Abstract
Mantle cell lymphoma (MCL) carries an unfavorable prognosis and requires new treatment strategies. The associated t(11:14) translocation results in enhanced cyclin D1 expression and cyclin D1–dependent kinase activity to promote cell-cycle progression. A pharmacodynamic study of the selective CDK4/6 inhibitor PD0332991 was conducted in 17 patients with relapsed disease, using 2-deoxy-2-[18F]fluoro-D-glucose (FDG) and 3-deoxy-3[18F]fluorothymidine (FLT) positron emission tomography (PET) to study tumor metabolism and proliferation, respectively, in concert with pre- and on-treatment lymph node biopsies to assess retinoblastoma protein (Rb) phosphorylation and markers of proliferation and apoptosis. Substantial reductions in the summed FLT-PET maximal standard uptake value (SUVmax), as well as in Rb phosphorylation and Ki-67 expression, occurred after 3 weeks in most patients, with significant correlations among these end points. Five patients achieved progression-free survival time of > 1 year (range, 14.9-30.1+ months), with 1 complete and 2 partial responses (18% objective response rate; 90% confidence interval, 5%-40%). These patients demonstrated > 70%, > 90%, and ≥ 87.5% reductions in summed FLT SUVmax and expression of phospho-Rb and Ki67, respectively, parameters necessary but not sufficient for long-term disease control. The results of the present study confirm CDK4/6 inhibition by PD0332991 at a well-tolerated dose and schedule and suggest clinical benefit in a subset of MCL patients. This study is registered at www.clinicaltrials.gov under identifier NCT00420056.
Introduction
Mantle cell lymphoma (MCL) is an uncommon subtype of B-cell lymphoma1 with median survival times of approximately 5 years.2 Initial treatment options include standard-dose or intensive chemo-immunotherapy with or without consolidation with autologous stem-cell transplantation.3,4 However, most patients will relapse with incurable disease. Although bortezomib, temsirolimus, and lenalidomide are active agents,5 novel approaches are needed, particularly those that target critical biologic pathways associated with MCL pathogenesis.6,7
The hallmark of MCL is the oncogenic t(11;14)(q13;q32) translocation that results in aberrant expression of cyclin D1, a D-type cyclin not ordinarily expressed in normal B cells.8-13 Cyclin D1 complexes with its catalytic partners, cyclin-dependent kinases (CDKs) 4 and 6, driving retinoblastoma (Rb) protein phosphorylation and G1 cell-cycle progression.14 Although cyclin D1 may have CDK-independent effects, nearly all cyclin D1 is found in complex with CDK4 in MCL cell lines and primary samples.15 In addition, in MCL cells, CDK4 activity is further enhanced by concomitant homozygous deletion of CDKN2A, encoding the CDK4/6-selective inhibitor p16INK4A.16,17 Alternatively, p16INK4A expression may be down-regulated as a result of BMI-1 amplification, causing overexpression of a transcriptional repressor of the CDKN2A locus.18 In addition to direct phosphorylation of Rb, cyclin D1-CDK4 complexes sequester p27Kip1 and p21Waf1/Cip1 away from cyclin E-CDK2, further facilitating the G1/S transition.19
The dysregulation of the cell-cycle machinery suggests that inhibition of CDK4 should have antiproliferative effects in MCL, and has prompted the preclinical and clinical evaluation of CDK inhibitors in this disease. The agents tested to date, including flavopiridol,20,21 seliciclib,22 and SNS-032,23 have greater potency against other CDK family members, including the transcriptional CDK9, and may be of interest for their ability to deplete antiapoptotic proteins from malignant hematopoietic cells.14
PD0332991, a potent, selective CDK 4/6 inhibitor, causes G1 arrest in MCL and other cell lines by blocking phosphorylation of Rb at CDK4/6-specific sites, including Ser807/811 and Ser780.15,24,25 In vitro, PD0332991 induces G1 cell-cycle arrest without substantial apoptosis, although its effects in preclinical in vivo MCL models have not yet been tested. The selectivity of this agent for CDK4 and CDK6 is reflected by IC50 values in the 5-10 ng/mL range, whereas IC50 values for CDK1, CDK2, and CDK5 exceed 2870 ng/mL.25 Phase 1 studies of PD0332991 administered orally have been conducted in patients with advanced cancers that express Rb, establishing recommended phase 2 doses of 125 mg daily for 3 weeks on/1 week off (the 3/1 schedule) or 200 mg daily for 2 weeks on/1 week off (the 2/1 schedule).26,27 Dose-limiting toxicities were neutropenia and thrombocytopenia. Other side effects were mild, including fatigue, diarrhea, anemia, and nausea, with overall good tolerability on both dosing schedules. PD0332991 exhibited linear pharmacokinetics, with a median time to maximal concentration (Tmax) of 4 hours, and was eliminated with a mean plasma half-life of 26.5 hours. In the phase 1 experience, stable disease was noted in 19 of 74 patients with advanced solid tumors, with 9 patients receiving 10 or more cycles on either the 3/1 or 2/1 schedule. A partial response in a patient with growing teratoma syndrome has also been described previously.28
Its safety and tolerability, as well as the compelling preclinical rationale, prompted us to conduct a phase 1b, multicenter, exploratory translational evaluation of PD0332991 in previously treated MCL patients using the 125-mg daily dose in the 3/1 schedule. The principal objective was to assess the pharmacodynamics of CDK4/6 inhibition by PD0332991. This was accomplished using 3-deoxy-3[18F]-fluorothymidine (FLT)29,30 and 2-deoxy-2-[18F]fluoro-D-glucose (FDG) positron emission tomography/computed tomography (PET/CT) imaging on 2 consecutive days before treatment and during the third week of study as measures of cellular proliferative and glucose metabolic responses, respectively. In addition, tumor biopsies were performed shortly after the pre- and posttreatment PET/CT scans to document reduced CDK4-mediated Rb phosphorylation by PD0332991 and to further measure antiproliferative effects with Ki-67 staining. Additional study objectives included assessing the safety and antilymphoma activity of PD0332991 in this patient population.
Methods
Patient eligibility
In this Pfizer-sponsored study (www.clinicaltrials.gov identifier NCT00420056), patients were 18 years of age or older with MCL, histologically documented as CD19+ or CD20+, CD5+, CD23−, with at least 1 of the following: cyclin D1 positivity by immunostaining, t(11;14) translocation on cytogenetic analysis, or molecular evidence of bcl-1/IgH rearrangement. Additional criteria for study entry included: bidimensionally measurable disease (ie, tumor size of ≥ 1.5 × 1.5 cm), a maximum standard uptake value (SUVmax) of ≥ 5 for at least 1 tumor lesion by both FLT-PET and FDG-PET at baseline; ≥ 1 prior therapy completed at least 3 weeks before study enrollment with recovery from acute toxic effects; Eastern Cooperative Oncology Group performance status of 0-1; and adequate organ function.
Exclusion criteria included: major surgery, radiation, or systemic treatment within 4 weeks of enrollment; prior radiation therapy to > 25% of the BM; major acute cardiovascular illness within the prior 12 months (within 6 months for pulmonary embolus); and QTc interval > 470 ms. The protocol was approved by the site institutional review boards, and all patients provided written informed consent before enrollment in accordance with the Declaration of Helsinki. The trial was conducted in accordance with Good Clinical Practice guidelines.
Protocol treatment and clinical protocol assessments
Baseline evaluation included history and physical examination, laboratory evaluations, multigated acquisition scan, 12-lead electrocardiogram, and imaging by CT or magnetic resonance imaging (MRI). PD0332991 was administered orally once daily in the fasted state at a dose of 125 mg. Treatment cycles comprised 3 consecutive weeks on therapy followed by a 1-week rest period. Physical examination was repeated on day 1 of each cycle; hematology and chemistry laboratory assessments were repeated on days 1, 8, 15, and 22 of each 28-day cycle. Triplicate electrocardiograms were taken at baseline and at days 1 and 21 of the first cycle.
Treatment continued until disease progression or unacceptable toxicity. Adverse events were assessed at baseline, throughout the trial, and during the 28-day period after treatment discontinuation and were assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0. PD0332991 was held for grade 3 or 4 hematologic toxicity and resumed with recovery to ≤ grade 2 (with a 25-mg dose reduction if grade 4 toxicity had occurred). Dosing was also interrupted for grade 3 or 4 nonhematologic toxicity and resumed with recovery to ≤ grade 1 with or without dose reduction depending on severity and/or recurrence of the event.
CT or MRI scans for the assessment of objective tumor responses were performed at baseline, after cycle 2, and every other cycle thereafter until disease progression. Standard response criteria were used for classification of objective tumor responses.31
Pharmacokinetic analyses
Blood samples were collected on days 1, 15, and 21 and analyzed using a validated LC-MS/MS assay (PPD Inc). Day 1 and day 15 samples were collected before dose; day 21 samples were collected at the time of the tumor biopsy.
Correlative imaging assessments
For assessment of cell-cycle and metabolic imaging responses, FLT-PET/CT and FDG-PET/CT were performed at baseline and during the third week of study treatment no more than 2 days apart. A single commercial provider (PETNET Solutions) manufactured the investigational FLT radiopharmaceutical for all sites. Consistent PET/CT image acquisition techniques were performed across sites according to a specified imaging manual. The imaging core laboratory at the Dana-Farber Cancer Institute performed prospective PET/CT scanner qualification and central image review and analysis.
For each patient, SUVmax was calculated in up to 10 lesions and the summed SUVmax of all lesions was calculated for each subject. The changes in the posttreatment overall summed SUVmax values were calculated relative to the pretreatment baseline values. PET response was also evaluated by calculating the average of the relative changes of individual lesions for each patient. Metabolic and proliferative responses were categorized using the ± 25% thresholds in relative changes for FDG-PET and FLT-PET summed SUVmax based on the European Organization for Research and Treatment of Cancer (EORTC) FDG-PET criteria.32
Correlative tissue assessments
Tumor biopsies were performed at baseline and on cycle 1 day 21 (while the patient was receiving PD0332991) and assessed for standard histology (with H&E staining). Immunohistochemistry was performed for total Rb (1:75; BD Pharmingen), phospho-Rb (pS807/S811; 1:100; Cell Signaling Technology), and Ki-67 (M7240 Clone MIB-1, 1:500; Dako), as described previously.33 TUNEL staining was performed with the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Millipore). For double immunofluorescence, cyclin D1 Ab34 diluted in DaVinci Green diluent was applied before the second Ab, the latter detected with Alexa Fluor 555 goat anti–mouse or anti-rabbit (Invitrogen). DAPI antifade mounting medium (Invitrogen) was then applied. Slides were scanned using a Zeiss Axio Imager.Z1 microscope (Carl Zeiss Inc) with filters detecting DAPI, Cy5, and Cy7 fluorochromes. Images were taken with Zeiss EC Plan-Neofluor objectives (40×/0.15 primary objective and 10× ocular objective) at room temperature in the DAPI, Cy5 and Cy7 channels using a PCO PixelFly camera (Zeiss), and processed with Tissue FAXS Version 2.0.4.0147 software (Tissue Gnostics). Image quantitation was performed using TissueQuest Version 3.0.1120.0138 software (Tissue Gnostics). Images from all cases were acquired and analyzed using identical hardware and software settings.
Statistical analyses
The planned sample size of 16 provided approximately 80% power to detect a change in SUV of 0.5 for both FLT-PET with FDG-PET, assuming a baseline SUV of 5.0, SD of 0.8, and α of 0.10. Posttreatment changes in FLT-SUVmax and FDG-SUVmax, as well as in expression of total Rb, phospho-Rb (pS807/S811), and Ki-67, were calculated and assessed for statistical significance using the paired Student t test. A ≥ 50% reduction in phospho-Rb expression in > 30% of evaluable patients was identified a priori as the primary criterion for achieving proof of mechanism. The relationships between percent changes in SUVmax, phospho-Rb, and Ki-67 with each other were assessed by the Pearson correlation coefficient. Kaplan-Meier estimates were used for progression-free survival and the log-rank test was used to assess the ability of binary proliferative biomarkers to predict progression-free survival. Nominal P values are presented. Descriptive statistics were used to summarize patient characteristics,and for the analysis of pharmacokinetic, tumor-response, and safety data.
Results
Patient demographics
Seventeen patients were enrolled between May 2007 and August 2008 (Table 1). The median age was 66 years (range, 40-79). Patients had received a median of 3 prior therapies (range, 1-8). Nine patients (53%) had received prior bortezomib and 3 patients (18%) had undergone prior stem cell transplantation. Although 16 patients (94%) had an Eastern Cooperative Oncology Group performance status of 0 or 1, most patients (59%) had high- to intermediate-risk disease according to the International Prognostic Index,35 and 11 patients (65%) had 3 or more involved disease sites (Table 1). Most patients had intermediate-risk scores by the Mantle Cell Lymphoma International Prognostic Index (MIPI) or by simplified MIPI criteria36 (Table 1 and supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). However, lymph node biopsy shortly before the start of PD0332991 showed that the majority of patients harbored lymphomas with high Ki-67 index (Table 1), an estimate of the proliferation signature,37 and adjustment of MIPI scores with the Ki-67 index to generate a combined biologic score demonstrated that the majority of enrolled patients had high-risk disease36 (Table 1 and supplemental Table 1).
Safety and tolerability
Treatment was well tolerated (Table 2). The most common grade 3 or 4 adverse events were hematologic and included neutropenia and thrombocytopenia. Fatigue and diarrhea were the most frequently occurring nonhematologic adverse events and were generally mild, save for 1 patient who experienced grade 3 diarrhea and grade 2 dehydration. Additional serious adverse events include grade 3 pneumonia and grade 5 cardiac arrest (1 patient each); the latter event was considered unrelated to PD0332991 and occurred more than 20 days after its discontinuation and after the start of a subsequent treatment regimen.
Nine patients received treatment at the full dose without interruption and 8 had therapy stopped temporarily primarily because of grade 3 or grade 4 hematologic or nonhematologic toxicities. One patient required dose reduction. For this patient, treatment was initially interrupted for grade 4 neutropenia and resumed at 100 mg daily after recovery. A second dose reduction to 75 mg daily was subsequently required as a result of grade 3 diarrhea and grade 2 dehydration. No patient discontinued study treatment because of adverse events, and all 15 patients who have been removed from the study were discontinued as a result of disease progression.
Antitumor activity
As of June 2010, 16 patients were evaluable for tumor response using the International Working Group criteria31 (supplemental Table 2). The 17th patient was not evaluable for response based on the absence of a baseline CT scan and was counted as a nonresponder. One patient (6%) achieved a complete response and 2 patients (12%) achieved a partial response, resulting in an objective response rate of 18% (90% confidence interval [90% CI], 5%-40%). In the first patient, partial response was documented after 4 cycles and complete response after 8 cycles. This patient remains in the study after more than 24 months (725+ days). In the other patients, partial responses were documented after 8 cycles. One of these patients remained on PD0332991 for 18.8 months (565 days); the other patient continues on study after more than 30 months (903+ days). An additional 7 patients (41%) had stable disease as the best response, whereas the remaining 6 patients (35%) experienced progressive disease. Both median time to progression and progression-free survival were 4 months (90% CI, 2.0-14.7 months). Five patients received study treatment for more than 1 year, with a probability of being event free at month ≥ 12 of 29% (90% CI, 12%-52%).
Correlative PET/CT imaging
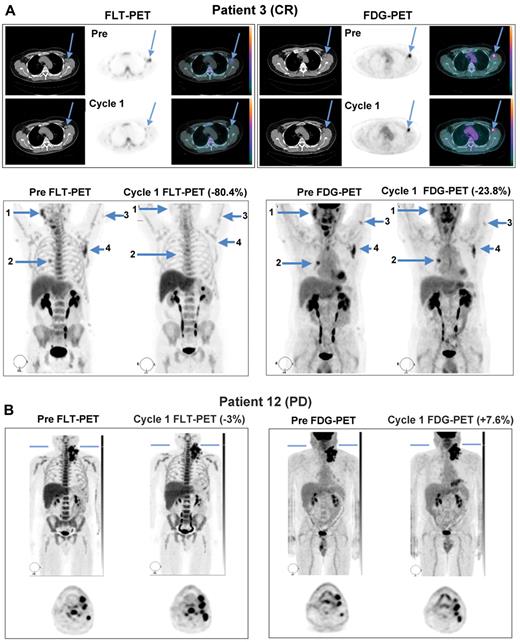
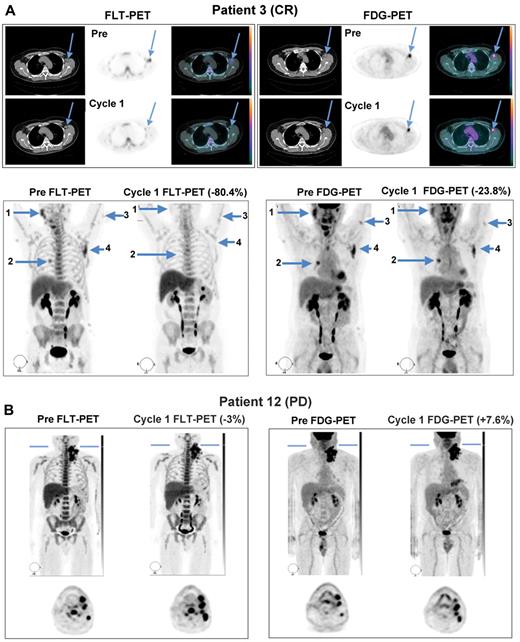
All patients enrolled in the study demonstrated FDG- and FLT-avid disease in the baseline PET/CT scans, with at least one tumor having an SUVmax ≥ 5. Sixteen patients were evaluable for both FLT-PET and FDG-PET analyses at both time points. Representative PET images from 2 patients obtained both before study treatment and during the third week of the first cycle are shown in Figure 1. Patient 3, who achieved partial anatomic response after 4 cycles and ultimately a durable, complete anatomic response had a > 80% reduction in tumor proliferation by FLT-PET and an approximately 24% reduction in glucose metabolism on FDG-PET during the third week of continuous study treatment (Figure 1A). Quantification of SUVmax among individual lesions in this patient demonstrated concordant reduction of FLT uptake among multiple lymph node stations at this time point (Figure 2A). In contrast, patient 12, who experienced disease progression by day 46, had only a 3% reduction in tumor proliferation on average, with most lesions demonstrating an increase in FLT uptake while on treatment, which was matched by a slight increase in glucose metabolism during the third week of PD0332991 (Figures 1B and 2B).
Representative FLT-PET/CT and FDG-PET/CT images at baseline and during the third week of PD0332991 in responding and progressing patients. (A) Images from patient 3, who went on to achieve complete response, demonstrating substantial improvements in FLT uptake and more modest changes in FDG uptake. (B) Images from patient 12, whose best response was disease progression, demonstrating interval increases in the number of lesions and in tracer uptake on both sets of scans.
Representative FLT-PET/CT and FDG-PET/CT images at baseline and during the third week of PD0332991 in responding and progressing patients. (A) Images from patient 3, who went on to achieve complete response, demonstrating substantial improvements in FLT uptake and more modest changes in FDG uptake. (B) Images from patient 12, whose best response was disease progression, demonstrating interval increases in the number of lesions and in tracer uptake on both sets of scans.
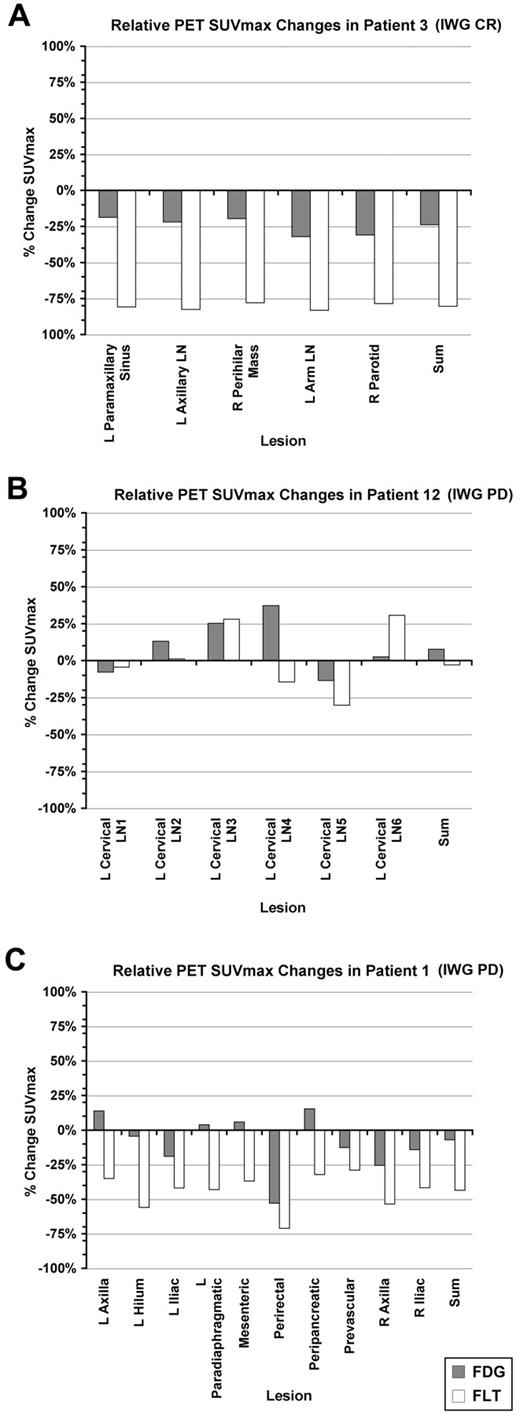
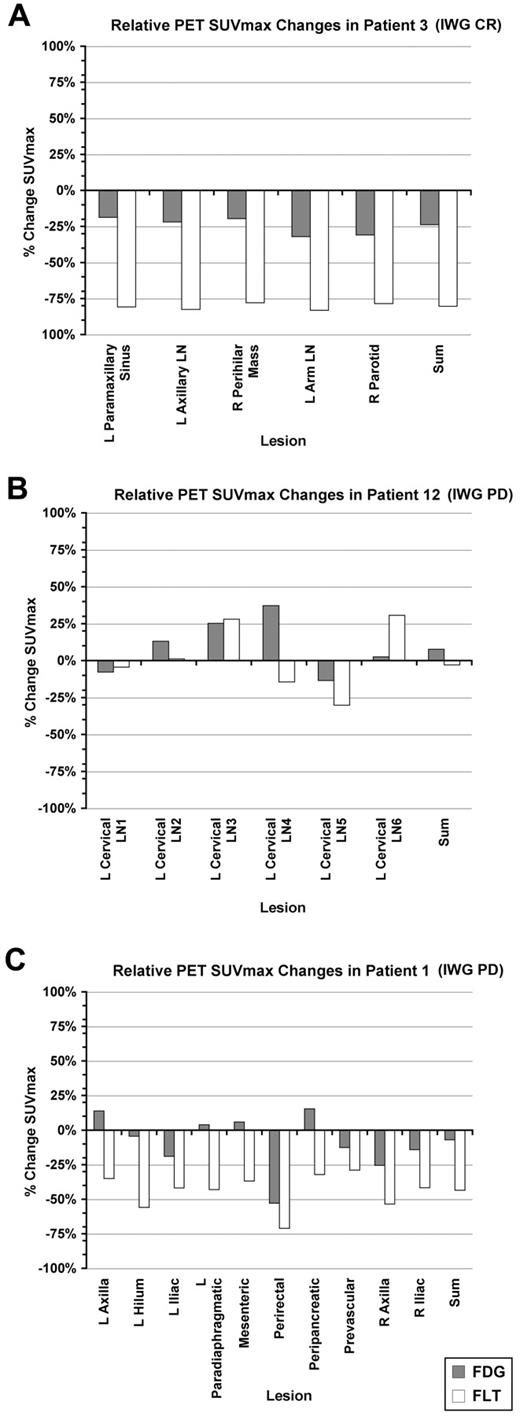
Percent change in FDG- and FLT-PET SUVmax for individual lesions for representative patients. The percentage change in summed SUVmax for the designated lesions is also shown. (A) Patient 3. (B) Patient 12. (C) Patient 1. L indicates left; LN, lymph node; and R, right.
Percent change in FDG- and FLT-PET SUVmax for individual lesions for representative patients. The percentage change in summed SUVmax for the designated lesions is also shown. (A) Patient 3. (B) Patient 12. (C) Patient 1. L indicates left; LN, lymph node; and R, right.
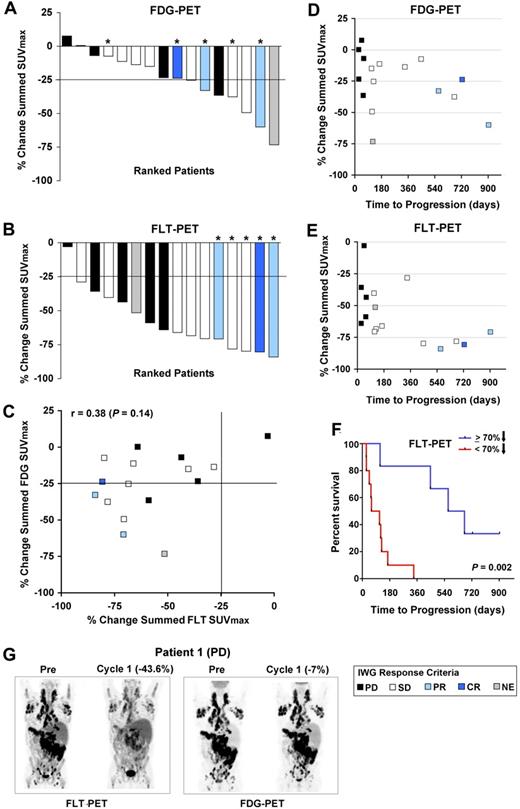
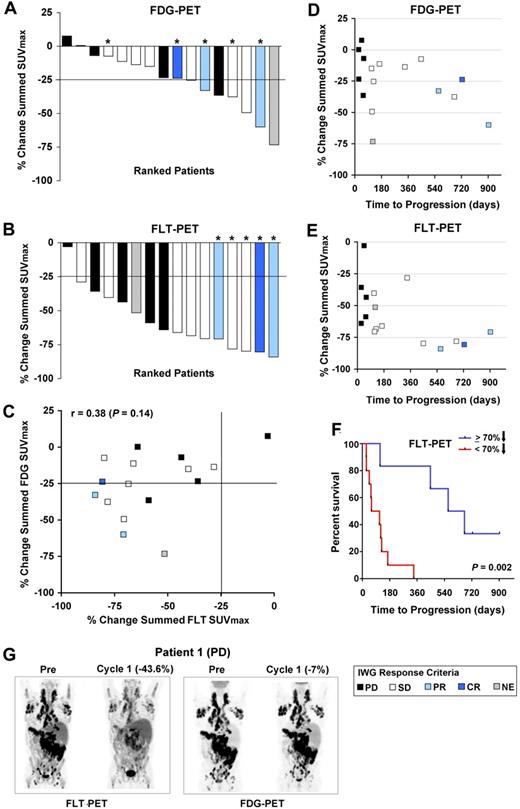
By EORTC criteria,32 7 patients achieved partial metabolic response by FDG-PET during the third week of the first cycle, with a > 25% reduction from pretreatment in the sum of SUVmax values (Figure 3A and supplemental Table 2). Using similar criteria, 15 patients achieved a proliferative FLT-PET response (Figure 3B and supplemental Table 2). Overall, reductions in FLT were not well correlated with those in FDG (r = 0.38; P = .14; Figures 2 and 3C, and supplemental Table 3).
Quantification of FDG- and FLT-PET changes on PD0332991 and correlation with each other and time to progression. (A) Percent change in summed SUVmax for FDG among 16 evaluable patients. (B) Percent change in summed SUVmax for FLT among 16 evaluable patients. In panels A and B, the bar colors represent the best anatomic response achieved by International Working Group response criteria, as indicated in the legend. Asterisks denote patients who remained enrolled on PD0332991 for more than 1 year. (C) Percent changes in summed SUVmax of FDG and FLT plotted against one another. (D) Percent change in the summed FDG SUVmax in relation to time to progression. (E) Percent change in the summed FLT SUVmax in relation to time to progression. In panels C through E, the colors of the data points designate the best anatomic response by International Working Group criteria, as indicated in the legend. (F) Kaplan-Meier analysis of time to progression among patients who achieved < or ≥ 70% reduction in summed FLT SUVmax. (G) Representative images from patient 1, who achieved a reduction in FLT uptake after 3 weeks of PD0332991, but no metabolic response by FDG-PET, and whose best response was disease progression.
Quantification of FDG- and FLT-PET changes on PD0332991 and correlation with each other and time to progression. (A) Percent change in summed SUVmax for FDG among 16 evaluable patients. (B) Percent change in summed SUVmax for FLT among 16 evaluable patients. In panels A and B, the bar colors represent the best anatomic response achieved by International Working Group response criteria, as indicated in the legend. Asterisks denote patients who remained enrolled on PD0332991 for more than 1 year. (C) Percent changes in summed SUVmax of FDG and FLT plotted against one another. (D) Percent change in the summed FDG SUVmax in relation to time to progression. (E) Percent change in the summed FLT SUVmax in relation to time to progression. In panels C through E, the colors of the data points designate the best anatomic response by International Working Group criteria, as indicated in the legend. (F) Kaplan-Meier analysis of time to progression among patients who achieved < or ≥ 70% reduction in summed FLT SUVmax. (G) Representative images from patient 1, who achieved a reduction in FLT uptake after 3 weeks of PD0332991, but no metabolic response by FDG-PET, and whose best response was disease progression.
FDG-PET was not associated with time to progression, with similar reductions in the summed FDG SUVmax noted in patients who experienced response, stable disease, or progression (Figure 3D). Although the majority of patients demonstrated pharmacodynamic activity with a greater than 25% reduction in FLT uptake, the 5 patients who remained on PD0332991 for more than 1 year all achieved a > 70% reduction in the summed FLT SUVmax (Figure 3E), with 3 of these patients demonstrating anatomic response in later cycles (1 complete and 2 partial responses) and 2 remaining with stable disease. The median time to progression among patients demonstrating a ≥ 70% reduction in tumor proliferation was 618.5 days, whereas that of patients with < 70% reduction was 86.5 days (P = .002; Figure 3F). However, an FLT response by EORTC criteria, or even a reduction in the summed FLT SUVmax approximating 70%, did not predict for long-term (> 1 year) anatomic disease stability or response (Figure 3E). This is illustrated by patient 1 (Figures 2C and 3G), who achieved an initial proliferative response to PD0332991 by FLT-PET, but no significant decrease in FDG uptake, and who went on to experience progressive disease by anatomic imaging criteria after 60 days, as well as by other patients who achieved reductions in summed FLT SUVmax at or near 70%, with times to progression of < 1 year (Figure 3E and supplemental Table 2).
Correlative biopsy data
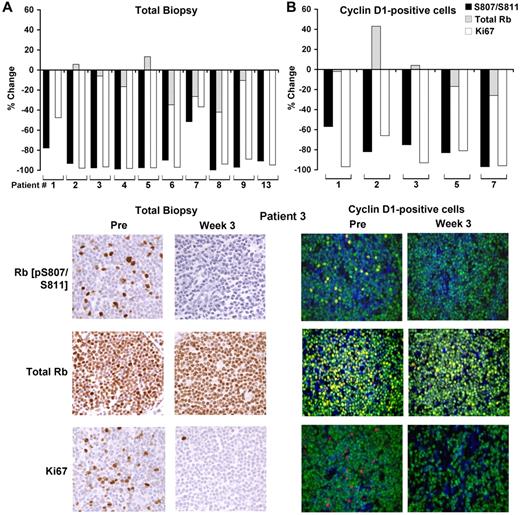
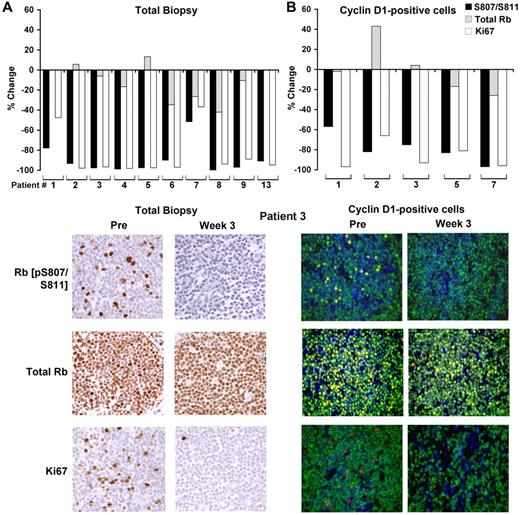
Of the 17 paired biopsy samples, 10 yielded pre- and on-treatment data for total Rb, phospho-Rb (pS807/811), and Ki-67 staining (Figure 4A and supplemental Table 2). In an additional 5 samples, Ki-67 alone was analyzed. For 2 patients, there was insufficient tissue for the paired assessment; the remaining failed analyses were related to inadequate staining for phosphorylated Rb. Among the informative samples, the average reduction in the percentage of cells positive for phospho-Rb (pS807/811) between baseline and week 3 was 89% (n = 10; range, 51.6%-100%; paired t test P = .000072), whereas total Rb staining was largely preserved. Similarly, Ki-67 staining was substantially reduced, with an average decrease of 74% on treatment (n = 15; range, 5%-98.2%; paired t test P = .0000018; Figure 4A and supplemental Table 2). In 5 patients, results were confirmed by double immunofluorescence in which cells were simultaneously stained for cyclin D1, and results were quantified only in cyclin D1–positive cells (Figure 4B and supplemental Table 2).
PD0332991-induced changes in Rb phosphorylation and Ki-67 expression in pre- and on-treatment lymph node biopsies. (A) Percent change in expression in phospho-Rb (pS807/S811), total Rb and Ki-67 in the on-treatment biopsy compared with the pre-treatment biopsy (top panels) and representative immunohistochemical images from patient 3, who eventually achieved a complete response (bottom panels). (B) Percent change in expression in phospho-Rb (pS807/S811), total Rb, and Ki-67 in cyclin D1–positive cells in the on-treatment biopsy compared with the pretreatment biopsy and representative immunofluorescence images from patient 3 (bottom panels). Blue indicates DAPI staining; green, cyclin D1; and red/yellow, the indicated Rb or Ki-67 Ab.
PD0332991-induced changes in Rb phosphorylation and Ki-67 expression in pre- and on-treatment lymph node biopsies. (A) Percent change in expression in phospho-Rb (pS807/S811), total Rb and Ki-67 in the on-treatment biopsy compared with the pre-treatment biopsy (top panels) and representative immunohistochemical images from patient 3, who eventually achieved a complete response (bottom panels). (B) Percent change in expression in phospho-Rb (pS807/S811), total Rb, and Ki-67 in cyclin D1–positive cells in the on-treatment biopsy compared with the pretreatment biopsy and representative immunofluorescence images from patient 3 (bottom panels). Blue indicates DAPI staining; green, cyclin D1; and red/yellow, the indicated Rb or Ki-67 Ab.
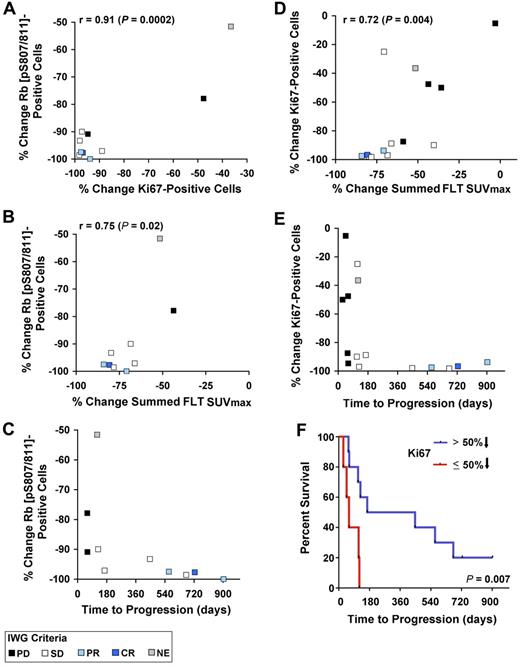
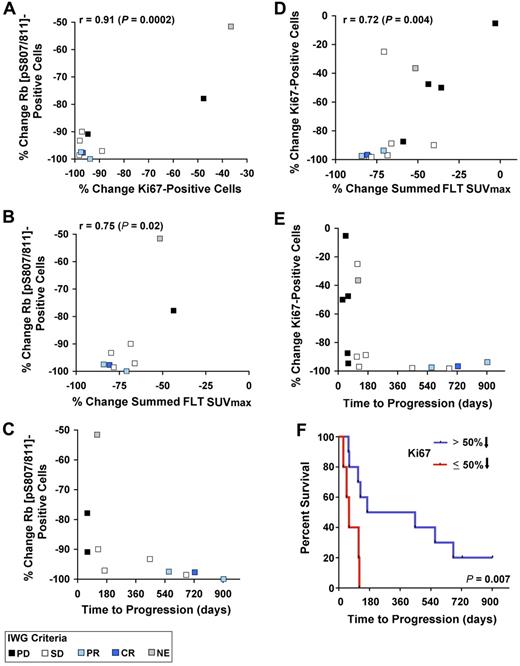
On treatment, the degree of reduction of phospho-Rb was correlated with that of Ki-67 (r = 0.91; P = .0002; Figure 5A and supplemental Table 3). In addition, reductions in phospho-Rb (pS807/S811) staining were correlated with the percentage change in the summed FLT SUVmax (r = 0.75, P = .02; Figure 5B and supplemental Table 3). For example, a ≥ 70% reduction in FLT-measured proliferation was generally associated with a > 90% reduction in phospho-Rb (pS807/S811) expression. Among the 5 patients who received PD0332991 for more than 1 year, all had a 90% or greater reduction in phospho-Rb (pS807/S811) expression (Figure 5C). Similarly, there was a correlation between reduction in Ki-67 on treatment and reduction in the summed FLT SUVmax (r = 0.72, P = .004; Figure 5D and supplemental Table 3). As with phospho-Rb, all 5 patients who remained on treatment for more than 1 year demonstrated profound reductions in Ki-67 staining during the third week of treatment (Figure 5E) and the median time to progression of those with > 50% reduction in Ki-67 (all of these patients achieving ≥ 87.5% reduction) was substantially longer than that of patients with ≤ 50% reduction (P = .007; Figure 5F). However, similar to the results for FLT-PET, substantial reductions in Ki-67 or phospho-Rb (pS807/S811) expression during the third week was inadequate to predict long-term disease stability or response to PD0332991, because other patients whose biopsies demonstrated similar degrees of change progressed after shorter periods of time (Figure 5C-E). There was no association between reductions in phospho-Rb or Ki-67 and the reduction in summed FDG SUVmax values (supplemental Table 3).
Correlations between immunohistochemical and radiographic pharmacodynamic parameters. (A-C) Percent change in phospho-Rb on PD0332991 treatment compared with the percentage change in Ki-67, the percentage decrease in summed FLT SUVmax, and time to progression. (D-E) Percent change in Ki-67 expression on PD033291 treatment compared with the percentage decrease in summed FLT SUVmax and time to progression. (F) Kaplan-Meier analysis of time to progression among patients who achieved > 50% reduction in Ki-67 (all with ≥ 87.5% decrease) on PD0332991 compared with other patients who achieved a ≤ 50% decrease.
Correlations between immunohistochemical and radiographic pharmacodynamic parameters. (A-C) Percent change in phospho-Rb on PD0332991 treatment compared with the percentage change in Ki-67, the percentage decrease in summed FLT SUVmax, and time to progression. (D-E) Percent change in Ki-67 expression on PD033291 treatment compared with the percentage decrease in summed FLT SUVmax and time to progression. (F) Kaplan-Meier analysis of time to progression among patients who achieved > 50% reduction in Ki-67 (all with ≥ 87.5% decrease) on PD0332991 compared with other patients who achieved a ≤ 50% decrease.
The proportion of apoptotic cells was assessed by TUNEL analysis in paired biopsies from 5 patients, which demonstrated only a small percentage of apoptotic cells in pretreatment samples (0%-3%), with no substantial increase in the proportion of apoptotic cells on treatment (supplemental Figure 1).
Pharmacokinetics
During the first 3 weeks, plasma pharmacokinetic samples were collected before treatment on day 1 and before dose on day 15 and time-matched with biopsies on day 21 to determine the systemic exposure to PD0332991. Trough plasma concentrations on day 15 ranged from 26.2-151 ng/mL; those taken on day 21 were similar, ranging from 35.7-173 ng/mL (supplemental Table 4). PD0332991 plasma concentrations on day 21 were not correlated with the percentage change from baseline in summed FLT SUVmax, phospho-Rb, or Ki-67 (supplemental Figure 2).
Discussion
The data presented herein provide evidence for modulation of pharmacodynamic biomarkers by PD0332991, using MCL as a disease model. We observed substantial reductions in tumor-cell proliferation and, to a lesser extent, in tumor metabolism as assessed by the percentage change in FLT and FDG-PET SUVmax, respectively, from baseline to week 3 of the first treatment cycle. The antiproliferative effect of PD0332991 was also demonstrated by a marked decrease in the percentage of phospho-Rb positive cells. Together with relatively preserved staining of total Rb on treatment, these data provide direct evidence of CDK4/6 inhibition by PD0332991 in human cancer cells on a safe and well tolerated dose administration schedule. The reduction in phospho-Rb staining was accompanied by a similar reduction in Ki-67 staining.
The majority of patients enrolled in this study had tumors with high proliferative index, likely related to the multiply relapsed nature of the population, nearly half having received > 3 prior systemic therapies, as well as the aggressive histology noted on many of the pretreatment biopsies.38 The 5 patients who have remained on PD0332991 for more than 1 year had baseline lymph node biopsies demonstrating 30%-80% Ki-67 positivity; therefore, long-term disease control was observed in patients with a range of initial estimates of proliferative index.36,37 These patients demonstrated substantial reductions in summed FLT-SUVmax and phospho-Rb and Ki-67 staining during the first cycle of study treatment. The data suggest that an initial > 70% reduction in summed FLT SUVmax and approximately 90% reduction in phospho-Rb and Ki-67 (ie, nearly complete inhibition of CDK4/6 activity) are necessary but not sufficient to predict long-term clinical benefit, findings that will require prospective confirmation in future studies. In other patients, evidence of reduced tumor proliferation and CDK4/6 inhibition during the third week of treatment was not correlated with disease stabilization, response, prolonged progression-free survival, or initial metabolic response by FDG-PET. In this subset of patients, resistance to PD0332991 often occurs within 5-6 months. In preclinical models, resistance to selective pharmacologic or genetic reduction in CDK4/6 activity may occur by increased activation of CDK239,40 ; further work will be required to determine whether a similar mechanism operates in the MCL population and whether serial FLT-PET imaging can predict the development of resistance before it is detected anatomically.
Steady-state trough PD0332991 plasma levels on day 15 of cycle 1 were in agreement with those observed at the 125-mg dose level (in the 3/1 schedule) in the phase 1 study,26 indicating similar exposures in solid tumor and lymphoma populations at this dose level and schedule. We found no correlation between PD0332991 plasma levels and change from baseline in radiographic or immunohistochemical pharmacodynamic parameters. The timing of pharmacokinetic sample collection was matched to tumor biopsy and therefore varied relative to dosing time between patients. However, this was unlikely to be the cause of the lack of pharmacokinetic-pharmacodynamic correlation. The range of pharmacokinetic concentrations observed on day 15 (at trough) and day 21 were nearly identical. The median Tmax of 4 hours and a mean terminal half-life of 26.5 hours indicate slow absorption and relatively slow elimination, resulting in a relatively shallow pharmacokinetic profile over the dosing interval of 24 hours, with a peak/trough ratio of less than 2.26
It will be of interest to determine in future studies whether intratumoral drug concentrations are correlated with the degree of modulation of biomarker and imaging parameters. Preclinical studies for PD0332991 in mice have shown that ratio of average steady-state concentrations in tumor tissue to plasma range from 5-121 across different tumor models and dose levels, so that drug levels are typically much higher in tumor tissue than in plasma, perhaps accounting for the lack of correlation between plasma pharmacokinetics and markers in tumor tissue seen in the current clinical trial.
Because some of the greatest reductions in pharmacodynamic end points were seen in patients with lower PD0332991 plasma levels, it is likely that the recommended phase 2 dose used in this study achieved a concentration above the minimum biologic effective dose.
The IC50 for inhibition of the cyclin D1/CDK4 or CDK6 holoenzymes ranges from 0.011-0.015μM (ie, 6.4-8.6 ng/mL) in vitro, whereas in tumor cells, the IC50 for reduction of Rb phosphorylation ranges from 0.063-0.066μM (ie, approximately 35 ng/mL), so that concentrations achieved in this clinical trial were sufficient to cause modulation of pharmacodynamic markers. Nonetheless, biologic characteristics of individual tumors that are yet to be defined may also have contributed to variability in the degree of response of the markers.
Preclinical data in MCL cell lines and primary tumor cells predicted a cytostatic response to PD0332991.15 However, in colon cancer models, only G1 arrest was observed in vitro, whereas tumor regressions did occur in vivo.24 The mechanism for this effect remains unclear. It has been postulated that tumors represent a balance of proliferating and apoptotic cells, and that inhibition of the proliferative compartment allows naturally dying cells to predominate. Alternatively, inhibition of CDK4/6 activity via the p16INK4A tumor-suppressor protein has been shown to reduce VEGF expression and to compromise angiogenic signaling,41 observations that may account for the effects only being observed in vivo. In addition, it has been demonstrated that selective CDK4 inhibition may repress NFκB-driven transcription, providing another mechanism by which a CDK4 inhibitor may induce apoptosis in certain cell types.42 Whatever the mechanism, in the present study, durable cytotoxic responses were observed in 3 patients after 4-8 months of PD0332991 as a single agent, indicating that chronic inhibition of CDK4/6 activity may be lethal to subsets of MCL cells. Cytotoxicity likely required prolonged exposure in these cases, because apoptosis was not detected early during the course of CDK inhibitor exposure.
Similarly, more prolonged drug exposure may have been required to detect changes in FDG uptake posttreatment that would be well correlated with those of FLT responses. Preliminary data from this study and others suggest that further study of FLT-PET in MCL is warranted.43 Although changes in FLT and FDG uptake were poorly correlated in individual patients in our study, the early time point examined was designed to quantify the immediate antiproliferative pharmacodynamic effect of CDK4/6 inhibition, which was expected to be measured with FLT but not necessarily demonstrated with FDG. However, later imaging may have produced improved the FLT-FDG correlation, with FDG demonstrating continued reductions in SUV over time, reflective of decreases in the number of viable cells in patients achieving prolonged stability or response.
Flavopiridol, another CDK inhibitor, has also been evaluated clinically in MCL with a variety of schedules. Modest activity has been reported, with cases of partial response and prolonged disease stability reported in previously treated patients using schedules likely to produce pharmacokinetic levels capable of overcoming plasma protein binding.20,21 When administered as a 1-hour bolus for 3 consecutive days every 21 days, 3 of 28 patients (11%) achieved partial responses. Twenty patients had stable disease as their best response; the durability of response or stable disease ranged from 1.4-13.2 months.20 Flavopiridol inhibits multiple CDK family members, most notably CDK9, with greatest potency, an activity not shared by PD0332991. This activity depletes mRNAs and proteins of short half-life, including Mcl-1, predisposing MCL cells to apoptosis.14 However, the potency of flavopiridol against CDK4 is likely inferior to that of PD0332991,21 raising the possibility that a combination of these CDK inhibitors may effectively disrupt cell-cycle progression and lower the apoptotic threshold simultaneously.
It is possible that the association of CDK4 inhibition with repression of NFκB activity contributes to the synergism of PD0332991 with bortezomib44 and dexamethasone,45 which was reported recently in multiple myeloma cells. These agents also may have propensity to induce apoptosis during the G1 phase of the cell cycle. Such results have prompted the development of combinations of bortezomib and PD0332991, which are currently undergoing evaluation in both myeloma and MCL. In addition, confirmation of target inhibition has supported preclinical and phase 2 evaluation of PD0332991 in a variety of solid tumor types.46-50
In summary, our results suggest that the approach of combining FLT-PET imaging and immunohistochemical studies may inform the development of a CDK inhibitor such as PD0332991, especially when cytostatic outcomes predominate. The absence of predicted changes at an early time point portends disease progression. However, acquired resistance may compromise the ability of early changes in noninvasive and invasive pharmacodynamics to predict long-term outcome. Nonetheless, these assessments provide early evidence of antitumor effects that would not otherwise be detected and offer a useful strategy for establishing proof-of-mechanism for agents that target the cell cycle.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Isan Chen and Deborah Womble of Pfizer for collaborative efforts during the inception of the study and protocol management; Anna Kreshock, Tyler Caron, and Donna Skinner of the Dana-Farber/Harvard Cancer Center Pathology Core for technical assistance; Christine Arris, PhD, of ACUMED (Tytherington, United Kingdom) for manuscript editing and preparation funded by Pfizer; all participating patients and their families; and the network of investigators, research nurses, study coordinators, and operations staff at the participating institutions.
This work was sponsored and funded by Pfizer Clinical Development and Medical Affairs, a Lymphoma Research Foundation Mantle Cell Lymphoma Correlative Science grant (MCLC-07-015 to G.I.S.), and a Mantle Cell Lymphoma Research Initiative grant (MCLI-03-011 to G.I.S.).
Authorship
Contribution: J.P.L., A.S.L., M.R.S., A.N., P.M., and M.M.M. enrolled and cared for the patients; A.S.L., J.T.Y., and G.I.S. designed the study in collaboration with K.D.W. and other Pfizer investigators; L.R.C. and S.J.R. directed the pathologic analyses of tissue samples with preliminary and methodologic contributions from S.A.E. and S.Y.C.-K.; P.E. and D.S.N. performed the biostatistics; A.D.V.D.A., and J.T.Y. directed the FLT-PET and FDG-PET analyses at the Dana-Farber Cancer Institute, which served as the coordinating site; J.Q.Y., S.V., and H.S. oversaw the acquisition of PET images at their respective institutions; R.C. and N.S. performed the pharmacokinetic analyses; J.P.L., S.R., A.D.V.D.A., J.T.Y., and G.I.S. wrote the manuscript; S.R. and G.I.S. oversaw and coordinated the entire project; and all authors had access to primary clinical trial data and reviewed and approved the manuscript content.
Conflict-of-interest disclosure: P.E., R.C., N.S., K.D.W., and S.R. are employed by Pfizer. J.P.L. and P.M. have served as consultants for Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey T. Yap, Department of Imaging, Dana L101, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: jeffrey_yap@dfci.harvard.edu; or Geoffrey I. Shapiro, Department of Medical Oncology, Early Drug Development Center, Mayer 446, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: geoffrey_shapiro@dfci.harvard.edu.
References
Author notes
J.P.L. and A.S.L. contributed equally to this work.