Natural killer (NK) cells have gained significant attention in adoptive immunotherapy for cancer. Consequently, novel methods of clinical-grade expansion of NK cells have emerged. Subsets of NK cells express a variety of chemokine receptors. However, to expand the scope of adoptively transferred NK cell homing to various malignancies, expression of corresponding chemokine receptors on NK cells is essential. Here, we have explored the use of trogocytosis as a tool to transiently express the chemokine receptor CCR7 on expanded human NK cells with the aim to enhance their homing to lymph nodes. We generated a K562-based “donor” cell line expressing CCR7, Clone9.CCR7, to transfer CCR7 onto NK cells via trogocytosis. CCR7 expression occurred in 80% of expanded NK cells within 1 hour after coculture with Clone9.CCR7. After removal of the donor cells from the coculture, the CCR7 expression on NK cells steadily declined to baseline levels by 72 hours. The acquired CCR7 receptors mediated in vitro migration of NK cells toward CCL19 and CCL21 and increased the lymph node homing by 144% in athymic nude mice. This is the first report on exploiting trogocytosis to rapidly and transiently modify lymphocytes, without direct genetic interven-tion, for adoptive transfer.
Introduction
Natural killer (NK) cells are part of the innate arm of immune system because of their hallmark “readiness” to combat viral infections and involvement in tumor immune surveillance without prior Ag priming. However, recent insights into NK cell function have led to the understanding that these cells participate in innate as well as adaptive immune responses.1 This increases the attractiveness of NK cells as effectors in adoptive immunotherapy for cancer.
Until recently, the main limiting factor to the clinical application and efficacy of NK cells was the difficulty in obtaining sufficient cell numbers for adoptive transfer. Development of novel methods of expanding primary human NK cells ex vivo has renewed interest in NK cells for immunotherapy for cancer.2,,,–6 Expanded NK cells have enhanced expression of activating receptors,4,7,8 that in turn improves their antitumor cytotoxicity. Where activating receptors did not sufficiently elicit an antitumor response, researchers augmented the antitumor effect of NK cells by expression of chimeric Ag receptors.9,–11 Ultimately, the success of NK cell adoptive immunotherapy for cancer depends not only on target recognition but also on homing of NK cells to the tumor target in vivo. Thus, the effector cells must express the appropriate chemokine receptors.
Cancer cells express a wide array of chemokines and chemokine receptors that are instrumental in tumor survival12 and metastatic spread.13 Lymph nodes, particularly the tumor-draining nodes, are the foci of metastatic spread of tumors for a vast number of cancer types.13,14 The expression of CCR7, a member of the G protein–coupled receptor family, on lymphocytes directs their homing to lymph node, coordinates primary immune responses, and induces peripheral immune tolerance.15 CCR7 expression on tumor cells has been reported and shown to play a pivotal role in lymph node metastasis of various cancers such as breast,16 pancreatic,17 thyroid,18 and colorectal19 cancers; oral squamous cell carcinoma20 ; melanoma21 ; and lymphoma.22 Lymph node involvement in these cancers is generally associated with poor prognosis.
Peripheral NK cells express a variety of chemokine receptors such as CXCR1, CXCR3, and CXCR4 with subsets expressing CCR1, CCR4, CCR5, CCR6, CCR7, CCR9, CXCR5, and CXCR6. Expression of CCR7 on NK cells can facilitate homing to lymph nodes, which, in the context of adoptive immunotherapy for various cancers, would offer a main advantage in targeting lymph node metastases. However, CD56brightCD16− NK cells, which primarily secrete cytokines, express CCR7, but CD56dimCD16+ NK cells, which are primarily responsible for cytotoxicity, do not.23 In a previous study, we reported that expanded NK cells are predominantly of CD56+CD16bright phenotype and did not express CCR7.7
In this study, we sought to express CCR7 on expanded NK cells ex vivo to facilitate lymph node homing on adoptive transfer. Although investigators have used viral vectors to gene modify NK cell lines10,24 and primary NK cells,9,25 because of safety concerns over integrating viral vectors there has been a recent shift in emphasis toward nonviral methods of gene transfer, particularly nonintegrating, mRNA-based electroporation approaches.11 However, electroporation of NK cells has been difficult in that the transfection efficiency and viability of NK cells are low, and high-throughput electroporation methods for gene modifying clinically relevant NK cell numbers are currently lacking.
Marcenaro et al showed that NK cells could acquire CCR7 expression in vitro via transfer of membrane patches from APCs, especially mature dendritic cells.26 They suggested that this process can occur in vivo and may be involved in lymph node homing of NK cells from inflamed peripheral tissue. Uptake of membrane molecules by NK cells from their targets was first described for the transfer of human leukocyte Ag-C,27 by a process later termed as trogocytosis.28
Trogocytosis is a fast, contact-dependent uptake of membrane fragment and molecules contained therein, from “donor” cell by the “acceptor” cell, and is distinct from other mechanisms of intercellular exchanges such as nanotubes and exosomes. Trogocytosis was first described in 1973.29 Since then, it has been identified as an active process that depends on ATP, calcium mobilization, and actin cytoskeleton reorganization and is regulated by Src kinases.30 Recently, direct evidence was presented for functional incorporation of receptor molecules acquired through trogocytosis.31 Acquisition of molecules by immune cells through trogocytosis has diverse consequences such as augmented or diminished immune function and generation of immunosuppressive or regulatory cells.32,,,,,,–39 Trogocytosis can also be bidirectional as reported in the context of NK cells, resulting in tumor immune evasion.40 In addition, it can be a dynamic process that involves multiple cell types, resulting in serial transfer of Ags from donor cells to acceptor immune cells41 across innate and adaptive arms of immune systems in local and distant microenvironments. These observations have shed light on the role of trogocytosis in generating complex adaptations in immune cells and the ensuing immune plasticity beyond genetic and epigenetic programming.42,43
Although the process of trogocytosis has been well studied both in vitro and in vivo with the use of various cell types, to date there have been no reports that trogocytosis can be exploited as a tool for engineering expression of cell membrane molecules on immune cells for therapeutic applications. We proposed the use of trogocytosis to engineer CCR7 expression on expanded human NK cells for adoptive immunotherapy applications. In the present study we showed that CCR7 acquired by NK cells via trogocytosis enhances the lymph node homing of these cells on adoptive transfer to athymic nude mice. To the best of our knowledge, this is the first report to suggest and validate trogocytosis as a tool for modifying lymphocytes with chemokine receptors in place of gene transfer for the purposes of adoptive immunotherapy.
Methods
Cells and cell lines
The K562-based artificial APC line CJLCKT64.86.41BBL.CD19.MBIL21 (Clone9.mbIL21) was described previously for expansion of NK cells ex vivo.7,8 CCR7 cDNA (CCR7/pCMV-XL4) was obtained from Origene, and the coding sequence was amplified (primers listed in Table 1) and cloned into the Sleeping Beauty transposon vector pSBSO. Clone9.mbIL21 was electroporated with CCR7/pSBSO and the Sleeping Beauty transposase SB11 (pKan-CMV-SB11) at 3:1 ratio with the use of Amaxa Nucleofector I device (Nucleofector Kit V, program T16). CCR7+ cells were sorted to generate a stable CCR7 donor cell line, Clone9.mbIL21.CCR7 (Clone9.CCR7).
Anonymized normal human donor buffy coats were obtained from the Gulf Coast Regional Blood Center under protocol approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. NK cells were expanded from buffy coat–derived PBMCs as described previously.7 Briefly, PBMCs were stimulated weekly by coculturing with irradiated (100 Gy) Clone9.mbIL21 in an NK cell media (RPMI 1640 [Cellgro] supplemented with 10% FBS [Invitrogen] and 50 IU/mL IL-2 [Proleukin; Novartis Vaccines and Diagnostics]). The CD56+CD3− NK cells were purified from the expansion culture at the end of second stimulation with the use of a RosetteSep human NK cell–enrichment cocktail (StemCell Technologies). Purified NK cells were subjected to a third stimulation with irradiated Clone9.mbIL21 and cryopreserved 7 days later.
Abs and flow cytometry
Anti-CD16 PE-Cy5 (Clone 3G8), anti-CD56 PE-Cy5 (Clone B159), anti-CCR7 FITC (Clone 3D12), anti-CD19 PE-Cy5 (Clone HIB19), anti-CD64 FITC (Clone 10.1), anti-CD86 allophycocyanin (Clone 2331 FUN-1), anti-CD137L PE (Clone C65-485), and anti-CD107a PE (Clone H4A3) Abs were obtained from BD Biosciences, and anti–IL-21 PE (Clone eBio3A3-N2) Ab was obtained from eBioscience. Flow cytometric data acquisition was performed with BD FACSCalibur, and the data were analyzed with FlowJo Version 9.2 software (TreeStar Inc).
Modifying NK cells by trogocytosis
Expanded NK cells were thawed and allowed to rest overnight in NK cell culture media. Trogocytosis of CCR7 was induced by coculturing NK cells with γ-irradiated (100 Gy) Clone9.CCR7 cells, at an acceptor-to-donor cell ratio of 1:1 in a 24-well plate. The plate was spun for 2 minutes at 100g to initiate cell contact and incubated at 37°C in 5% CO2. NK cells cocultured with Clone9.mbIL21 cells served as control. The cells from the cocultures were recovered for analysis at the times indicated and pipetted to dissociate cell synapses, fixed for 15 minutes at room temperature with the use of a 1% formaldehyde solution in PBS, and stained for CD16 PE-Cy5, CD56 PE-Cy5, and CCR7 FITC. The percentage of the NK cells that acquired CCR7 was determined with the use of flow cytometry. Uptake of multiple surface receptors from Clone9.CCR7 cells by NK cells was also assessed with flow cytometry. NK cells with (cocultured with Clone9.CCR7 cells) and without (cocultured with Clone9.mbIL21 cells) acquired CCR7 are henceforth referred to as CCR7+ NK and CCR7− NK cells, respectively.
To evaluate the kinetics of persistence of acquired CCR7, NK cells from 3 healthy persons were cocultured for 1 hour as described earlier with donor cells that were subjected to freezing and thawing. Donor cells (5 × 106 cells/mL of culture medium) were frozen in a dry-ice/ethanol bath for 2 minutes and then thawed in a 37°C water bath. Optimal amount of freezing and thawing was determined when > 95% of the cells stained positive for trypan blue without significant disruption of the structural integrity of the cells. The NK cells were then separated from the donor cells by centrifugation at 400g for 20 minutes on Ficoll-Paque PLUS medium (GE Healthcare). The NK cells were returned to culture in NK cell medium as described earlier, and CCR7 expression on the cells was assessed over time.
RT-PCR analysis
Transcription of CCR7 mRNA in donor as well as NK cells was assessed with RT-PCR. Briefly, total RNA was isolated from untreated NK cells, K562 donor cell lines (Clone9.mbIL21, Clone9.CCR7), CCR7+ NK cells, and CCR7− NK cells and T cells (positive control), and cDNA was then synthesized with oligo(dT) primers with SuperScript III first-strand synthesis supermix (Invitrogen). To detect CCR7 transgene expression in donor cells, RT-PCR was performed with primers that bind within the coding region of the transcript. To detect the endogenous CCR7 transcripts in the acceptor NK cells, a different primer set was used with the reverse primer designed to bind in the 3′-untranslated region of the mRNA, which was lacking in the CCR7 transgene. The PCR products were run on a 1.2% agarose gel. The primers used in this analysis are listed in Table 1.
Fluorescence microscopy
NK cells were cocultured with Clone9.CCR7 cells for 15 minutes at 37°C, gently recovered to prevent disturbing the cell aggregates, centrifuged at 80g for 5 minutes, and fixed in a 1% formaldehyde solution. The cells were then stained with anti-CCR7 FITC, anti-CD16 PE, and anti-CD56 PE Abs. Next, the cells were transferred onto glass slides with the use of a cytospin (Cytopro 7620 cytocentrifuge; Wescor) and imaged with a Nikon Eclipse TE2000-U fluorescence microscope.
Transwell migration assay
NK cells were cocultured with freeze/thaw-treated Clone9.mbIL21 or Clone9.CCR7 cells for 1 hour at 37°C, and NK cells were separated by Ficoll-Paque centrifugation as described in “Modifying NK cells by trogocytosis.” CCR7+ and CCR7− NK cells were tested for acquired migratory properties by a transwell migration assay that used a 3-μm polycarbonate 24-well transwell chamber plate (HTS; Corning Costar). A culture medium (RPMI, supplemented with 10% FBS) containing 300 ng/mL CCL19 or CCL21 was added to the lower chamber of the transwell plate, and 100 μL of serum-free RPMI medium containing 5 × 105 CCR7+ or CCR7− NK cells was added to the upper chamber; the plate was then incubated for 6 hours at 37°C in 5% CO2. Triplicate wells were set up for each cytokine condition, and triplicate cell counts were performed per well. NK cells expanded from 4 different persons were used in the study. The CCR7+ and CCR7− NK cells that migrated to the lower chamber were counted with a hemocytometer. Data are represented as percentage of migration based on original cell input.
In vivo lymph node homing of NK cells
Male athymic nude mice (B6.Cg-Fox1nu/J; 4-6 weeks old) were obtained from the Department of Experimental Radiation Oncology at MD Anderson and housed in individually ventilated barrier cages and given sterilized alfalfa-free diet. For adoptive transfer, NK cells were stained with 50μM XenoLight DiR membrane dye (Caliper Life Sciences) for 15 minutes, washed, and subjected to coculture with γ-irradiated Clone9.mbIL21 or Clone9.CCR7 cells for 1 hour. The NK cells were recovered and resuspended in PBS at 10 × 106 cells per 200 μL. Next, 10 × 106 CCR7+ or CCR7− NK cells were injected intravenously in 5 mice each. Twenty-four hours after adoptive transfer, the mice were anesthetized with isoflurane and killed by cervical dislocation, and peripheral lymph nodes (inguinal, axillary, and cervical) were dissected from both the left and right flanks. NK cell homing to the lymph nodes in the mice was assessed with fluorescence imaging of the resected lymph nodes with the use of Xenogen IVIS Spectrum (Caliper Life Sciences), equipped with 710/760 nanometer excitation-emission filter set. Uninjected mice served as controls. All animal experiments were performed in accordance to the protocols approved by the MD Anderson Office of Research Administration and Institutional Animal Care and Use Committee.
Statistical analysis
Statistical analyses were performed with GraphPad Prism Version 5.0 software with the use of nonparametric, Wilcoxon test. Correlation analysis was done with the nonlinear curve fit method, and significance of correlation was assessed by the Pearson test. P values < .05 were considered statistically significant.
Results
Expanded human NK cells acquire CCR7 from K562-based donor cells via trogocytosis
We genetically modified the Clone9.mbIL21 cell line to express CCR7 with the use of Sleeping Beauty transposon/transposase vectors (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and generated the Clone9.CCR7 cell line by sorting for CCR7+ cells 2 weeks after electroporation. After 1 month of culture, we confirmed that the Clone9.CCR7 cell line had stable expression of CCR7 (supplemental Figure 1B).
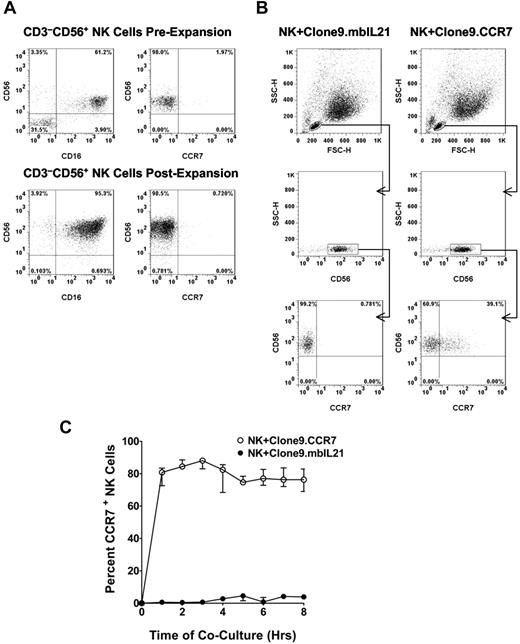
We generated the NK cells used in the study by expanding buffy coat–derived PBMCs on Clone9.mbIL21 cells as we described previously.7 This method yielded an average 21 000-fold expansion of NK cells over 3 weeks with a CD3−CD56+ NK cell purity of 99%. The buffy coat–derived NK cells lacked the expression of CCR7 before and after expansion (Figure 1A).
Analysis of CCR7 uptake by NK cells. (A) A representative flow cytometric analysis of CD56, CD16, and CCR7 expression on buffy coat–derived CD3−CD56+ NK cells before and after expansion. (B) Flow cytometric analysis of CCR7 acquisition by NK cells after 30 minutes of coculture with Clone9.CCR7 cells compared with Clone9.mbIL21 cells. NK cells were initially discriminated from the K562 cells on the basis of the forward (FSC-H) and side (SSC-H) scatter properties and further gated on CD56+ NK cells to ensure analysis of CCR7 in NK cell population. (C) Kinetics of CCR7 uptake by NK cells from irradiated Clone9.CCR7 cells as analyzed with flow cytometry. The uptake of CCR7 was analyzed hourly for ≤ 8 hours compared with NK cells cocultured with Clone9.mbIL21. The data are presented as the median ± range for 3 unrelated NK cell donors.
Analysis of CCR7 uptake by NK cells. (A) A representative flow cytometric analysis of CD56, CD16, and CCR7 expression on buffy coat–derived CD3−CD56+ NK cells before and after expansion. (B) Flow cytometric analysis of CCR7 acquisition by NK cells after 30 minutes of coculture with Clone9.CCR7 cells compared with Clone9.mbIL21 cells. NK cells were initially discriminated from the K562 cells on the basis of the forward (FSC-H) and side (SSC-H) scatter properties and further gated on CD56+ NK cells to ensure analysis of CCR7 in NK cell population. (C) Kinetics of CCR7 uptake by NK cells from irradiated Clone9.CCR7 cells as analyzed with flow cytometry. The uptake of CCR7 was analyzed hourly for ≤ 8 hours compared with NK cells cocultured with Clone9.mbIL21. The data are presented as the median ± range for 3 unrelated NK cell donors.
To assess the feasibility of CCR7 uptake by expanded NK cells, we cocultured expanded NK cells with live Clone9.mbIL21 cells or Clone9.CCR7 donor cells. Flow cytometric analysis after 30 minutes of coculture showed that ∼ 40% of the NK cells had surface expression of CCR7 when cocultured with Clone9.CCR7 cells (Figure 1B), whereas those NK cells cocultured with Clone9.mbIL21 cells lacked CCR7 expression.
We further evaluated the kinetics of CCR7 uptake by NK cells from irradiated Clone9.mbIL21 or Clone9.CCR7 cells as a potential clinically applicable approach and, again, observed uptake of CCR7 by NK cells. By 1 hour of coculture, a median of 80% of NK cells were positive for CCR7 (Figure 1C). The uptake peaked by 3 hours, followed by a marginal decrease and stabilization at a median of 76% of NK cells ≤ 8 hours. No expression of CCR7 on NK cells cocultured with Clone9.mbIL21 was observed during this period. These data suggested that 1-3 hours of NK cell coculture with Clone9.CCR7 was optimal for achieving maximal uptake of CCR7 by expanded NK cells in our system. In subsequent experiments, we used 1 hour as the standard coincubation period.
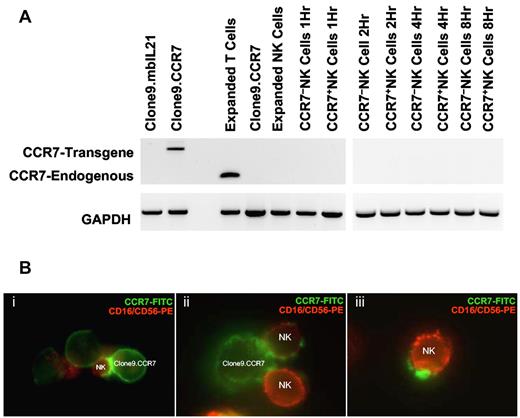
To exclude induction of gene expression, we assessed for onset of endogenous CCR7 transcription in NK cells after coculture with K562 donor cells with the use of RT-PCR. Because of potential contamination of donor cell debris in NK cells separated from the cocultures by Ficoll-Paque centrifugation, we designed 2 sets of primers to distinguish between transgene and endogenous CCR7 transcripts (Table 1). mRNA derived from T cells served as a positive control for the endogenous CCR7 transcript. Although CCR7+ NK cells had surface expression of CCR7, expanded NK cells, CCR7− NK cells, and CCR7+ NK cells all lacked transcript for endogenous CCR7 over the entire 8-hour period of coculture (Figure 2A), providing evidence that onset of endogenous gene transcription was not the mechanism for CCR7 expression on CCR7+ NK cells.
CCR7 expression on NK cells is because of membrane uptake from donor cells rather than expression of the endogenous CCR7 gene. (A) RT-PCR analysis of CCR7 expression in NK cells. The donor cells Clone9.CCR7 were positive for CCR7 transgene mRNA expression and served as negative control for endogenous CCR7 transcript, and T cells served as positive control. CCR7+ NK and CCR7− NK cells both lacked the expression of endogenous CCR7 transcript over the entire 8-hour period of coculture. GAPDH was used as control for quality of cDNA synthesis. (B) Fluorescence microscopic analysis of CCR7 uptake. After coculture of NK and Clone9.CCR7 cells for 15 minutes, the cells were stained with an anti-CCR7 FITC Ab (Clone9.CCR7 cells, in green) and with anti-CD16 and anti-CD56 PE Abs (NK cells, in red), spun onto glass slides with the use of cytospin and mounted slides using Vectashield mounting media, and visualized CCR7 uptake by NK cells with the use of a fluorescence microscope (Nikon Eclipse TE2000-U) equipped with Coolsnap HQ camera/Roper Scientific photometries and Metamorph image acquisition software.
CCR7 expression on NK cells is because of membrane uptake from donor cells rather than expression of the endogenous CCR7 gene. (A) RT-PCR analysis of CCR7 expression in NK cells. The donor cells Clone9.CCR7 were positive for CCR7 transgene mRNA expression and served as negative control for endogenous CCR7 transcript, and T cells served as positive control. CCR7+ NK and CCR7− NK cells both lacked the expression of endogenous CCR7 transcript over the entire 8-hour period of coculture. GAPDH was used as control for quality of cDNA synthesis. (B) Fluorescence microscopic analysis of CCR7 uptake. After coculture of NK and Clone9.CCR7 cells for 15 minutes, the cells were stained with an anti-CCR7 FITC Ab (Clone9.CCR7 cells, in green) and with anti-CD16 and anti-CD56 PE Abs (NK cells, in red), spun onto glass slides with the use of cytospin and mounted slides using Vectashield mounting media, and visualized CCR7 uptake by NK cells with the use of a fluorescence microscope (Nikon Eclipse TE2000-U) equipped with Coolsnap HQ camera/Roper Scientific photometries and Metamorph image acquisition software.
To investigate whether trogocytosis was the mechanism of CCR7 uptake by NK cells, we imaged NK cells 15 minutes after coculture with Clone9.CCR7 cells with the use of fluorescence microscopy. We observed 3 stages of CCR7 acquisition by NK cells. First, CCR7 expression on NK cells was found around the synapse with the donor cells, potentially representing an early stage of CCR7 uptake. Second, we observed that patches of acquired CCR7 spread across the NK cell surface, and, third, NK cells that acquired CCR7 on their surfaces had detached from the donor cells (Figure 2B). These data confirmed that CCR7 is indeed acquired from donor cells via trogocytosis.
Persistence of acquired CCR7 expression on NK cells
To determine the kinetics of loss of acquired CCR7 receptors from NK cells, we cocultured NK cells with freeze/thaw-treated Clone9.mbIL21 and Clone9.CCR7 cells to facilitate Ficoll-Paque separation of NK cells. A single cycle of freeze/thaw of K562-based donor cells at a cell density of 5 × 106/mL of RPMI culture medium yielded cells with compromised membranes that stained for trypan blue but maintained cell integrity as shown in supplemental Figure 2Ai. Further freezing and thawing cycles resulted in complete cell lysis and generation of cell debris (supplemental Figure 2Aii-iii). We observed that NK cells were able to extract CCR7 from Clone9.CCR7 cells subjected to a single freezing/thawing cycle although at a lower level than from live Clone9.CCR7 cells (supplemental Figure 2B). Donor cells treated with further freeze/thaw cycles did not support uptake of CCR7 by trogocytosis (data not shown). To understand the reason for diminished trogocytosis we assessed whether trogocytosis depended on NK cell lysis of the targets. We stained NK cells for CCR7 and CD107a, a marker for degranulation, after coculture with live and freeze/thawed donor Clone9.CCR7 cells (supplemental Figure 2C). Equal proportions of NK cells cocultured with live donor cells were dual positive for CCR7 and CD107a (1:1 ratio). After freeze/thaw treatment there was a decrease in overall trogocytosis and degranulation by NK cells and increase in CCR7−CD107a− cell population (supplemental Figure 2D). However, we noted that the percentage of CCR7+ NK cells remained higher than CD107a+ NK cells, and the ratio shifted to 1:0.58 with a median of 31% NK cells that are CCR7+CD107a−. These data suggest that trogocytosis in this system is not absolutely dependent on donor cell lysis, in agreement with previous findings.26 The data however show that membrane integrity of target cells may play an important role in NK cell engagement, resulting in both degranulation and trogocytosis.
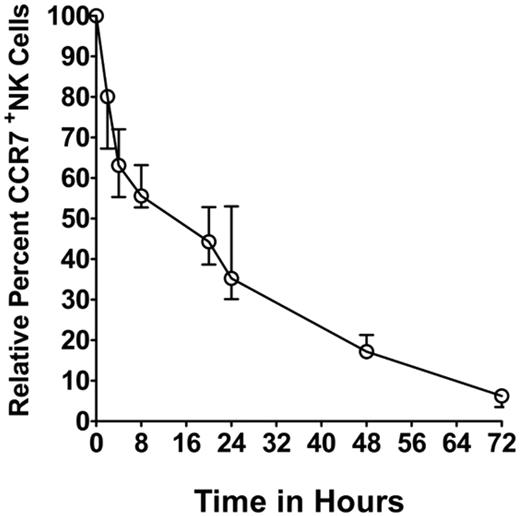
To assess the kinetics of loss of acquired receptors, we separated NK cells from the coculture by Ficoll-Paque separation (designated as time = 0 hour) and assessed for CCR7 expression by flow cytometry over the next 72 hours. Relative to CCR7 expression at 0 hour, we found a median of 20% reduction in CCR7+ NK cells over the first 2 hours. Subsequent loss was more gradual, with 45.0% reduction at 8 hours (Figure 3), 65% at 24 hours, and 83% at 48 hours. CCR7 expression on NK cells eventually fell to background levels by 72 hours.
Persistence of acquired CCR7 receptors on NK cells. Kinetics of persistence of acquired CCR7 receptors (after Ficoll-Paque separation) from 3 independent experiments performed with different donor-derived NK cells. The percentage of CCR7+ NK cells was adjusted to 100% at time 0 hour, and the subsequent loss of acquired CCR7 relative to 0 hour was assessed over 72 hours. NK cells cocultured with Clone9.mbIL21 cells were used as negative controls and for background correction. The data are presented as median ± range (n = 3).
Persistence of acquired CCR7 receptors on NK cells. Kinetics of persistence of acquired CCR7 receptors (after Ficoll-Paque separation) from 3 independent experiments performed with different donor-derived NK cells. The percentage of CCR7+ NK cells was adjusted to 100% at time 0 hour, and the subsequent loss of acquired CCR7 relative to 0 hour was assessed over 72 hours. NK cells cocultured with Clone9.mbIL21 cells were used as negative controls and for background correction. The data are presented as median ± range (n = 3).
Acquired CCR7 expression confers migratory properties to NK cells in vitro
We assessed the functional integration of acquired CCR7 in NK cells by studying their migration potential toward chemokine ligands CCL19 and CCL21 with the use of the transwell migration assay. We separated NK cells cocultured with freeze/thaw-treated Clone9.mbIL21 or Clone9.CCR7 cells on Ficoll-Paque and assessed them for CCR7 uptake before setting up the migration assay. As shown in Figure 4, a median of 5% and 5.5%, of the CCR7− NK cells exhibited spontaneous migration toward CCL19 and CCL21, respectively, whereas a median of 33.3% and 35% of CCR7+ NK cells migrated toward CCL19 and CCL21, respectively. This 6-fold increase in migration (P < .0001) suggests that the CCR7 receptors acquired by NK cells were functional at conferring migratory property.
Transwell migration assay. The functional incorporation of the acquired CCR7 receptors on NK cells was assessed with transwell migration assay. The percentage of migration was assessed by the formula (no. of cells that migrated to the lower chamber/no. of cells seeded in the upper chamber) × 100. The data were acquired by setting up triplicate wells per chemokine condition, and triplicate counts were performed per each well. NK cells obtained from 4 different donors were used in the analysis (n = 4). The statistical significance was assessed with a nonparametric Wilcoxon test, and the data are presented as median ± range.
Transwell migration assay. The functional incorporation of the acquired CCR7 receptors on NK cells was assessed with transwell migration assay. The percentage of migration was assessed by the formula (no. of cells that migrated to the lower chamber/no. of cells seeded in the upper chamber) × 100. The data were acquired by setting up triplicate wells per chemokine condition, and triplicate counts were performed per each well. NK cells obtained from 4 different donors were used in the analysis (n = 4). The statistical significance was assessed with a nonparametric Wilcoxon test, and the data are presented as median ± range.
Acquired CCR7 aids homing of adoptively transferred NK cells to lymph nodes in mice
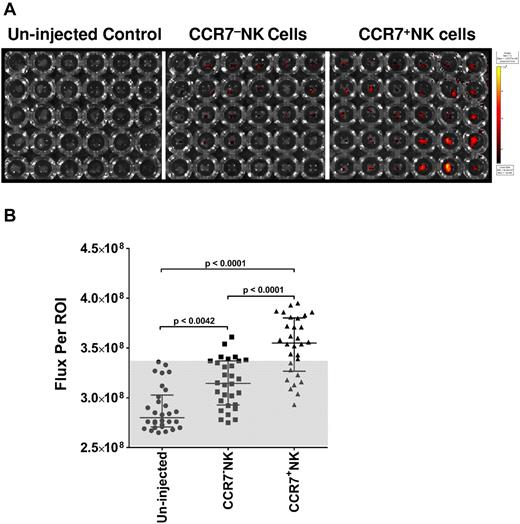
To assess the practical feasibility of using trogocytosis for adoptive immunotherapy we studied lymph node homing of NK cells with acquired CCR7 in athymic nude mice. Expanded NK cells, prestained with fluorescent lipophilic dye XenoLight DiR, were cocultured with irradiated Clone9.mbIL21 or Clone9.CCR7 cells. We adoptively transferred the CCR7+ NK cells or CCR7− NK cells to athymic nude mice via the intravenous route. First, we assessed the cumulative fluorescence signals from the live mice immediately after adoptive transfer to ensure uniform administration (supplemental Figure 3A) and found no statistical difference in the intensity of fluorescence signal Flux (photons/second) between the 2 groups (supplemental Figure 3B). The uninjected group served as control for background fluorescence. Twenty-four hours after the adoptive transfer of NK cells we killed the mice by cervical dislocation after isoflurane anesthesia and resected from each group (n = 5) inguinal, axillary, and superficial cervical lymph nodes from left and right flanks and imaged the nodes (30 nodes per group) in a 96-well plate with the use of Xenogen IVIS Spectrum (Figure 5A). Figure 5B shows that CCR7+ NK cells migrated to lymph nodes in vivo at a significantly higher rate than did CCR7− NK cells. The mean percentage of increase in fluorescence signal from NK cells in the lymph nodes of mice that received CCR7+ NK cells was 144% greater than those mice that received control CCR7− NK cells and was calculated with the formula (flux CCR7+ NK − flux CCR7− NK)/(flux CCR7− NK − flux uninjected) × 100.
Effect of acquired CCR7 on homing of NK cells to peripheral lymph nodes. (A) Xenogen IVIS Spectrum fluorescence image of XenoLight DiR-labeled NK cells that homed to peripheral lymph nodes 24 hours after intravenous administration. Individual lymph nodes resected from either the uninjected group or the group injected with XenoLight DiR-labeled CCR7− or CCR7+ NK cells were placed in a 96-well plate; the columns of the plate represent right and left inguinal, axillary, and superficial cervical lymph nodes; and the rows represent individual animals. (B) Assessment of flux per region of interest drawn around each well that contained a single lymph node. Statistical analysis was performed with a nonparametric Wilcoxon test, and data are presented as median ± interquartile range (n = 30). The gray area depicts the range of flux because of autofluorescence.
Effect of acquired CCR7 on homing of NK cells to peripheral lymph nodes. (A) Xenogen IVIS Spectrum fluorescence image of XenoLight DiR-labeled NK cells that homed to peripheral lymph nodes 24 hours after intravenous administration. Individual lymph nodes resected from either the uninjected group or the group injected with XenoLight DiR-labeled CCR7− or CCR7+ NK cells were placed in a 96-well plate; the columns of the plate represent right and left inguinal, axillary, and superficial cervical lymph nodes; and the rows represent individual animals. (B) Assessment of flux per region of interest drawn around each well that contained a single lymph node. Statistical analysis was performed with a nonparametric Wilcoxon test, and data are presented as median ± interquartile range (n = 30). The gray area depicts the range of flux because of autofluorescence.
Multiple receptors are acquired simultaneously by NK cells from a single donor-cell population
Trogocytosis is the process of transfer of membrane patches and not individual receptors, so to demonstrate the robustness and scope of application of trogocytosis in NK cell modification for adoptive immunotherapy we sought to determine whether NK cells simultaneously take up multiple membrane-bound receptors. In this experiment, we cocultured NK cells with parental K562 (control) and Clone9.CCR7 for 30 minutes. The Clone9.CCR7 line had been genetically modified to express wild-type CD64, CD86, CD134L,44 truncated CD19 (tCD19) Ag, and membrane-bound IL-21 to support NK- and T-cell expansion in vitro. As expected, NK cells cocultured with parental K562 cells did not gain expression of any of the transgenes (tCD19, CD64, CD86, CD134L, mbIL21, and CCR7), whereas NK cells cultured with Clone9.CCR7 cells exhibited uptake of all these molecules (Figure 6A). The acquisition of each molecule by NK cells correlated with the expression of the molecule on the donor cells, as measured by mean fluorescence intensity (Figure 6B-C). Expression of endogenously expressed CD16 and CD56 remained unaltered during the coculture period (Figure 6D). We assessed the kinetics of loss of acquired wild-type receptors CD64, CD86, and CD137L. The decline in NK cells positive for acquired receptors to a median of 50% (t½), relative to time 0 hour, was most rapid for CD64 (∼ 4 hours), intermediate for CD137L (∼ 8 hours), and slowest for CD86 (∼ 24 hours; Figure 6E). These data show that receptors acquired through trogocytosis may decay at varying rates.
Correlation of uptake of multiple receptors by NK cells with expression of the receptors on donor cells. (A) Flow cytometric analysis of NK cells after 30 minutes of coculture with parental K562 (black histogram) and clone9.CCR7 (unfilled histogram) cells. NK cells acquired CCR7, mbIL21, CD137L, CD64, truncated CD19, and CD86 transgenes that were expressed on Clone9.CCR7 cells.(B) Transgene expression on parental K562 (black histogram) and Clone9.CCR7 (unfilled histogram) cells. (C) Nonlinear regression (curve fit) analysis of the correlation between the mean fluorescence intensity (MFI) of acquired receptors on NK cells and their corresponding MFI on donor cells. The correlation as calculated by Pearson test was statistically significant (P < .0034). (D) Expression of CD16 and CD56 in NK cells before (black histogram) and after (unfilled solid histogram) coculture with parental K562 or Clone9.CCR7 cells (unfilled dotted histogram). (E) Kinetics of loss of CD64, CD86, and CD137L from NK cells derived from 3 different persons (n = 3). The data are represented as median ± range.
Correlation of uptake of multiple receptors by NK cells with expression of the receptors on donor cells. (A) Flow cytometric analysis of NK cells after 30 minutes of coculture with parental K562 (black histogram) and clone9.CCR7 (unfilled histogram) cells. NK cells acquired CCR7, mbIL21, CD137L, CD64, truncated CD19, and CD86 transgenes that were expressed on Clone9.CCR7 cells.(B) Transgene expression on parental K562 (black histogram) and Clone9.CCR7 (unfilled histogram) cells. (C) Nonlinear regression (curve fit) analysis of the correlation between the mean fluorescence intensity (MFI) of acquired receptors on NK cells and their corresponding MFI on donor cells. The correlation as calculated by Pearson test was statistically significant (P < .0034). (D) Expression of CD16 and CD56 in NK cells before (black histogram) and after (unfilled solid histogram) coculture with parental K562 or Clone9.CCR7 cells (unfilled dotted histogram). (E) Kinetics of loss of CD64, CD86, and CD137L from NK cells derived from 3 different persons (n = 3). The data are represented as median ± range.
Discussion
In this study, we proposed using trogocytosis to modify NK cells in place of gene transfer and showed the feasibility of this approach in conferring to NK cells CCR7-mediated ability to migrate to lymph nodes after adoptive transfer. We also showed the robustness of trogocytosis by simultaneously transferring multiple receptors to NK cells.
Because of the importance of chemokines and chemokine receptors in cancer progression and lymphocyte migration to tumors, we seek to improve homing of adoptively transferred NK cells to tumors or sites of tumor metastasis in vivo. In the present study, we used CCR7 as a generic chemokine receptor model that is relevant to many tumor types that metastasize to lymph nodes driven by their expression of CCR7 or CXCR4.14,45 We propose that expression of CCR7 on adoptively transferred NK cells will facilitate targeting of such lymph node metastasis by improving NK cell localization with the tumors. Although treatment of NK cells with IL-18 has been shown to induce CCR7 expression, it does so only in ∼ 30% of NK cells,46 and the current approaches to genetic modification of NK cells have limitations, so we evaluated trogocytosis as a means to express CCR7 on expanded human NK cells.
The K562 cell line is a classic NK cell target because of its expression of NK cell–activating ligands and lack of major histocompatibility complex class I expression. In addition, K562 is an established feeder cell for expansion of NK cells and is now being used in human clinical trials. Here, we showed that K562 cells can function as donors for trogocytosis of CCR7 by expanded NK cells. The rapid onset of CCR7 expression on expanded NK cells after coculture with Clone9.CCR7 cells and its absence on NK cells cocultured with Clone.mbIL21 lacking CCR7 indicated an exogenous acquisition mechanism. This was confirmed by RT-PCR showing that NK cells did not express mRNA transcripts for CCR7. In addition, we observed that the uptake process occurred at the synapse, using fluorescence imaging. Taken together, these data strongly support that trogocytosis of CCR7 from Clone9.CCR7 is the mechanism of CCR7 expression on NK cells in this system. Others have shown that trogocytosis may occur in NK cells activated with IL-2 alone35 ; IL-2 and PHA26 ; IL-2, PHA, and irradiated peripheral blood lymphocytes as feeders30 ; and IL-2 with irradiated 721.221 as feeders,27 but much less so in resting NK cells,30,35 establishing trogocytosis as a generalized function of activated NK cells.
Herein, we also showed for the first time that NK cells can capture receptors from dead cells and functionally incorporate the acquired receptors. We treated the donor cells with freeze/thaw to facilitate separation of NK cells on Ficoll-Paque so that continued uptake of CCR7 by NK cells did not occur and interfere with our evaluation of persistence of acquired CCR7 receptors. The expression of acquired CCR7 on NK cells was transient; however, it lasted longer (≤ 48 hours) than previously reported.26 This duration of expression was sufficient for adoptively transferred cells to migrate to the target tissues in vivo. NK cells can simultaneously take up multiple receptors, and the kinetics of loss of these acquired receptors are varied and may depend on such factors as amount of receptor uptake, recycling, degradation, and the rate of NK cell division.
Binding of chemokine ligands to CCR7 initiates signal transduction via phosphorylation of the MAPK family of proteins, ERK1/2 and p38 and the PI3K/Akt pathway,47,48 which are also involved in the signaling cascade of NK cell–activating receptors. Because coculture of NK cells with K562 cells engages NK cell–activating receptors during trogocytosis, we relied on functional assays of in vitro and in vivo migration to assess CCR7 signaling in NK cells. To evaluate in vivo homing of NK cells to lymph nodes, we chose the clinically relevant approach of intravenous cell injection and observed a significant (144%) increase in lymph node homing of CCR7+ NK cells compared with CCR7− NK cells. These data validate the feasibility of using trogocytosis to modify NK cells in place of gene transfer for adoptive immunotherapy.
Given the current regulatory, funding, and patient community concerns about the clinical safety of infusing genetically modified effector cells, we propose the use of this approach as an alternative to modifying lymphocytes before adoptive transfer. This relatively simple coculture method builds on an established platform of T- and NK-cell expansion and can easily be scaled up to modify large numbers of NK cells and support uptake of large, multiple, multiple subunit proteins with relative ease with the use of a single donor cell line. The data presented in this report also confirm that the acquired receptor density on NK cells can be regulated as needed by selecting for K562-based donor cells with corresponding expression levels of the desired target receptor(s).
The transitory nature of this approach may be appealing for certain clinical applications in which the acquired receptors affect fast responses such as homing to target tissues, or in cases where prolonged persistence may yield undesired off-target effects. However, the limitation of this method becomes apparent when long-term expression of the acquired molecule is essential to achieve therapeutic efficacy such as expression of chimeric Ag receptors. Nevertheless, in the context of NK cells, transient persistence of acquired receptors may not be a significant disadvantage, considering that adoptively transferred NK cells may have limited lifespan49 of ∼ 10-12 days after infusion even with cytokine support.50 It may be possible to administer multiple infusions of expanded NK cells modified via trogocytosis to overcome the limitations of receptor persistence. Because trogocytosis involves transfer of membrane fragments, this method is restricted to developing therapeutic approaches that exploit modifying lymphocytes with membrane-bound molecules. The use of trogocytosis as described in this study has broad applications in modifying other lymphocytes that participate in uptake of membrane patches from target cells via trogocytosis.
On the basis of our data in this study, we propose trogocytosis as a tool to transiently modify expanded human NK cells with chemokine receptors and other membrane-bound molecules without using gene transfer for adoptive immunotherapy for cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Perry Hackett for use of his Sleeping Beauty vectors, and Simon Olivares for his vector modifications.
This work was supported by the MD Anderson Physician Scientist Training Program, St Baldrick's Foundation, the Sunbeam Foundation, the Farrah Fawcett Foundation, and MD Anderson's Cancer Center Support (grant CA016672).
Authorship
Contribution: S.S.S. designed and performed the experiments and wrote the paper, A.S. designed and performed the experiments, D.A.L. offered critical reviews of the data and wrote the paper, and L.J.N.C. offered critical reviews of the data and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Srinivas S. Somanchi, Division of Pediatrics, Unit 853, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: sssomanchi@mdanderson.org.