.
Cell lines and transfection
COS7 and COSphoxFcγR cells (a generous gift of Dr Mary Dinauer, Indianapolis, IN) were grown in Dulbecco modified Eagle medium with GlutaMAX I (Invitrogen) supplemented with 10% (w/v) FBS, 50 units/mL penicillin, and 50 μg/mL streptomycin in a 5% humidified CO2 incubator at 37°C. Media for FcγRIIa-expressing COSphoxFcγR cell lines also included 0.2 mg/mL hygromycin, 0.8 mg/mL neomycin, and 1 μg/mL puromycin as described.21 PLB-985 cells were grown in RPMI-1640 media (Lonza) supplemented with serum and antibiotics as previously described. PLB cells were differentiated into neutrophilic granulocytes for 7 days in the presence of 0.5% dimethylformamide. Differentiation was controlled on the basis of CD11b expression, using phycoerythrin-labeled anti-CD11b antibodies (DAKO), as described.22 Venous blood was drawn from healthy adult volunteers according to procedures approved by the Institutional Review Board of Semmelweis University, and human neutrophilic granulocytes were prepared as described.23 Human monocytes were obtained from venous blood of healthy volunteers by dextran sedimentation followed by Ficoll-Paque gradient centrifugation. Cells were finally resuspended in RPMI-1640 media supplemented with 10% (w/v) FBS, 50 units/mL penicillin and 50 μg/mL streptomycin. Macrophages were differentiated in the presence of 50 ng/mL macrophage colony-stimulating factor (M-CSF, Peprotech). Human T cells and B cells from tonsils were a kind gift from Mariann Kremlitzka and Andrea Balogh (Department of Immunology, Eötvos Lorand University of Sciences, Budapest, Hungary). Transient transfection of COS7 and COSphoxFcγR cells was performed using Fugene HD (Roche Applied Science) according to the manufacturer's instructions. Cells were transfected with wild-type or mutant ARHGAP25 constructs or the empty CFP-C1 vector. PLB cells were transfected using the siSTRIKE U6 hairpin cloning system (Promega) to produce ARHGAP25-silenced stable cell clones. The target sequence on ARHGAP25 mRNA started at nucleotide 244 from the start codon. The effective shRNA oligos and the control shRNA oligos, containing minimal modifications were ordered from Sigma-Aldrich (supplemental Table 1). Transfection was performed using the Amaxa Nucleofector kits and devices (Lonza). After transfection, cells were cloned and selected with 10 μg/mL puromycin to generate stable clones. Transient transfection of human macrophages was carried out with the Amaxa Human Macrophage Nucleofector Kit and device (Lonza) according to the manufacturer's instructions. Supplemental Table 1 contains the sequences of effective and control siRNAs.
Immunoblotting
Cells were lysed on ice in lysis buffer (30mM Na-HEPES, 100mM NaCl, 2% (w/v) Triton-X-100, 20mM NaF, 1mM Na-EGTA, 1mM Na-EDTA, 100mM benzamidine, 0.02% (w/v) diisopropyl phosphorofluoridate, 1% (w/v) aprotinine, 1% (w/v) protease inhibitor cocktail (Sigma-Aldrich), 1% (w/v) phosphatase inhibitor cocktail 2 (Sigma-Aldrich), and 1% (w/v) phenyl methyl sulfonyl fluoride; pH 7.5).
Neutrophil crude membrane and cytosol fractions were prepared as previously described.24,25 The lysates were boiled and run on 10% (w/v) polyacrylamide gels. Separated proteins were then transferred to nitrocellulose membranes. After blocking for 1 hour in PBS containing 5% defatted milk powder and 0.1% (w/v) tween 20, blots were incubated with anti-ARHGAP25 polyclonal antibody (see also supplemental Figure 1 for details) in 1:2000 dilution or anti-p50RhoGAP polyclonal antibody in 1:1000 dilution or anti-GST antibody (Invitrogen) in 1:1000 dilution or anti–β-actin mAb (Sigma-Aldrich) in 1:10 000 dilution. Bound antibody was detected with enhanced chemiluminescence using horseradish peroxidase-conjugated anti–rabbit-Ig (from donkey) or anti–mouse-Ig (from sheep) secondary antibodies (GE Healthcare) used in 1:5000 dilution.
Measurement of the GTPase activity of small G proteins
The GTP-ase activity was measured by the nitrocellulose filter-binding assay as described.26 Loading of recombinant Rac1, RhoA, or Cdc42 (1-4 μg of E coli protein) was performed for 5 minutes at room temperature with a high specific radioactivity of [γ-32P]GTP (more than 5000 Ci/mM) in low magnesium buffer (16mM Tris-HCl, pH 7.5, 20mM NaCl, 0.1mM dithiothreitol [DTT], 5mM EDTA, and 100nM [γ-32P]GTP [5 μCi]). Thereafter, MgCl2 was added at 20mM to diminish further nucleotide exchange. The solution was kept on ice to decrease nucleotide hydrolysis. The GTPase reaction was initiated by the addition of 3 μL of small G protein loaded with [γ-32P]GTP to 27 μL of a warmed (20°C) buffer (16mM Tris-HCl, pH 7.5, 0.1mM DTT, 1 mg/mL bovine serum albumin, and 1mM unlabeled GTP) containing the different domains of ARHGAP25 or the full-length protein. Aliquots (5 μL) were taken at regular intervals and filtered through nitrocellulose membranes (0.45-μm pore size), followed by washing 3 times with 2 mL of cold buffer consisting of 50mM Tris-HCl and 5mM MgCl2; pH 7.7. The filters were dried and the radioactivity was measured by the Cerenkov effect in a Beckman LS 5000TD liquid-scintillation spectrometer. GAP activity is presented as the decrease in protein-bound radioactivity retained on the filters in time. The in situ GTPase activity was confirmed with microscopic analysis of EGF-stimulated COS7 cells overexpressing the ARHGAP25, the mutant GAP protein (ARHGAP25R192A), the p50RhoGAP or the control vector as previously described.27
Quantification of phagocytosis
One gram of Saccharomyces cerevisiae (yeast) was boiled in 300 mL of distilled water for 30 minutes, and then was washed 4 times in 50 mL of PBS. Yeast cells (5 × 106) were labeled with 2μM Cell Tracker Green (Invitrogen) at 37°C for 20 minutes. After washing with PBS, yeast cells were opsonized with pooled human serum or heat-inactivated (56°C) human serum at 37°C for 1 hour. Thereafter, 5 × 106 yeast cells and 5 × 105 PLB-985 or human macrophage cells were mixed and co-incubated at 37°C for 10 minutes. Phagocytosis was measured with a Cell Lab Quanta SC flow cytometer. Percentage of phagocytosed fluorescent yeast is presented. To verify that only internalized (and no bound) particles were counted, control measurements were carried out where surface fluorescence was quenched by addition of 0.2% (w/v) Trypan blue (Sigma-Aldrich) in PBS, pH 5.5. No measureable difference was detected between quenched and nonquenched samples. For microscopic analysis of phagocytosis, 3 × 105 COSphoxFcγR cells were co-incubated on a coverslip at 37°C for 1 hour with 5 × 106 opsonized yeast cells labeled with 2μM Cell Tracker Red. After washing with PBS, the cells were fixed with 4% (w/v) paraformaldehyde and analyzed with a Zeiss LSM510 laser scanning microscope using a 40×/1.3 oil immersion objective (Plan-Neofluar, Zeiss) and Zeiss LSM Image Browser acquisition software (Zeiss). Hundred COSphoxFcγR cells were counted and we determined the number of cells that had ingested 2 or more yeast cells (phagocytosis %) and the number of yeast cells phagocytosed per 100 COSphoxFcγR cells (phagocytosis index).
Measurement of F-actin level
Differentiated ARHGAP25-silenced and control PLB cells were washed twice with FACS buffer (0.1% (w/v) bovine serum albumin and 0.1% Na-azide in PBS), and then fixed with fixative buffer (4% (w/v) paraformaldehyde, 0.1% (w/v) saponin, in FACS buffer) at room temperature (RT) for 10 minutes. After washing, filamentous actin was labeled with Alexa-488–conjugated phalloidin (Invitrogen) dissolved in FACS buffer containing 0.1% (w/v) saponin, and incubated at RT for 20 minutes. After washing twice with FACS buffer, fluorescence intensity was measured with a Cell Lab Quanta SC flow cytometer. Relative fluorescence intensity was calculated as the quotient of the fluorescence intensity of nontreated and ARHGAP25 shRNA- or control shRNA-treated cells.
Measurement of O2·− production
Extracellular superoxide production of PLB-985 cells was measured by the superoxide dismutase inhibitable reduction of cytochrome c as described.23 Intracellular O2·− production was determined by lucigenin-based chemiluminescence. The cells (107/mL) were suspended in H-medium containing 5.1 mg/mL lucigenin (dissolved in DMSO). Aliquots (180 μL) of the suspension were added into wells of a 96-well plate and prewarmed at 37°C for 5 minutes in a shaking Fluoroskan Ascent FI luminometer (Labsystems). As stimulus solution, 15 mg of zymosan was opsonized in 1 mL of normal or heat-inactivated (at 56°C for 50 minutes) pooled human serum at 37°C for 20 minutes. Thereafter, the cells were stimulated with the appropriate stimuli by the addition of 20 μL of the stimulus suspension. Changes in the luminescence were recorded for 15 minutes at 37°C with gentle shaking.
Results
Expression of ARHGAP25 mRNA
Katoh and Katoh identified ARHGAP25 in silico as a novel member of the ARHGAP gene family homologous to human ARHGAP22 and ARHGAP24.17 Chromosomal localization, coding sequence, exon-intron structure, and predicted protein domain structure were determined.17 ARHGAP25 consists of an N-terminal PH domain followed by a GAP domain and a C-terminal coiled coil sequence (Figure 1A). Results of the EST database search suggested that ARHGAP25 is a leukocyte-specific GAP, which led us to investigate its tissue-expression profile. The full sequence of the ARHGAP25 was used as a probe in Northern blot analysis to determine the expression level of ARHGAP25 mRNA in human tissues. As shown in Figure 1B, we found the highest level of ARHGAP25 mRNA in the spleen and in peripheral leukocytes, whereas significantly lower expression was detected in other tissue types. This finding agrees with results of the EST database. We found only one remarkable signal at 1.8 kb, which suggests that ARHGAP25 probably has no splice variants.
ARHGAP25 expression in human tissues and in leukocytes. (A) Domain structure of ARHGAP25. (B) Northern blot analysis of the tissue distribution of ARHGAP25 mRNA. Human multiple-tissue Northern blot was hybridized with radiolabeled full-length ARHGAP25 cDNA as a probe. (C) Analysis of the expression of ARHGAP25 protein in leukocytes isolated from human peripheral blood (PMN, PBMC) or from human tonsils (T cells and B cells), using purified polyclonal ARHGAP25 antibody. WCL means whole cell lysate. Negative and positive controls are provided in Figures 3A and 4B and supplemental Figure 1.
ARHGAP25 expression in human tissues and in leukocytes. (A) Domain structure of ARHGAP25. (B) Northern blot analysis of the tissue distribution of ARHGAP25 mRNA. Human multiple-tissue Northern blot was hybridized with radiolabeled full-length ARHGAP25 cDNA as a probe. (C) Analysis of the expression of ARHGAP25 protein in leukocytes isolated from human peripheral blood (PMN, PBMC) or from human tonsils (T cells and B cells), using purified polyclonal ARHGAP25 antibody. WCL means whole cell lysate. Negative and positive controls are provided in Figures 3A and 4B and supplemental Figure 1.
Detection of ARHGAP25 protein in peripheral leukocytes
Polyclonal antibody was prepared against the coiled coil domain of ARHGAP25 to detect protein expression in whole cell lysate of different types of peripheral leukocyte. The specificity of the antibody was tested with Western blot analysis of ARHGAP25 fragments expressed as recombinant GST fusion proteins. The anti-coiled coil antibody specifically recognized the full-length ARHGAP25 and the coiled coil domain (supplemental Figure 1). Western blot experiments for testing the overexpression or silencing of the protein also confirmed the specificity of the antibody (Figures 3A and 4B). According to our results (Figure 1C), ARHGAP25 was present in polymorphonuclear cells and peripheral blood mononuclear cells, as well as CD3+ T cells and CD19+ B cells from tonsils. The presence of ARHGAP25 was shown both in cytosolic and membrane fractions of neutrophils (Figure 1C).
GTPase activating effect of ARHGAP25
We expressed the full-length ARHGAP25 and its truncated fragments as GST fusion proteins (Figure 2A) to test their GTPase-activating effect in a classic radioactive GAP assay. Nonprenylated, [γ-32P]GTP labeled GST-Rac, Rho, and Cdc42 had a slow, intrinsic GTP-hydrolyzing activity (Figure 2B-D). After 15 minutes ∼ 80% of the [γ-32P]GTP was hydrolysed by Rac or Cdc42, whereas GTPase activity of Rho was 2-fold slower. In 4 separate experiments, addition either of the full-length or truncated forms of GST-tagged ARHGAP25 induced significant increase in GTP hydrolysis on Rac, and all of the fragments had similar efficiency (Figure 2B). In contrast, none of the ARHGAP25 constructs had any effect on GTPase activity of RhoA or Cdc42 (Figure 2C-D). Mutation of the arginine in position 192 to alanine resulted in loss of the RacGAP activity (supplemental Figure 2). To confirm the GTPase activating effect of ARHGAP25 on Rac in vivo, COS7 cells were transfected transiently with cyan fluorescent protein (CFP)–tagged full-length ARHGAP25. Overexpression of CFP-ARHGAP25 abolished the EGF-induced ruffling (Figure 2G,K), which is a Rac-dependent function,27,28 whereas ruffling was observed on the cells transfected with the control vector or the GAP-deficient mutant ARHGAP25R192A, or on the nontransfected cells (Figure 2I-K). In transfected cells ARHGAP25 and ARHGAP25R192A showed diffuse distribution in the cytoplasm (Figure 2F-G,J-K); however the control vector localized mostly in the nucleus (Figure 2E,I). We counted the percentage of ruffled cells from hundred transfected cells. In 4 separate experiments, overexpressed ARHGAP25 caused 4-fold decrease in ruffling compared with control cells and ARHGAP25R192A-transfected cells (Figure 2M). No difference was observed between the cells transfected with the mutant GAP and the control cells that contained the empty vector. It should be noted that COS7 cells transfected with ARHGAP25 did not exhibit the elongated phenotype, which is usually characteristic of cells over-expressing RhoGAPs29 (Figure 2G,K). Both CFP-ARHGAP25 and CFP-ARHGAP25R192A colocalized with endogenous Rac in COS7 cells (supplemental Figure 3D-F,G-I), whereas cyan fluorescent protein itself did not colocalize with Rac (supplemental Figure 3A-C). All of these findings are in agreement with the in vitro effect of ARHGAP25 on Rac.
ARHGAP25 regulates the GTPase activity of Rac. (A) The investigated fragments of ARHGAP25. (B-D) Effect of full-length or truncated forms of ARHGAP25 on in vitro [γ-32P]GTP-hydrolysis of Rac (B), Rho (C), and Cdc42 (D). Mean ± SEM of 4 separate experiments is shown (*P < .05 vs control). (E-L) Inhibition of EGF-induced ruffling by ARHGAP25 and p50RhoGAP. COS7 cells were transfected with control vector (E,I), cyan fluorescent protein (CFP)–tagged ARHGAP25R192A (F,J), CFP-ARHGAP25 (G,K), or CFP-p50RhoGAP (H,L). ARHGAP25, ARHGAP25R192A, p50RhoGAP, and CFP from control vector are shown in blue. Actin was stained with Alexa-568–labeled phalloidin (red). Stimulation was carried out with 0.1 μg/mL EGF for 20 minutes (I-L). EGF-induced ruffling is indicated by arrows. (M) Quantification of EGF-induced ruffling. Percentage of ruffled cells from 100 transfected cells was calculated. Mean + SEM of 4 (control vector, ARHGAP25R192A, and ARHGAP25) or 3 (p50RhoGAP) separate experiments are shown (*P < .05 compared with control).
ARHGAP25 regulates the GTPase activity of Rac. (A) The investigated fragments of ARHGAP25. (B-D) Effect of full-length or truncated forms of ARHGAP25 on in vitro [γ-32P]GTP-hydrolysis of Rac (B), Rho (C), and Cdc42 (D). Mean ± SEM of 4 separate experiments is shown (*P < .05 vs control). (E-L) Inhibition of EGF-induced ruffling by ARHGAP25 and p50RhoGAP. COS7 cells were transfected with control vector (E,I), cyan fluorescent protein (CFP)–tagged ARHGAP25R192A (F,J), CFP-ARHGAP25 (G,K), or CFP-p50RhoGAP (H,L). ARHGAP25, ARHGAP25R192A, p50RhoGAP, and CFP from control vector are shown in blue. Actin was stained with Alexa-568–labeled phalloidin (red). Stimulation was carried out with 0.1 μg/mL EGF for 20 minutes (I-L). EGF-induced ruffling is indicated by arrows. (M) Quantification of EGF-induced ruffling. Percentage of ruffled cells from 100 transfected cells was calculated. Mean + SEM of 4 (control vector, ARHGAP25R192A, and ARHGAP25) or 3 (p50RhoGAP) separate experiments are shown (*P < .05 compared with control).
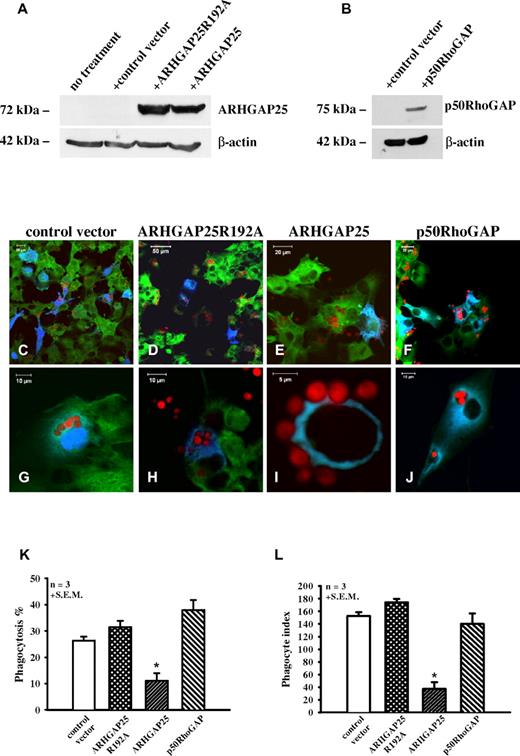
ARHGAP25 regulates the phagocytosis of COSphoxFcγR cells
To explore the potential involvement of ARHGAP25 in phagocytosis, the effect of ARHGAP25-expression was examined with opsonized, fluorescently labeled yeast. COSphoxFcγR cells express all the components of the phagocyte NADPH oxidase and FcγRIIa, but they do not contain endogenous ARHGAP25 (Figure 3A). The wild-type and the loss-of-function mutant of ARHGAP25 could be expressed in COSphoxFcγR cells at similar level (Figure 3A).
GTPase activating effect of ARHGAP25 is essential for its negative regulatory role in phagocytosis. (A-B) Western blot analysis of whole cell lysate of COSphoxFcγR cells transfected with control vector, cyan fluorescent protein (CFP)–tagged ARHGAP25R192A, CFP-ARHGAP25, or CFP-p50RhoGAP. (C-J) Opsonized yeast uptake by COSphoxFcγR cells. Phagocytosis of cells transfected with control vector (blue; C,G), CFP-ARHGAP25R192A (D,H), or CFP-p50RhoGAP (F,J) is similar to nontransfected cells (E). Cells expressing CFP-ARHGAP25 (blue) are able to bind yeast particles (red); however, the engulfment is inhibited (E,I). P40Phox is shown in green. (K-L) Quantification of phagocytosis by COSphoxFcγR cells. Panel K shows the number of cells that had phagocytosed 2 or more yeast particles from hundreds of cells transfected with the indicated constructs (phagocytosis %). Panel L shows the number of yeast particles phagocytosed per 100 COSphoxFcγR cells (phagocytosis index). Mean + SEM of 3 separate experiments is shown (*P < .05 compared with control).
GTPase activating effect of ARHGAP25 is essential for its negative regulatory role in phagocytosis. (A-B) Western blot analysis of whole cell lysate of COSphoxFcγR cells transfected with control vector, cyan fluorescent protein (CFP)–tagged ARHGAP25R192A, CFP-ARHGAP25, or CFP-p50RhoGAP. (C-J) Opsonized yeast uptake by COSphoxFcγR cells. Phagocytosis of cells transfected with control vector (blue; C,G), CFP-ARHGAP25R192A (D,H), or CFP-p50RhoGAP (F,J) is similar to nontransfected cells (E). Cells expressing CFP-ARHGAP25 (blue) are able to bind yeast particles (red); however, the engulfment is inhibited (E,I). P40Phox is shown in green. (K-L) Quantification of phagocytosis by COSphoxFcγR cells. Panel K shows the number of cells that had phagocytosed 2 or more yeast particles from hundreds of cells transfected with the indicated constructs (phagocytosis %). Panel L shows the number of yeast particles phagocytosed per 100 COSphoxFcγR cells (phagocytosis index). Mean + SEM of 3 separate experiments is shown (*P < .05 compared with control).
We measured the phagocytosis of COSphoxFcγR cells over-expressing the CFP-tagged full-length ARHGAP25, the CFP-ARHGAP25R192A, or transfected only with the control vector. Typically, the CFP-ARHGAP25 transfected COSphoxFcγR cells were not able to phagocytose opsonized yeast; however, the binding of the particles was visible (Figure 3E,I). Yeast uptake in the cells over-expressing the CFP-ARHGAP25R192A or only CFP was not inhibited (Figure 3C-D,G-H). We determined the percentage of phagocytosis and phagocytosis index of COSphoxFcγR cells. In 3 separate experiments, ∼ 10% of the ARHGAP25 transfected cells phagocytosed 2 or more yeast particles (phagocytosis %; Figure 3K). The total number of phagocytosed yeast particles in 100 transfected cells (phagocytosis index) was only ∼ 30 (Figure 3L). In contrast, percentage of phagocytosis of control vector-treated and ARHGAP25R192A-transfected cells was ∼ 30% (Figure 3K), and the phagocytosis index was ∼ 160 (Figure 3L).
GAP-deficient ARHGAP25 was typically detected around the phagocytosed yeast particles (supplemental Figure 4B,E). In the rare cases when phagocytosis did occur in COSphoxFcγR cells transfected with wild-type ARHGAP25, also the wild-type protein appeared to be enriched around the phagosomes (supplemental Figure 4C,F).
To investigate the specificity of ARHGAP25 on phagocytosis, we transfected COSphoxFcγR cells also with p50RhoGAP, that has been characterized earlier to have RacGAP activity in human neutrophils.10 The RacGAP activity of p50RhoGAP has been verified in the EGF-induced ruffling assay (Figure 2H, L-M). Nevertheless, expression of p50RhoGAP in COSphoxFcγR did not interfere with phagocytosis of opsonized yeast particles (Figure 3F,J-L).
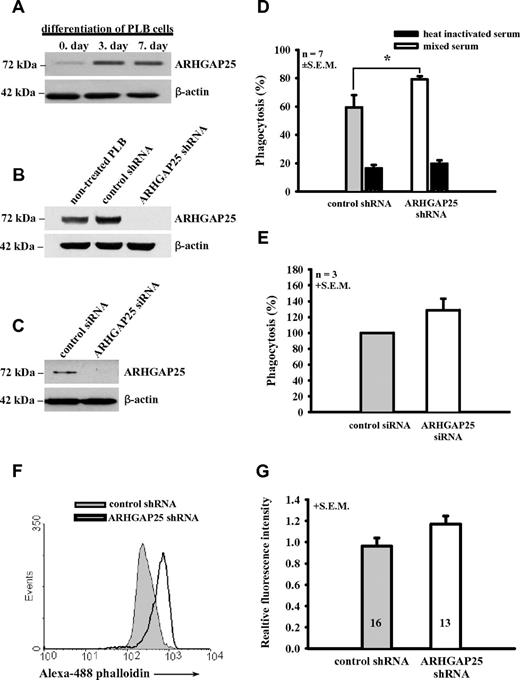
ARHGAP25 regulates the phagocytosis of PLB-985 cells and primary macrophages
Endogenous ARHGAP25 was detected in the PLB-985 cell line, which can be differentiated to neutrophilic granulocyte-like cells. After 7 days of differentiation, ARHGAP25 expression was significantly increased (Figure 4A). For functional studies, stable ARHGAP25 knockdown PLB clones were generated. Although significant amount of the protein was detectable in cells treated with control shRNA and in nontreated cells, effective shRNA caused marked decrease of ARHGAP25 level (Figure 4B). By densitometry, ARHGAP25 expression in each of the 6 isolated clones was decreased more than 5-fold by ARHGAP25 knockdown (data not shown). ShRNA treatment did not interfere with the differentiation of PLB cells (supplemental Figure 5).
ARHGAP25 regulates phagocytosis of ARHGAP25-silenced cells. (A) Expression of ARHGAP25 in PLB-985 cell line during the 7-day differentiation to neutrophils. (B) Western blot signal is significantly reduced in ARHGAP25-silenced PLB cells compared with control shRNA treated and nontreated cells. (C) Western blot analysis of ARHGAP25-silencing by siRNA in human macrophages. In Western blot experiments, staining for β-actin was used as loading control. (D) Yeast cells were labeled with Cell Tracker Green and were opsonized with pooled human serum (empty columns) or heat-inactivated human serum (hatched columns) for 1 hour. After 10 minutes' co-incubation, the number of PLB cells that had engulfed one or more yeast particles was counted by flow cytometer and normalized to 100 cells. Mean + SEM of 7 separate experiments is shown (*P < .05 vs control shRNA treatment). (E) Pooled serum opsonized yeast uptake of ARHGAP25-silenced human macrophages. The number of macrophages phagocytosed one or more yeast particles was calculated by flow cytometer and normalized to control siRNA treated cells. Mean + SEM of 3 separate experiments is shown. (F-G) F-actin level in ARHGAP25-silenced PLB cells, compared with control shRNA-treated cells. F-actin was stained with Alexa-488–labeled Phalloidin, F-actin level was measured with flow cytometer. Panel F shows a representative result of the experiment. In panel G mean + SEM of 16 (control shRNA-treated) and 13 (ARHGAP25-silenced) separate experiments are shown.
ARHGAP25 regulates phagocytosis of ARHGAP25-silenced cells. (A) Expression of ARHGAP25 in PLB-985 cell line during the 7-day differentiation to neutrophils. (B) Western blot signal is significantly reduced in ARHGAP25-silenced PLB cells compared with control shRNA treated and nontreated cells. (C) Western blot analysis of ARHGAP25-silencing by siRNA in human macrophages. In Western blot experiments, staining for β-actin was used as loading control. (D) Yeast cells were labeled with Cell Tracker Green and were opsonized with pooled human serum (empty columns) or heat-inactivated human serum (hatched columns) for 1 hour. After 10 minutes' co-incubation, the number of PLB cells that had engulfed one or more yeast particles was counted by flow cytometer and normalized to 100 cells. Mean + SEM of 7 separate experiments is shown (*P < .05 vs control shRNA treatment). (E) Pooled serum opsonized yeast uptake of ARHGAP25-silenced human macrophages. The number of macrophages phagocytosed one or more yeast particles was calculated by flow cytometer and normalized to control siRNA treated cells. Mean + SEM of 3 separate experiments is shown. (F-G) F-actin level in ARHGAP25-silenced PLB cells, compared with control shRNA-treated cells. F-actin was stained with Alexa-488–labeled Phalloidin, F-actin level was measured with flow cytometer. Panel F shows a representative result of the experiment. In panel G mean + SEM of 16 (control shRNA-treated) and 13 (ARHGAP25-silenced) separate experiments are shown.
To explore the potential involvement of endogenous ARHGAP25 in phagocytosis, the effect of ARHGAP25-silencing was examined with opsonized, fluorescently labeled yeast. Phagocytosis was evaluated using a flow cytometer. When opsonization was carried out with pooled normal serum, phagocytosis in differentiated PLB-985 cells attained 60%. Uptake of normal serum-opsonized yeast in ARHGAP25-silenced PLB cells was 20% higher than in untreated or control shRNA-treated cells (Figure 4D). In normal pooled serum both complement components and immunoglobulins are present, thus phagocytosis can occur both via complement and Fc receptors. Heat inactivation of pooled serum blocks the enzymes of the complement system, resulting in loss of complement receptor-dependent phagocytosis. Efficiency of phagocytosis of heat-inactivated serum-opsonized yeast in PLB-985 cells was approximately 20%, and no significant difference could be observed between ARHGAP25-silenced and control shRNA-treated cells (Figure 4D). In view of the observed differences depending on the mode of opsonization, we investigated the expression of FcγR on differentiated PLB cells. In flow cytometry analysis we could detect only very weak expression of FcγRIII, and the expression level of FcγRII was more than 100-fold lower than on PMN (data not shown). Our observation agrees with a previous study carried out in PLB cells after DMFA-differentiation.30 These results explain the low phagocytic capacity of PLB cells in the absence of complement components.
The effect of ARHGAP25 silencing on phagocytosis was also investigated in primary macrophages differentiated from monocytes of fresh blood. An increase of ∼ 30% was detected (Figure 4C,E).
ARHGAP25 is a potential regulator of actin remodeling
Phagocytosis requires significant reorganization of the actin cytoskeleton, a process dependent on small G proteins, particularly Rac.31 To test whether ARHGAP25 has any effect on filamentary actin formation, F-actin was labeled fluorescently with Alexa-488–conjugated phalloidin and measured by flow cytometry. F-actin levels showed a tendency to be higher in ARHGAP25 knock-down PLB cells (Figure 4F), but in 13 (silenced) and 16 (control shRNA) separate experiments, the difference was not statistically significant (Figure 4G). Stimulation of the ARHGAP25-silenced and control shRNA treated PLB cells with phorbol ester before actin-staining did not affect the F-actin level in 5 separate experiments, but the difference between silenced and control treated cells remained observable (data not shown).
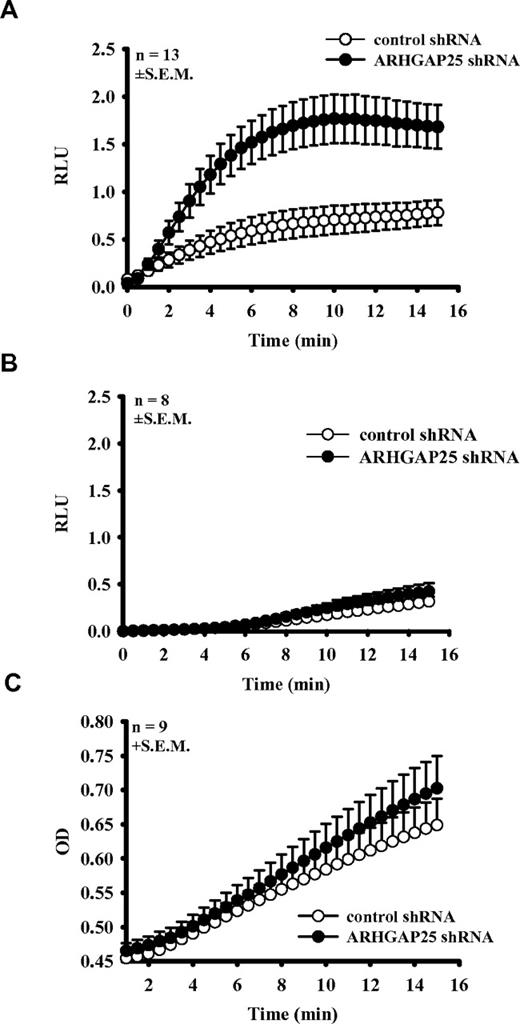
Superoxide production in ARHGAP25 knockdown PLB cells
ARHGAP25-silenced and control PLB cells were stimulated with opsonized zymosan or phorbol ester (PMA). The opsonization protocol was the same as in the previous phagocytosis experiments. As shown in Figure 5A, an ∼ 3-fold increase was detected in the rate of superoxide production on normal pooled serum-opsonized zymosan (OPZ) in silenced cells, compared with control shRNA-treated cells. Opsonization of zymosan with heat-inactivated serum (hOPZ) abolished the difference. HOPZ stimulus caused a relatively slow superoxide production both in knock-down and control cells that started to increase after a 5-minute lag phase (Figure 5B). The increase after 15 minutes was similar to the response of control PLB cells after OPZ stimulation. In 9 experiments, superoxide production induced by PMA showed no significant difference between silenced and control shRNA-treated cells (Figure 5C).
Superoxide-production of ARHGAP25-silenced PLB-985 cells. Superoxide production of PLB cells transfected with ARHGAP25 specific (●) or control shRNA (○) was stimulated with pooled human serum-opsonized zymosan (mean ± SEM of 13 separate experiments; A), with heat-inactivated serum (mean + SEM of 8 separate experiments; B), and with 0.1μM phorbol ester (mean + SEM of 9 separate experiments; C). RLU indicates relative fluorescence unit; and OD, optical density.
Superoxide-production of ARHGAP25-silenced PLB-985 cells. Superoxide production of PLB cells transfected with ARHGAP25 specific (●) or control shRNA (○) was stimulated with pooled human serum-opsonized zymosan (mean ± SEM of 13 separate experiments; A), with heat-inactivated serum (mean + SEM of 8 separate experiments; B), and with 0.1μM phorbol ester (mean + SEM of 9 separate experiments; C). RLU indicates relative fluorescence unit; and OD, optical density.
Discussion
Phagocytosis is a complex process: binding of the particles to specific receptors initiates diverse signaling pathways that lead to actin polymerization, alteration of the phosphoinositide metabolism, and assembly of the NADPH oxidase complex. Small GTPases of the Rac/Rho subfamily are involved in all these processes.32,33 Fine spatial and temporal coordination of the activity of small GTPases depends on the regulatory proteins GAPs, GEFs, and GDIs.4 In previous studies, 3 different GAPs have been identified in human neutrophils.10,14 However, data on the function of these GAPs issue mainly from experiments on genetically modified mice,11,12 and in case of p190RhoGAP, observations on human and murine neutrophils are contradictory.14,15
We have expressed the full-length ARHGAP25 protein for the first time and show that under in vitro conditions it affects GTP-hydrolysis only on Rac and not on Cdc42 or Rho (Figure 2B-D). This property is different from Bcr, p50RhoGAP, and p190RhoGAP, which were all shown to act on more than one member of the Rho-family GTPases.34,–36 Full-length ARHGAP25 and the 2 GAP-domain containing fragments were similarly efficient RacGAPs (Figure 2B), indicating, that—unlike several other Rho-family GAPs—ARHGAP25 is not subject to autoinhibitory regulation.36,,–39 We determined that arginine192 is critical for the RacGAP activity of the protein (supplemental Figure 2).
Investigation of the tissue-specific expression of ARHGAP25 mRNA confirmed the data of the EST database and indicated high expression in spleen and leukocytes. With help of our polyclonal antibodies we could detect the protein in all peripheral blood leukocytes and in the granulocyte-like cell line PLB-985, but not in COS cells (Figures 1C, 3A, and 4A). Expression of wild-type and GAP-deficient ARHGAP25 in COS cells supported the Rac-specificity of the protein also under cellular conditions (Figure 2E-G,I-K,M).
The functional role of ARHGAP25 was investigated in 2 model cell lines and in primary macrophages. COSPhoxFcγR cells express all the subunits of the NADPH oxidase and FcγRIIa, but no complement receptors and no ARHGAP25 (Figure 3A). Overexpression of ARHGAP25 in COSphoxFcγR cells caused significant decrease of phagocytosis of serum-opsonized yeast particles compared with control cells (Figure 3C-E,G-I,K,L). Moreover, mutation of the critical arginine in the GAP domain (ARHGAP25R192A) abolished the inhibitory effect of ARHGAP25 on phagocytosis of COSphoxFcγR cells, which proves that the GAP activity of the protein is required for the observed effect. Expression of another GAP for the Rac/Rho family that was shown to be present and have intensive RacGAP activity in human neutrophils,10 did not impair phagocytosis of opsonized yeast particles in COSPhoxFcγR (Figure 3F,J-L) indicating that not all RacGAPs exert identical effects.
Differentiated PLB-985 cells express endogenous ARHGAP25 (Figure 4A-B.) In these cells silencing of ARHGAP25 resulted in significant increase of uptake of opsonized yeast particles (Figure 4D) and phagocytosis-related superoxide-production (Figure 5A). Because of the low expression of Fc-receptors, phagocytosis occurred mostly via complement receptors. The 2 models of neutrophilic functions used in this study allowed different approaches. In PLB cells silencing of endogenous ARHGAP25 resulted in an increase of phagocytosis, whereas in COSphoxFcγR cells overexpression of ARHGAP25 clearly inhibited phagocytosis. In addition, the effect of silencing of ARHGAP25 has been verified also in primary cells (Figure 4E). The complementary effects observed in the 2 different models plus the findings in macrophages strongly suggest that ARHGAP25 functions as a negative regulator of phagocytosis.
In classic studies carried out on macrophages, it has been shown that Rac and Cdc42 have regulatory roles in FcγR-mediated phagocytosis, whereas RhoA is involved in complement receptor-signaling.40 However, later studies have shown that in neutrophilic granulocytes complement receptor ligation induces activation of Rac and Cdc42 as well.41 Thus our finding that a RacGAP is involved in the signaling pathway of both FcγR and complement receptors is in line with previous experimental data.
The domain structure of ARHGAP25 is similar to that of ARHGAP24 (also called FilGAP), another Rac-specific GAP with 40% to 61% homology between the relevant domains of the 2 proteins.18 FilGAP was shown to bind to the actin-crosslinking protein filamin A, and to regulate lamellae formation in fibroblast-like cells.18 In our experiments on differentiated PLB-985 cells, silencing of ARHGAP25 resulted in an increase of the F-actin level (Figure 4F-G). On the other hand, ARHGAP25 overexpressed in COS cells colocalized with endogenous Rac (supplemental Figure 3) and in phagocytosing cells it was enriched around the phagosomes (supplemental Figure 4). The hypothesis that ARHGAP25 may regulate phagocytosis via local modulation of the actin cytoskeleton is compatible with observations made in all investigated cells.
Taken together, we suggest that ARHGAP25, a novel leukocyte-specific RacGAP is a negative regulator of phagocytosis in neutrophilic granulocytes potentially acting via Rac-dependent F-actin reorganization. ARHGAP25 may be involved in a step that is common in the signaling pathway of Fc and complement receptors, but upstream of the assembly of the NADPH oxidase complex. Investigation of the interacting partners and regulation of ARHGAP25 will be the subject of future studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to Prof M. C. Dinauer for access to the COSPhoxFcγR cells and for helpful discussion; Prof A. Hall for the clones of Rac, Rho, and Cdc42; Profs D. Roos, A. Kapus, and A. Mócsai for critical reading of the paper; Dr G. Szanda for help with confocal microscopy; N. Gyöngyösi for help in statistical analysis; Mariann Kremlitzka and Andrea Balogh for isolation of T and B lymphocytes; Judit Szabó, Éva Wisniewski, and Enikő Lázár for help in some experiments; and Györgyi Domonkosné Járai, Erzsébet Horváthné Seres, and Edit Fedina for excellent and devoted technical assistance.
This work has been financially supported by the Hungarian Research Fund (OTKA grants K81277 and K75084 to E.L.). R.C.-K. was supported by TÁMOP-4.2.1/B-09/1/KMR-2010-0001.
Authorship
Contribution: R.C.-K. designed and carried out the majority of experiments, and prepared writing of the paper; G.S. carried out part of the experiments; M.G. first discovered ARHGAP25 in databases as potential leukocyte-specific GAP, and provided advises for the experiments; and E.L. supervised, coordinated, and financed the experimental work and had a major role in writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Erzsébet Ligeti, Department of Physiology, Semmelweis University, 1094 Budapest, Tűzoltó u 37-47, Hungary; e-mail: ligeti@puskin.sote.hu.


![Figure 2. ARHGAP25 regulates the GTPase activity of Rac. (A) The investigated fragments of ARHGAP25. (B-D) Effect of full-length or truncated forms of ARHGAP25 on in vitro [γ-32P]GTP-hydrolysis of Rac (B), Rho (C), and Cdc42 (D). Mean ± SEM of 4 separate experiments is shown (*P < .05 vs control). (E-L) Inhibition of EGF-induced ruffling by ARHGAP25 and p50RhoGAP. COS7 cells were transfected with control vector (E,I), cyan fluorescent protein (CFP)–tagged ARHGAP25R192A (F,J), CFP-ARHGAP25 (G,K), or CFP-p50RhoGAP (H,L). ARHGAP25, ARHGAP25R192A, p50RhoGAP, and CFP from control vector are shown in blue. Actin was stained with Alexa-568–labeled phalloidin (red). Stimulation was carried out with 0.1 μg/mL EGF for 20 minutes (I-L). EGF-induced ruffling is indicated by arrows. (M) Quantification of EGF-induced ruffling. Percentage of ruffled cells from 100 transfected cells was calculated. Mean + SEM of 4 (control vector, ARHGAP25R192A, and ARHGAP25) or 3 (p50RhoGAP) separate experiments are shown (*P < .05 compared with control).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/2/10.1182_blood-2010-12-324053/5/m_zh89991184320002.jpeg?Expires=1765022946&Signature=fgT5FqoHgSkBOC7H9zfjPp7RWkYM3kZQ6YD2kynsg-lNS4dZwD9zeK2O38KMFAMTUrCLs5wq5vXxVtXOxK2UheEtwMVKNw1VPmXYJ4o-iCC~wUWZpQZumFib891y-kvssIqtuhP07AaQ1eHWbohds1aW9jR3cKJ9CdrSMzEa1U-ul6k2DKXZ2gcXkP~a5fvAtkJqEbwmxIdzv8ucfv9J-DmQEfeAa8ZFM8D63XXpNprbzxxUcUp9nKe-d6uda5AyG-pn5I6v4O3WzsvkfKOUVcxPxWaVG1q5aeJ3Jmr6A1B60p~qBXKakejwUZSavLh~cXRI1aiqsV-qQ2vJM54Wtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)