Abstract
This multicenter phase 1/2 trial investigated the combination of bendamustine, lenalidomide, and dexamethasone in repeating 4-week cycles as treatment for relapsed refractory multiple myeloma (MM). Phase 1 established maximum tolerated dose (MTD). Phase 2 assessed overall response rate at the MTD. Secondary endpoints included progression-free survival (PFS) and overall survival (OS). A total of 29 evaluable patients were enrolled. Median age was 63 years (range, 38-80 years). Median number of prior therapies was 3 (range, 1-6). MTD was bendamustine 75 mg/m2 (days 1 and 2), lenalidomide 10 mg (days 1-21), and dexamethasone 40 mg (weekly) of a 28-day cycle. Partial response rate was 52%, with very good partial response achieved in 24%, and minimal response in an additional 24% of patients. Median follow-up was 13 months; median OS has not been reached. One-year OS is 93% (95% confidence interval [CI], 59%-99%). Median PFS is 6.1 months (95% CI, 3.7-9.4 months) with one-year PFS of 20% (95% CI, 6%-41%). Grade 3/4 adverse events included neutropenia, thrombocytopenia, anemia, hyperglycemia, and fatigue. This first phase 1/2 trial testing bendamustine, lenalidomide, and dexamethasone as treatment of relapsed refractory MM was feasible and highly active. This study is registered at www.clinicaltrials.gov as #NCT01042704.
Introduction
Recent advances in the therapy of multiple myeloma (MM), including high-dose chemotherapy, autologous stem cell transplantation (SCT), and incorporation of thalidomide, lenalidomide, and bortezomib into treatment regimens, have improved outcomes.1,2 Nevertheless, 5-year overall survival (OS) remains less than 40%,3 and there are few effective salvage regimens available for patients with disease resistant to novel agents. Therefore, there is a clear need for improved salvage regimens in MM.
Lenalidomide is an analog of thalidomide that binds to cereblon4 and subsequently affects transcription factors critical for MM growth, such as C/EBPβ5 and IRF4.6 In a pooled analysis of 2 large phase 3 trials (MM-009 and MM-010)7,8 with a median follow-up of 48 months, lenalidomide plus dexamethasone significantly improved overall response rate (ORR; 60.6% vs 21.9%; P < .001), complete response rate (CR; 15% vs 2%; P < .001), and median (38.0% vs 31.6 months; P = .045) compared with dexamethasone alone. Median time to progression during treatment with lenalidomide-dexamethasone was 13.4 months (vs 4.6 months for dexamethasone; P < .001).9
Bendamustine, a bifunctional agent, shares properties of alkylating agents and purine analogs.10 In a phase 3 study comparing bendamustine and prednisone versus melphalan and prednisone (MP) in previously untreated MM patients, comparable ORRs were observed; however, a significantly higher number of patients treated with bendamustine and prednisone achieved CR (32% vs 13%; P = .007).11 In a phase 1 trial of bendamustine in patients with progressive disease (PD) after autologous SCT, the maximally tolerated dose (MTD) was 100 mg/m2 on days 1 and 2 of repeating 28-day cycles. The ORR was 55%.12 A lower dose of bendamustine (60 mg/m2) was used together with thalidomide and prednisolone in a phase 1 or 2 study involving patients with relapsed or refractory MM after autologous SCT or chemotherapy. The ORR in this study was 86%.13
Given the different mechanisms of action ascribed to lenalidomide and bendamustine, as well as results from previous studies successfully combining immunomodulatory drugs and alkylating agents, such as MP plus thalidomide or MP plus lenalidomide,14-16 we sought to assess the feasibility and efficacy of a regimen combining bendamustine and lenalidomide. In addition, we wanted to explore whether this combination has efficacy in patients with prior exposure to lenalidomide. We report here a phase 1/2 clinical trial evaluating the safety, tolerability, and activity of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory MM.
Methods
Study design
This open-label, dose escalation study was performed at 2 centers in the United States: University of Pittsburgh Cancer Institute, Pittsburgh, PA; and Karmanos Cancer Institute at Wayne State University, Detroit, MI. The phase 1 portion was designed to determine the MTD (primary objective) of bendamustine and lenalidomide in combination with a fixed dose of dexamethasone for patients with relapsed or refractory MM. In the phase 2 portion of the study, an expansion cohort of patients was treated at the MTD to assess ORR. Secondary endpoints included evaluation of progression-free survival (PFS), OS, and time to response. Individual patients stayed at the same dose level throughout study treatment unless they required dose reduction because of toxicity.
Patients and eligibility
Eligible patients had relapsed or refractory MM, were 18 years of age or older, had an Eastern Cooperative Oncology Group performance status of 0 to 2, and had an expected survival greater than 6 months. All patients had symptomatic MM and had previously been treated with at least 1 line of therapy, after which the patient had progressive or refractory disease. Prior lenalidomide and/or autologous SCT were permissible. Patients had to have a total white blood cell count of at least 2 × 109/L with an absolute neutrophil count of at least 1 × 109/L, hemoglobin of at least 9 g/dL, platelet count of at least 75 × 109/L, international normalized ratio 2 or 3 if on anticoagulation, total bilirubin less than or equal to 2.5 mg/dL, aspartate aminotransferase and alanine aminotransferase less than or equal to 5 times the institutional upper limit of normal, and creatinine less than 2.5 mg/dL. Patients were excluded if they had nonsecretory MM, central nervous system involvement, other malignancy within 2 years before enrollment (excluding squamous/basal cell carcinoma of the skin or carcinoma in situ of the cervix), myocardial infarction within 6 months before enrollment or New York Heart Association class III or IV heart failure, prior allogeneic SCT, or allergic reaction to compounds of similar chemical or biologic composition to bendamustine or lenalidomide. In addition, patients were excluded if they received chemotherapy, radiotherapy (except in cases of severe bone pain or impending pathologic fracture), or investigational agents within one month of starting BLD. All patients gave written, informed consent before enrollment in the study. The institutional review boards at the University of Pittsburgh and Wayne State University, in accordance with federal regulations, the Declaration of Helsinki, and the International Conference on Harmonization Guidelines for Good Clinical Practice, approved the study and consent.
Determination of MTD and efficacy assessment
Patients were treated in repeating 28-day cycles, up to a maximum of 8 cycles, unless disease progression or dose-limiting toxicity (DLT) occurred earlier. DLTs were defined as sustained (≥ 7 days) grade 3 neutropenia, more than or equal to grade 3 neutropenia with fever (temperature ≥ 38.5°C), grade 4 neutropenia, platelets less than 25 × 109/L, or other grade 3 or 4 toxicity, which, in the judgment of the treating physician, was possibly related to bendamustine, lenalidomide, or dexamethasone. Bendamustine and lenalidomide doses were increased through 3 levels in a standard 3 + 3 dose escalation scheme. Lenalidomide was given orally at a dose of 5 or 10 mg daily on days 1 to 21 of each cycle. Bendamustine was administered intravenously at a dose of 75 or 100 mg/m2 on day 1 and day 2 of each cycle. The MTD was defined as the dose level at which less than or equal to 1 of 6 patients experienced DLT during the first cycle of therapy, with the next higher dose level having more than or equal to 2 of 3 or more than or equal to 2 of 6 patients with DLTs. After determining the MTD, an additional 14 patients were enrolled in a phase 2 MTD expansion cohort. Thus, altogether, 20 patients were treated at the MTD. All patients received a fixed dose of dexamethasone (40 mg weekly) as well as a gastroprotectant (either an H2 blocker or a proton pump inhibitor) and a daily aspirin (325 mg/day). Patients received additional supportive treatment as indicated, including G-CSF, if prolonged grade 3 or grade 4 neutropenia occurred during cycle 2 or subsequently. Study drug was held in subjects experiencing grade 3 or grade 4 adverse events until resolution of the adverse event. In those cases, a dose reduction was performed. Once a subject's dose was reduced, no re-escalation was permitted. Adverse events were monitored throughout the study and for up to 30 days after the last dose of the study drug. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (Version 3.0). Abnormal laboratory values were considered adverse events if the abnormality resulted in discontinuation from the study or required treatment modification. Evaluation of response was based on the International Working Group criteria and the International Uniform Response Criteria.17-20 Response assessment was performed after each cycle of therapy. After discontinuation of therapy, patients were followed every 3 months for the first 2 years, then every 6 months thereafter, or until death.
Statistical analysis
The cut-off date for response and survival data was June 1, 2011. The patient demographics and baseline clinical characteristics were summarized as the number and percentage of patients or as the median and range of values. Eligible patients who received at least one full cycle of BLD were evaluable for safety. Patients who received at least 2 full cycles were evaluable for efficacy. Exact binomial 90% confidence intervals (CIs) were reported for toxicities and responses.
For patients who responded, the time to response was calculated as the interval between the date on which the patient started protocol therapy and the date on which objective response (very good partial response [VGPR] or partial response [PR]) was first documented. PFS was calculated as the time interval between the date on which a patient first received regimen and the documented date of disease progression. OS was calculated as the time interval between the date on which a patient first received regimen and the documented date of death. Patients without an event were censored at the date they were last known to be in remission and alive for PFS, and alive for OS. PFS and OS functions were estimated by the Kaplan-Meier method. To evaluate an association between β2-microglobulin and response as well as between albumin, glomerular filtration rate (GFR), and grade 3 or 4 neutropenia, we used the Fisher exact test.
Results
Patient characteristics and determination of MTD
Between June 2008 and February 2011, 29 patients were enrolled. We enrolled 15 patients into the phase 1 study and 14 patients into the phase 2 part of the study. All patients (n = 29) had relapsed or refractory MM. Table 1 lists the baseline characteristics of these patients. The median number of prior treatments was 3 (range, 1-6). A total of 97% of patients were previously treated with lenalidomide, thalidomide, or both. A total of 66% (n = 19) of patients had prior treatment with bortezomib. Twenty patients (69%) had previously undergone autologous SCT.
Patient demographics and baseline clinical characteristics
| Characteristic . | No. of patients (n = 29) . |
|---|---|
| Age, y | |
| Median | 63 |
| Range | 38-80 |
| Male sex | 55 |
| International Staging System stage | |
| I | 10 (34) |
| II | 15 (52) |
| III | 4 (14) |
| Albumin, g/dL | |
| Median | 3.62 |
| Range | 2.6-4.5 |
| Paraprotein | |
| IgG | 13 (45) |
| IgA | 9 (31) |
| Free light chain | 15 (52) |
| Other (IgD and IgM) | 0 (0) |
| Eastern Cooperative Oncology Group performance status | |
| 0 | 16 (55) |
| 1 | 11 (38) |
| 2 | 2 (7) |
| No. of previous therapies | |
| Median | 3 |
| Range | 1-6 |
| Type of therapy | |
| Thalidomide only | 4 (14) |
| Lenalidomide only | 13 (45) |
| Lenalidomide + thalidomide | 11 (38) |
| No previous thalidomide or lenalidomide | 1 (3) |
| Bortezomib | 19 (66) |
| Autologous stem cell transplantation | 20 (69) |
| Characteristic . | No. of patients (n = 29) . |
|---|---|
| Age, y | |
| Median | 63 |
| Range | 38-80 |
| Male sex | 55 |
| International Staging System stage | |
| I | 10 (34) |
| II | 15 (52) |
| III | 4 (14) |
| Albumin, g/dL | |
| Median | 3.62 |
| Range | 2.6-4.5 |
| Paraprotein | |
| IgG | 13 (45) |
| IgA | 9 (31) |
| Free light chain | 15 (52) |
| Other (IgD and IgM) | 0 (0) |
| Eastern Cooperative Oncology Group performance status | |
| 0 | 16 (55) |
| 1 | 11 (38) |
| 2 | 2 (7) |
| No. of previous therapies | |
| Median | 3 |
| Range | 1-6 |
| Type of therapy | |
| Thalidomide only | 4 (14) |
| Lenalidomide only | 13 (45) |
| Lenalidomide + thalidomide | 11 (38) |
| No previous thalidomide or lenalidomide | 1 (3) |
| Bortezomib | 19 (66) |
| Autologous stem cell transplantation | 20 (69) |
IgG indicates immunoglobulin G; and IgA, immunoglobulin A.
The number of patients in each dosing cohort is detailed in Table 2. DLTs were not observed at dose level 1. At dose level 2, 1 of 6 patients experienced a grade 4 neutropenia. At dose level 3, 2 patients had a DLT, with 1 grade 4 neutropenia and 1 prolonged grade 3 thrombocytopenia, that was graded as a DLT. The MTD therefore was determined to be bendamustine 75 mg/m2 and lenalidomide 10 mg.
Patient disposition per dose level
| Dose level . | Bendamustine, mg/m2 . | Lenalidomide, mg . | Dexamethasone, mg . | No. of patients (N = 29) . |
|---|---|---|---|---|
| 1 | 75 | 5 | 40 | 3 |
| 2 | 75 | 10 | 40 | 20* |
| 3 | 100 | 10 | 40 | 6 |
| Dose level . | Bendamustine, mg/m2 . | Lenalidomide, mg . | Dexamethasone, mg . | No. of patients (N = 29) . |
|---|---|---|---|---|
| 1 | 75 | 5 | 40 | 3 |
| 2 | 75 | 10 | 40 | 20* |
| 3 | 100 | 10 | 40 | 6 |
Includes 6 initial patients plus 14 patients added at the level of the MTD; this provided a total of 20 patients at dose level 2.
Drug exposure and safety
Treatment-related adverse events for all cycles that occurred with a frequency of 25% or greater are listed in Table 3. The most common grade 3 or greater toxicities include neutropenia (62%; n = 18 patients), thrombocytopenia (38%; n = 11 patients), anemia (17%; n = 5 patients), and leukopenia (38%; n = 11 patients). To assess whether low albumin or decreased GFR contributes to increased toxicity of BLD, we correlated grade 3 or 4 neutropenia with albumin less than 3.5 g/dL and with GFR less than 50 mL/min. Neither a low albumin (P = .7021) nor a decreased GFR (P = .2315) was associated with increased risk of grade 3 or 4 neutropenia. Other treatment-related toxicity (all grades) in 25% or more patients included fatigue (45%), diarrhea (35%), hypocalcemia (31%), hyperglycemia (31%), and nausea (28%). Despite the high frequency of neutropenia, we observed only 1 grade 3 febrile neutropenia (3%). In addition, only 1 patient (3%) had a grade 3 infection (pneumonia) with normal absolute neutrophil count. Four of 29 patients discontinued the study because of disease progression or lack of response, and 9 patients (31%) discontinued therapy because of prolonged neutropenia and/or thrombocytopenia occurring subsequent to the first cycle of treatment. Twelve (41%) of 29 patients received G-CSF for prolonged grade 3 or 4 neutropenia. The dose of dexamethasone was reduced in 4 (14%) patients. Thromboembolic events were not observed. There were no deaths considered to be possibly related to the study drug.
Summary of all treatment-related adverse events (N = 29)
| Adverse event . | No. of patients by event grade . | Total patients, n (%) . | |||
|---|---|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | ||
| Treatment-related in ≥ 25% of patients | |||||
| Thrombocytopenia | 6 | 7 | 8 | 3 | 24 (83) |
| Neutropenia (ANC/AGC) | 1 | 4 | 11 | 7 | 23 (79) |
| Anemia | 2 | 10 | 5 | 0 | 17 (59) |
| Leukopenia | 1 | 5 | 10 | 1 | 17 (59) |
| Fatigue (asthenia, lethargy, malaise) | 7 | 3 | 3 | 0 | 13 (45) |
| Diarrhea | 7 | 1 | 2 | 0 | 10 (35) |
| Hypocalcemia | 6 | 2 | 1 | 0 | 9 (31) |
| Hyperglycemia | 3 | 2 | 4 | 0 | 9 (31) |
| Nausea | 5 | 2 | 1 | 0 | 8 (28) |
| Additional grades 3 or 4 | |||||
| Hypokalemia | 1 | 0 | 4 | 1 | 6 (21) |
| Dehydration | 0 | 0 | 2 | 0 | 2 (6) |
| Prolonged QTc interval | 0 | 0 | 1 | 0 | 1 (3) |
| Arrhythmia, atrial fibrillation | 0 | 0 | 1 | 0 | 1 (3) |
| Infection with normal (pneumonia) | 0 | 0 | 1 | 0 | 1 (3) |
| Febrile neutropenia | 0 | 0 | 1 | 0 | 1 (3) |
| Pain, joint | 0 | 0 | 1 | 0 | 1 (3) |
| Adverse event . | No. of patients by event grade . | Total patients, n (%) . | |||
|---|---|---|---|---|---|
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | ||
| Treatment-related in ≥ 25% of patients | |||||
| Thrombocytopenia | 6 | 7 | 8 | 3 | 24 (83) |
| Neutropenia (ANC/AGC) | 1 | 4 | 11 | 7 | 23 (79) |
| Anemia | 2 | 10 | 5 | 0 | 17 (59) |
| Leukopenia | 1 | 5 | 10 | 1 | 17 (59) |
| Fatigue (asthenia, lethargy, malaise) | 7 | 3 | 3 | 0 | 13 (45) |
| Diarrhea | 7 | 1 | 2 | 0 | 10 (35) |
| Hypocalcemia | 6 | 2 | 1 | 0 | 9 (31) |
| Hyperglycemia | 3 | 2 | 4 | 0 | 9 (31) |
| Nausea | 5 | 2 | 1 | 0 | 8 (28) |
| Additional grades 3 or 4 | |||||
| Hypokalemia | 1 | 0 | 4 | 1 | 6 (21) |
| Dehydration | 0 | 0 | 2 | 0 | 2 (6) |
| Prolonged QTc interval | 0 | 0 | 1 | 0 | 1 (3) |
| Arrhythmia, atrial fibrillation | 0 | 0 | 1 | 0 | 1 (3) |
| Infection with normal (pneumonia) | 0 | 0 | 1 | 0 | 1 (3) |
| Febrile neutropenia | 0 | 0 | 1 | 0 | 1 (3) |
| Pain, joint | 0 | 0 | 1 | 0 | 1 (3) |
ANC indicates absolute neutrophil count; and AGC, absolute granulocyte count.
Efficacy
Twenty-five patients who received more than or equal to 2 cycles were evaluable for efficacy (Table 4). PR or better was observed in 52% (n = 13) of patients. Six patients (24%) achieved a VGPR and 7 (28%) reached PR. Minimal response was observed in an additional 6 patients (24%); thus, a total of 76% of patients had some degree of objective improvement. Among the patients achieving at least a PR, the median time to response was 1.6 months (95% CI, 1.6-3.5 months; Table 5).
Response among evaluable patients and selected subgroups
| . | Patients (n = 25), n (%) . |
|---|---|
| Response | |
| VGPR | 6 (24) |
| PR | 7 (28) |
| MR | 6 (24) |
| Stable disease | 4 (16) |
| Progressive disease | 2 (8) |
| VGPR and PR in selected subgroups | 13 |
| Previous use of thalidomide only | 4 (31) |
| Previous use of lenalidomide only | 5 (38) |
| Previous use of thalidomide + lenalidomide | 3 (23) |
| No previous use of thalidomide or lenalidomide | 1 (8) |
| . | Patients (n = 25), n (%) . |
|---|---|
| Response | |
| VGPR | 6 (24) |
| PR | 7 (28) |
| MR | 6 (24) |
| Stable disease | 4 (16) |
| Progressive disease | 2 (8) |
| VGPR and PR in selected subgroups | 13 |
| Previous use of thalidomide only | 4 (31) |
| Previous use of lenalidomide only | 5 (38) |
| Previous use of thalidomide + lenalidomide | 3 (23) |
| No previous use of thalidomide or lenalidomide | 1 (8) |
VGPR indicates very good partial remission; PR, partial remission; and MR, minor remission.
Additional efficacy outcomes (among evaluable patients)
| Outcome . | Median (95% CI) . |
|---|---|
| Time to VGPR + PR, mo | 1.6 (1.6-3.5) |
| PFS, mo | 6.1 (3.7-9.4) |
| OS, mo | — (17=—) |
| Outcome . | Median (95% CI) . |
|---|---|
| Time to VGPR + PR, mo | 1.6 (1.6-3.5) |
| PFS, mo | 6.1 (3.7-9.4) |
| OS, mo | — (17=—) |
VGPR indicates very good partial remission; PR, partial remission; PFS, progression free survival; OS, overall survival; and —, not reached yet.
Efficacy in prognostic adverse groups
Twenty-five patients evaluable for response were assessed for relapsed/refractory disease status according to the International Myeloma Working Group criteria20 as well as chromosomal abnormalities at the time of study entry. A total of 52% (n = 13) of the patients had relapsed/refractory disease to lenalidomide and/or thalidomide. A total of 69% (n = 9) of the lenalidomide/thalidomide-relapsed/refractory patients responded to BLD achieving 1 VGPR, 5 PR, and 3 minimal response (MR). Ten (40%) of 25 patients had abnormalities by cytogenetic and/or FISH analysis (intermediate and high risk).20 This included 6 patients with del 13q, 1 patient with del 13q and 17p, 1 patient with t(8;14), 1 patient with complex chromosomal aberrations, and 1 patient with −Y, t(8;10). A total of 60% (n = 6) of the patients with abnormal cytogenetic/FISH analysis responded, achieving 2 VGPR, 3 PR, and 1 MR. We also correlated whether an increased β2-microglobulin (≥ 3.5 mg/L) was associated with poorer outcome in patients treated with BLD. We found no correlation between high β2-microglobulin and poor response (stable disease and progressive disease, P = .1753).
PFS and OS data
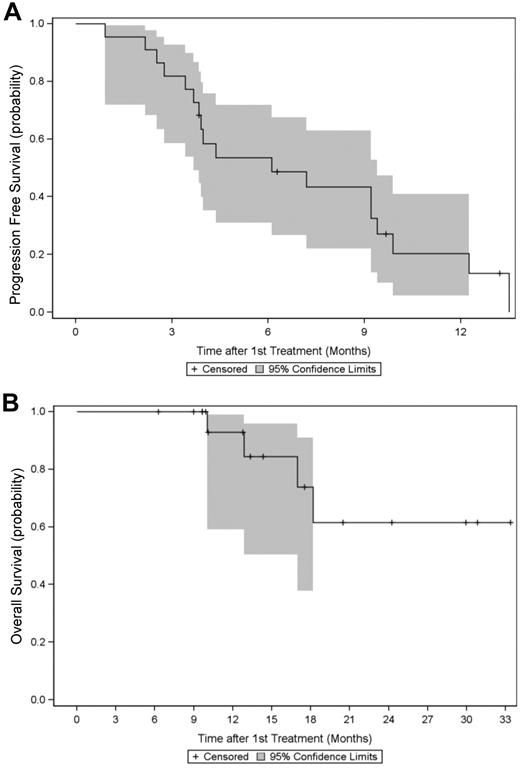
Among the 25 evaluable patients, the median PFS was 6.1 months (95% CI, 3.7-9.4 months). The one-year PFS rate was 20% (95% CI, 6%-41%; Figure 1A). The median OS rate was not reached at the time of data evaluation (June 1, 2011; Table 5). The estimated 1-year and 2-year OS rates are 93% (95% CI, 59%-99%) and 62% (95% CI, 25%-84%), respectively (Figure 1B). After a median follow-up of 13.1 months (range, 6-33 months), 4 patients (16%) died, all from progressive disease.
Progression free survival and overall survival. Kaplan-Meier estimates (in months) with 95% CI of (A) PFS and (B) OS in 25 patients treated with BLD for relapsed MM.
Progression free survival and overall survival. Kaplan-Meier estimates (in months) with 95% CI of (A) PFS and (B) OS in 25 patients treated with BLD for relapsed MM.
Discussion
Our findings indicate that the combination of BLD is a highly active regimen for patients with relapsed or refractory myeloma. The ORR, including MR, in our heavily pretreated patient population was 76% with PR or better in 52%. The time to response, including PR and VGPR, was only 1.6 months (95% CI, 1.6-3.5 months) in our study. A total of 69% of the patients with disease refractory/relapsed to lenalidomide and/or thalidomide responded to BLD, suggesting that this regimen overcomes resistance to immunomodulatory drugs. Further, 60% of patients with abnormal cytogenetic/FISH analysis responded to BLD. In addition, we found no correlation between high β2-microglobulin and poor outcome, suggesting that BLD might be effective in patients with adverse prognostic markers. Because of the small number of patients, the results have to be interpreted with caution and further studies are warranted to confirm our results. The 1-year OS was 93%, and the median OS was not reached at a median follow-up of 13 months (95% CI, 6-33 months). The frequent, rapid responses observed were very encouraging, especially because of the fact that these patients were heavily pretreated, including prior immunomodulatory drug therapy in 97% of the patients.
In the clinical trials MM-009 and MM-010, patients were treated with lenalidomide-dexamethasone alone, with achievement of response rates (ORR of 60%-61%) similar to the present trial. But in contrast to our study, patients enrolled in MM-009 and MM-010 were less heavily pretreated, were lenalidomide- and bortezomib-naive, and received higher doses of dexamethasone.7,8 Other trials using bendamustine in pretreated MM patients also produced high response rates. A dose escalation study of bendamustine alone, starting at 60 mg/m2 and increasing up to 100 mg/m2, showed an ORR of 55%, including MR, PR, and CR.12 Ponisch et al performed a phase 1 clinical trial testing the combination of bendamustine, prednisolone, and thalidomide for relapsed or refractory MM after autologous SCT or conventional chemotherapy.13 Using fixed doses of bendamustine (60 mg/m2) and prednisolone (100 mg) with escalating doses of thalidomide (50, 100, and 200 mg), the response rate was 86% with 14% CRs, but a direct comparison with our data is difficult because of the use of different response criteria without free light chains for response assessment.21 Further, patients in this trial were lenalidomide- and bortezomib-naive and were less heavily pretreated. Similar to our study, the major side effects were grade 3 or 4 neutropenia in 43% and grade 3 or 4 thrombocytopenia in 7% of patients. A dose escalation trial using bortezomib, dexamethasone, and bendamustine in patients with relapsed or refractory MM and who failed bortezomib and dexamethasone showed a PR rate of 57%, comparable with our trial.22 Taken together, these studies demonstrate the activity of bendamustine in MM, administered either as a single agent or in combination with other medications.
As expected and in accordance with other trials, neutropenia (62% grade 3/4) and thrombocytopenia (38% grade 3/4) were the most commonly observed adverse events in our trial. Neutropenia was manageable with administration of G-CSF, dose adjustments, or both. Patients with pre-existing low-grade myelosuppression seemed to experience more hematologic toxicity. But the occurrence of neutropenia was not associated with decreased albumin or GFR, suggesting that patients with hypoalbuminemia or impaired renal function do not have increased toxicity by BLD. This is in accordance with studies reporting that bendamustine can be given in patients with renal impairment without dose reduction.23,24 Despite the relatively high frequency of grade 3 and 4 neutropenia, only 1 patient (3%) developed neutropenic fever with this treatment combination. Therefore, the low rate of infection in our study does not support routine infection prophylaxis for relapsed MM patients treated with this regimen. Other side effects, such as diarrhea, were generally mild and could be treated with supportive therapy, such as loperamide. No thromboembolic events were documented. We did not observe unexpectedly high rates of skin rash, fatigue, or peripheral neuropathies. As the high incidence of neuropathy in patients with MM may limit the use of neurotoxic agents, the low frequency of treatment-emergent neuropathy observed in this trial makes it a potentially attractive option in patients with relapsed disease.
In conclusion, the combination of BLD is an effective treatment option frequently inducing rapid responses in patients with relapsed or refractory MM who were heavily pretreated. The high response rate in patients previously treated with lenalidomide and/or thalidomide suggests that bendamustine has no cross-resistance with those drugs. One of the most frequent side effects was myelosuppression. Based on our experience, we recommend prophylaxis with G-CSF for patients with relapsed or refractory MM regardless of baseline myelosuppression. The lack of treatment-emergent neuropathy makes this combination especially attractive for patients with pre-existing neuropathy.
Preliminary results were presented at the 53rd Annual Meeting of the American Society of Hematology, December 12, 2011, Orlando, FL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Robert Redner, Robert L. Volkin, and Frank Viverette for enrolling patients on this trial, Dr Rachel M Saunders for reviewing the manuscript, and Ms Rita Bhutta for excellent preparation and submission of the manuscript.
This work was supported by Cephalon and in part by the Pennsylvania Department of Health (PA DOH). This project used the UPCI Biostatistics Facility and was supported in part by the Cancer Center Support Grant (award P30CA047904).
Authorship
Contribution: S.L. designed the protocol and wrote the manuscript; A.O. and R.C.K. collected data and maintained records; L.D. and M. Abbas collected and analyzed data; S.L.P. and S.B. enrolled patients and collected data; M.B. helped to analyze data; G.D.R. enrolled patients and helped to design the protocol; M.Y.M. analyzed data; M. Agha, J.W., and J.A.Z. enrolled patients; and Y.S. and D.N. provided statistical support.
Conflict-of-interest disclosure: S.L. is a consultant for Celgene Corp, Onyx, and Genzyme and received research funding from Celgene. S.B. is on the Speakers Bureau for Celgene Corp and Cephalon. G.D.R. is a consultant for Amgen and Millennium Pharmaceuticals. M. Agha is a consultant for Novartis. The remaining authors declare no competing financial interests.
Correspondence: Suzanne Lentzsch, Columbia University Medical Center, Herbert Irving Pavilion, 161 Fort Washington Ave, 9th Floor, R937, New York, NY 10032; e-mail: sl3440@columbia.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal