Although natural killer (NK) T cells are present in about 1 in a 1000 T cells contained in hematopoietic cell transplants used for treatment of leukemia and lymphoma, their number is the dominant predictor of acute graft-versus-host disease (GVHD) in transplant patients according to the paper by Chaidos et al in this issue of Blood.1
The incidence of severe acute GVHD was reduced when higher levels of NKT cells were present among the infused donor cells. The level of NKT cells was the only significant predictor even when the age of the donor and recipient or their sex mismatch, content of conventional T cells or CD4+CD25hiFoxP3+ T regulatory (Treg) cells, or different conditioning regimens were taken into account. These findings point to the important role of NKT cells in suppressing GVHD that was initially described in preclinical studies.2,3 The striking conclusion of the study by Chaidos et al is that a very small number of the immune modulatory NKT cells determines whether the far more numerous conventional donor CD4+ and CD8+ T cells go on to attack the foreign target tissues of the host including the intestines and skin.1
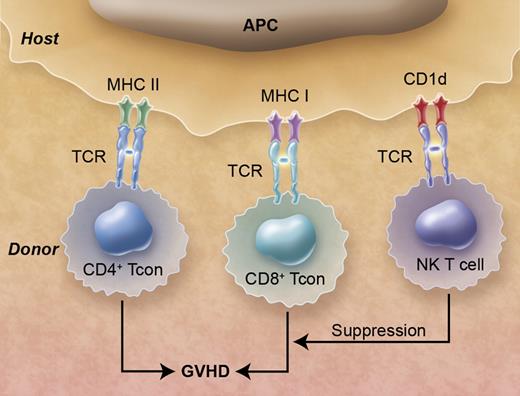
Donor natural killer (NK) T cells in hematopoietic cell transplants suppress graft-versus-host disease (GVHD) that is induced by donor CD4+ and CD8+ conventional T (Tcon) cells. The T-cell antigen receptors (TCR)s of the Tcon cells recognize class I and class II MHC receptors associated with peptides, and the NKT cells recognize CD1d receptors associated with glycolipids on the surface of antigen presenting cells (APCs). Professional illustration by Alice Y. Chen.
Donor natural killer (NK) T cells in hematopoietic cell transplants suppress graft-versus-host disease (GVHD) that is induced by donor CD4+ and CD8+ conventional T (Tcon) cells. The T-cell antigen receptors (TCR)s of the Tcon cells recognize class I and class II MHC receptors associated with peptides, and the NKT cells recognize CD1d receptors associated with glycolipids on the surface of antigen presenting cells (APCs). Professional illustration by Alice Y. Chen.
NKT cells are a class of regulatory T cells that shape and modify the immune responses of conventional T cells and can be divided into 2 main types, variant and invariant.4 The invariant NKT (iNKT) cells have several unique features. First, iNKT cells have an invariant T-cell antigen receptor (TCR) α chain that allows their identification in mice and humans by the presence of the invariant TCR by immunoflourescent staining.4 Second, the invariant TCR on the iNKT cell recognizes a nonpolymorphic antigen-presenting molecule, CD1d, associated with self or microbial glycolipids instead of recognizing highly polymorphic MHC class I and II molecules that present foreign peptides to conventional CD4+ and CD8+ T cells (see figure). Third, on activation iNKT cells can secrete large amounts of Th1 or Th2 type cytokines, depending on the antigen-presenting cells and their environment4 that can enhance or suppress immune-mediated reactions by conventional T cells. Invariant NKT cells can be further subdivided into CD4+ and CD4− (CD4−CD8−) subsets, and the study by Chaidos et al identified the donor CD4− iNKT cell in the hematopoietic cell graft as the key predictor for reduced acute GVHD.1
The first observations that donor NKT cells in a bone marrow transplant suppress GVHD were made in mouse studies reported in 1999 in which selective depletion of the CD4− iNKT cells from the marrow transplant resulted in high levels of acute GVHD, and the add-back of these cells protected against GVHD.2 Before this study, all T-cell subsets in marrow transplants were thought to contribute to GVHD. Host iNKT cells also protect against GVHD.3,5,6 The lymphoid tissues and spleen of murine marrow transplant recipients given nonmyeloablative conditioning with total lymphoid irradiation and anti-T cell antibodies become enriched for host iNKT cells and these mice were protected from GVHD after bone marrow transplantation, whereas iNKT knockout mice conditioned in the same manner died from lethal GVHD.5,6 The enrichment of iNKT cells after total lymphoid irradiation was also reported in clinical studies that showed marked protection against GVHD.3
In the preclinical models, suppressive donor and host iNKT cells produced both IFN-γ and IL-4, yet GVHD suppression was dependent on IL-4 production in both cases because experiments using IL-4 knockout donor or recipient mice died from GVHD.2,5,6 The important role of IL-4 in the suppression of GVHD by mouse iNKT cells was also reported in a recent study in which purified donor iNKT cells from the spleen were added to marrow transplants spiked with GVHD-inducing conventional T cells.7 In the murine models, the IL-4 secretion by iNKT cells promoted expansion of IL-10–secreting donor CD4+CD25+FoxP3+ T regulatory cells and polarized donor T cells toward a Th2 phenotype thereby reducing injury in the target tissues of GVHD (skin, liver, and gut).7-10 It is noteworthy that the cytokine-dependent mechanism of GVHD protection by the donor or host iNKT cells did not affect donor CD8+ T-cell cytolytic function and graft antitumor activity was preserved.9-11
The report by Chaidos et al validates in humans the finding from murine models of bone marrow transplantation that iNKT cells in the donor graft protect against GVHD. However, the mechanism of GVHD protection in humans is incompletely defined. Chaidos et al reported that in vitro assays using human donor iNKT cells inhibited the immune response of conventional T cells to alloantigens, and the CD4− iNKT cells induced cytolysis of antigen presenting cells (dendritic cells). These results point to another mechanism by which iNKT cells can suppress GVHD in addition to the cytokine-dependent pathways. However, in view of the rarity of CD4− iNKT cells in humans (less than 1 in 1000) and the high ratio of effector NKT cells to target dendritic cells required for the in vitro cytolysis assays, the cytokine pathway is more likely to contribute to GVHD protection in vivo. Although the exact mechanism(s) by which the rare iNKT cells modify the immune responses of the numerous conventional T cells is not fully defined, the report by Chaidos et al is an important clinical advance in support of the role of regulatory T cells suppressing GVHD, and will likely spark interest in developing clinical protocols in which purified donor NKT cells are infused into transplant recipients to prevent or treat GVHD.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■