Abstract
To better understand cellular basis of hemophilia, cell types capable of producing FVIII need to be identified. We determined whether bone marrow (BM)–derived cells would produce cells capable of synthesizing and releasing FVIII by transplanting healthy mouse BM into hemophilia A mice. To track donor-derived cells, we used genetic reporters. Use of multiple coagulation assays demonstrated whether FVIII produced by discrete cell populations would correct hemophilia A. We found that animals receiving healthy BM cells survived bleeding challenge with correction of hemophilia, although donor BM-derived hepatocytes or endothelial cells were extremely rare, and these cells did not account for therapeutic benefits. By contrast, donor BM-derived mononuclear and mesenchymal stromal cells were more abundant and expressed FVIII mRNA as well as FVIII protein. Moreover, injection of healthy mouse Kupffer cells (liver macrophage/mononuclear cells), which predominantly originate from BM, or of healthy BM-derived mesenchymal stromal cells, protected hemophilia A mice from bleeding challenge with appearance of FVIII in blood. Therefore, BM transplantation corrected hemophilia A through donor-derived mononuclear cells and mesenchymal stromal cells. These insights into FVIII synthesis and production in alternative cell types will advance studies of pathophysiological mechanisms and therapeutic development in hemophilia A.
Introduction
Hemophilia A is characterized by inability to clot blood because of FVIII gene mutations and deficiency of this coagulation factor.1 The potential for cell and gene therapy in hemophilia A is highly attractive because even small amounts of FVIII may substantially decrease bleeding risk. This requires sound knowledge of cell types capable of replacing FVIII, especially within proximity of von Willebrand factor (vWF), which protects FVIII from degradation.2 However, the cell-type origin of FVIII has been controversial.3 Correction of hemophilia after orthotopic liver transplantation (OLT) but not after kidney transplantation, despite FVIII mRNA expression in both organs,3,4 indicated that liver was a major site for FVIII production. Recently, the cell transplantation approach established that of various liver cell types, liver sinusoidal endothelial cells (LSECs) replaced FVIII in hemophilia mice.5,6 Nonetheless, extrahepatic organs probably contributed in FVIII production, as indicated by FVIII synthesis in spleen, lungs, or pancreatic islets.6-8 This was in agreement with lack of plasma FVIII deficiency after OLT with donor liver from dogs or people with hemophilia A because such donor liver cells would not have synthesized or secreted FVIII.9,10 Therefore, whether nonendothelial cells, and cells in extrahepatic organs, could also produce FVIII was not excluded. For instance, macrophages, which originate in bone marrow (BM), and contained FVIII mRNA,11 could be such a candidate. Although FVIII was cloned from T cells,12 and transplantation of lymphatic tissue was thought to correct hemophilia in dogs,13 whether lymphocytes did express FVIII was uncertain, because correction of hemophilia by transplanted organs (eg, spleen) included other cell types.7,14,15
Because BM cells may generate multiple lineages, the potential of BM-derived cells in FVIII production seemed relevant to us. Previously, BM transplantation studies in hemophilia A, 40 years ago, were limited to just 3 dogs and 2 persons.16-19 BM transplantation in hemophilia dogs was carefully performed, although there were limitations, such as suboptimal allograft tolerance, lack of studies showing engraftment and generation of various BM cell types, absence of FVIII expression analysis in donor BM-derived cells, and other issues.16,17 For instance, low plasma FVIII activity levels, up to 8% of normal, were observed, but could not be differentiated from untreated controls. One dog died of transplant-related complications after 34 days,16 another died of bleeding after BM aspiration, which suggests that FVIII activity was not corrected to high levels, but 1 dog remained healthy for > 2 years.17 Despite the impact of these dog studies on the field, whether FVIII could be replaced by cell types originating from donor BM was unresolved, that is, through production of endothelial cells,20 which could potentially have arisen from the shared hematopoietic and endothelial stem cell, the hemangioblast.21
More recently, availability of hemophilia mice lacking FVIII activity with mortality after bleeding challenge and development of sensitive FVIII assays offered robust alternative opportunities to establish the value of BM transplantation.5,6,22 Therapeutic correction in hemophilia required more than rare endothelial cells, because replacement of 5%-10% of LSECs was necessary in hemophilia A mice.5 Here, we discovered transplanted BM in mice did not produce endothelial cells, yet healthy donor BM-derived mononuclear cells (MNCs), macrophages, and mesenchymal stromal cells (MSCs) expressed FVIII, and corrected hemophilia.
Methods
Animals
The Animal Care and Use Committees of Albert Einstein College of Medicine and University of Piemonte Orientale approved studies. Donor mice were: C57BL/6-Gt(ROSA)26Sor/J; C57BL/6-Tg(ACTbEGFP)1Osb/J; B6.SJL-PTPRCPEP/BOY (CD45.1+); and TgN(Tie2GFP)287Sato/J (The Jackson Laboratory). C57BL/6 and FVB/N mice were from the National Cancer Institute. Hemophilia A mice were crossed into C57BL/6 background.22 Methods to harvest BM cells are given in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cells
Nonparenchymal liver cells were isolated as described.23 RBCs were lysed for 5 minutes on ice. Kupffer cells (KCs) were selected by anti-CD11b, hematopoietic cells were removed by anti-CD45, and LSECs were isolated by immunomagnetic sorting (Miltenyi Biotec; supplemental Methods).
Mouse MSCs
Cells were from humeri, tibiae, and femurs of 18- to 21-day-old mice as described,24 followed by flow cytometric characterization (supplemental Methods). Cells were cultured in α-minimum essential medium (MEM) with 10% serum. No FVIII or vWF was added to medium. Moreover, testing of medium used to culture MSCs by Coatest assay excluded FVIII activity.
Human cells
RNAs from total BM and BM fractions, human BM cells and MSCs were purchased (StemCell Technologies). Cord blood was from the New York Blood Center. Studies with human cells were approved by the Einstein Committee on Clinical Investigations. Human BM–derived MSCs were cultured at 37°C in 5% CO2 for up to 7 passages in Mesencult MSC basal medium plus mesenchymal stem cell stimulatory supplements (StemCell Technologies).
BM replacement
Flow cytometry used phycoerythrin (PE)–conjugated anti–mouse antibody for CD45.1 (Pharmingen, BD Biosciences), fluorescein di (β-D-galactopyranoside [FDG]; Sigma Chemical) staining for β-galactosidase (LacZ), or native green fluorescent protein (GFP) fluorescence.
Donor-derived cells in tissues
Immunostaining was performed for LacZ, GFP, and other antigens (supplemental Methods).
DNA PCR and RT-PCR
Vimentin and nestin staining
Mouse MSCs were immunostained at passage 3-4. Cells were fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) pH 7.4, for 10 minutes and permeabilized with 0.5% Tween 20 for 10 minutes at 4°C. After blocking with goat serum in PBS, vimentin antibody (1:200, V2122-10C; United States Biologic) or nestin antibody (1:200, Rat-401; Millipore) was applied for 1 hour at room temperature (RT). Cells were washed in PBS for 20 minutes and then secondary antibody was applied (1:500, goat anti–mouse Alexa Fluor 488 or 546; Molecular Probes, Invitrogen).
FVIII staining
LSECs and KCs were cultured for 24 hours on collagen-coated cover slips in M199 (LSECs) or IMDM (KCs) with 10% FBS and fixed in 4% PFA. BM-MSCs were cultured on cover slips (human cells in medium as previously described; mouse cells in α-MEM with 10% serum for 2 days and fixed in 4% PFA. Cells were stained with rabbit anti-FVIII (1:100, ab53703; Abcam), rat anti-FVIII (1:100, ab61390, Abcam), or mouse anti-FVIII (1:200, GM 8015; Green Mountain Antibodies) for 2 hours at RT. After washing in PBS, Alexa Fluor 488– or 546–conjugated goat anti–rabbit, anti–rat, or anti–mouse immunoglobulin (Ig)G (1:500; Molecular Probes) was added for 1 hour. Nuclei were stained with DAPI-Antifade (Molecular Probes). Specificity of FVIII antibodies was established by immunostaining of recombinant mouse B domain–deleted FVIII (from D. Sabatino, University of Pennsylvania) expressed by lentiviral vector in hemophilia A mouse fibroblasts without background FVIII expression (supplemental Methods).
FVIII activity and FVIII antigen assays
Assays were validated with plasma from wild-type (WT) and hemophilia A mice. FVIII activity was measured by fluorogenic thrombin generation test (FTGT), activated partial thromboplastin time (aPTT) or chromogenic assay, and FVIII antigen by immunoassay, as described (supplemental Methods).5,26,27 We used standard curves with pooled mouse plasma because FVIII reference standards for mouse are lacking. Results were compared with World Health Organization's 6th International Standard for human plasma FVIII (07/316), which was assigned 0.68 IU/mL FVIII activity and 1.04 IU/mL FVIII antigen content,28 with 1 IU defined as FVIII activity in 1 mL of normal pooled human plasma.
FVIII inhibitor assay
Bleeding assay
Statistical methods
Data are shown as mean ± SD. Significances were analyzed by t test, χ2 test, or ANOVA by SigmaStat (Jandel Scientific). A P value less than .05 was considered significant.
Results
Donor BM produced rare endothelial or parenchymal cells
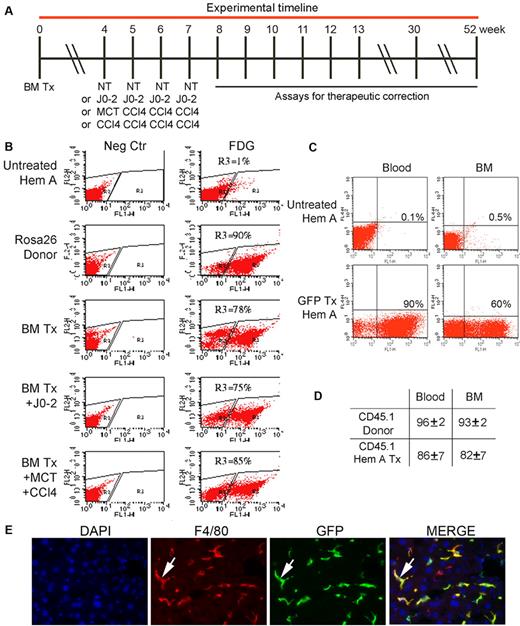
We transplanted BM in hemophilia A mice according to prospectively established experimental approach (Figure 1). BM recipients were given lethal total body irradiation followed by total nucleated donor BM cells intravenously. To study the effect of donor BM cell numbers, we transplanted 2 × 106 to 10 × 106 BM cells. To avoid immune responses, donor and recipient mice were in syngeneic C57BL/6 backgrounds. After BM transplantation, flow cytometry for LacZ, GFP, or CD45.1 indicated up to 90% of nucleated blood, and the BM cells in recipients were donor BM-derived (Figure 1B-D). Genomic DNA PCR showed BM-derived cells in multiple organs, as expected. In addition, tissue staining identified transplanted cells (eg, BM-derived GFP+ and LacZ+ cells were in organs, including the liver) largely in vascular spaces (Figure 1E, supplemental Figure 1). By contrast, donor BM-derived parenchymal cells (eg, hepatocytes) were extremely rare, and BM-derived LSECs were not found (see below). To determine whether transdifferentiation of BM-derived cells needed to be promoted, we administered apoptogenic J0-2 antibody, the endothelial toxin monocrotaline (MCT), and the toxin, carbon tetrachloride (CCl4), 4 weeks after transplanting 2 × 106 BM cells.32,33 Liver histology 1 to 3 days after these treatments confirmed tissue damage. However, again no donor BM-derived parenchymal or endothelial cells were found in organs. In view of the significance of endothelial cells in FVIII synthesis and release, we confirmed the absence of donor BM-derived endothelial cells by studies with Tie2-GFP transgenic donor FVB/N mice, where Tie2 promoter restricts GFP to endothelial cells.34 In these Tie2-GFP donors, endothelial cells in all organs, including large and small vessels, were known to widely express GFP.5,34 Recipients of Tie2-GFP cells were lethally irradiated syngeneic mice, also given J0-2, MCT and CCl4, as previously described (n = 36). We verified extensive BM replacement by donor cells but tissue surveys in FVB/N mice after 3, 6, or 8 months showed no GFP+ endothelial cells in liver, BM, kidneys, spleen, or heart.
Replacement of BM cells in hemophilia A mice after transplantation of 2 × 106 BM cells. (A) Timeline indicating animal groups subjected to BM transplantation (Tx), including no further treatment (NT) or J0-2 antibody, MCT, and CCl4 followed by assays for therapeutic correction. (B) Flow cytometry showing BM chimerism after 2 months in recipients of Rosa 26 BM with LacZ expression detected by FDG. Panels on left are negative controls. R3 gate in panels on right indicates BM repopulating cells. BM chimerism was extensive, including mice with BM Tx and J0-2, MCT, and CCl4. (C) Flow cytometry after 2 months in recipient of GFP+ BM cells showing chimerism of 90% in blood and 60% in BM. (D) Flow cytometry after 2 months in recipients (n = 7) of CD45.1 BM cells showing > 80% chimerism. (E) GFP+ donor-derived cells in liver sinusoids (green, arrow) 6 months after transplant. Stains included DAPI for nuclei (blue) and F4/80 for KC (red). Donor BM-derived KCs appear yellow in merged image (arrow, right panel). Original magnification ×400.
Replacement of BM cells in hemophilia A mice after transplantation of 2 × 106 BM cells. (A) Timeline indicating animal groups subjected to BM transplantation (Tx), including no further treatment (NT) or J0-2 antibody, MCT, and CCl4 followed by assays for therapeutic correction. (B) Flow cytometry showing BM chimerism after 2 months in recipients of Rosa 26 BM with LacZ expression detected by FDG. Panels on left are negative controls. R3 gate in panels on right indicates BM repopulating cells. BM chimerism was extensive, including mice with BM Tx and J0-2, MCT, and CCl4. (C) Flow cytometry after 2 months in recipient of GFP+ BM cells showing chimerism of 90% in blood and 60% in BM. (D) Flow cytometry after 2 months in recipients (n = 7) of CD45.1 BM cells showing > 80% chimerism. (E) GFP+ donor-derived cells in liver sinusoids (green, arrow) 6 months after transplant. Stains included DAPI for nuclei (blue) and F4/80 for KC (red). Donor BM-derived KCs appear yellow in merged image (arrow, right panel). Original magnification ×400.
To further demonstrate donor BM-derived hepatocytes, we transplanted 10 × 106 BM cells from GFP-transgenic mice into C57BL/6 mice after total body irradiation, and 2 months later, administered the hepatotoxin, acetaminophen. Generation of donor BM-derived hepatocytes was analyzed after another 3 months. Despite extensive BM replacement, donor BM-derived GFP+ hepatocytes were extremely rare. Flow cytometry of isolated liver cells showed that GFP+ BM-derived cells were < 0.2% of all cells, which indicates minimal hepatic replacement.
FVIII deficiency was corrected in relation with donor BM cell numbers
We analyzed therapeutic correction in hemophilia A mice after BM transplantation. Plasma FVIII activity was measured at the end by FTGT,5,27 which was chosen for its ultrasensitivity, because we anticipated plasma FVIII activity to be corrected, if at all, at low levels. FTGT was calibrated with plasma from C57BL/6 WT and hemophilia mice (supplemental Figure 2A). Results of FTGT were confirmed by chromogenic assay and aPTT. Comparison with human FVIII standard indicated that 100% of mouse FVIII activity was represented by assays as follows: FTGT, 1.02 IU/mL; chromogenic assay, 1.03 IU/mL; and aPTT, 1.04 IU/mL. In this immunoassay, 100% mouse plasma FVIII antigen represented 0.86 IU/mL of human FVIII standard. Neither FTGT nor chromogenic assay showed plasma FVIII activity in the hemophilia mouse colony. In untreated hemophilia A mice (n = 46), aPTT showed 3 ± 3% FVIII activity, representing 0.03 ± 0.03 IU/mL of human plasma FVIII standard, probably because of the tissue factor introduced during blood collection. In donor mice used in our studies, FVIII activity and antigen were at similar levels. The specificity of FVIII antibodies was verified by immunostaining of transduced mouse fibroblasts expressing recombinant mouse FVIII (supplemental Figure 2B). In addition, plasma assays and tissue staining excluded cross-reactivity with other proteins, such as vWF.
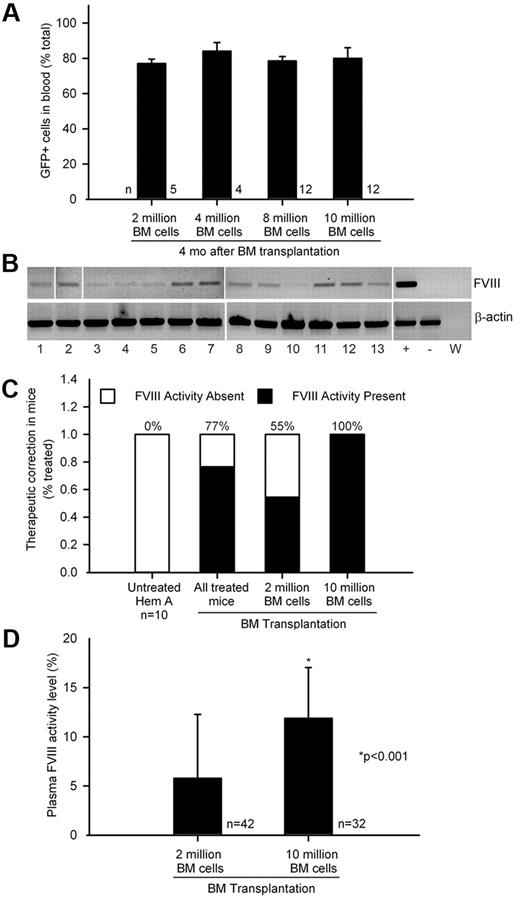
The extent of BM chimerism was comparable in hemophilia mice after transplantation of 2, 4, 8, or 10 × 106 BM cells (Figure 2A). We found that FVIII mRNA in BM in 33 of 36 randomly sampled hemophilia mice (Figure 2B). This was in agreement with ≥ 1% plasma FVIII activity in 77% and not 100% of 74 mice transplanted with healthy BM cells (Figure 2C). As differences in plasma FVIII activity levels were related to the number of BM cells transplanted, we compared hemophilia mice receiving 2 × 106 BM cells, n = 42, or 10 × 106 BM cells, n = 32. FTGT showed fewer mice with FVIII activity after 2 × 106 BM cells, 55% mice, versus FVIII activity in 100% mice after 10 × 106 BM cells (P < .0001; Fisher exact test). Remarkably, plasma FVIII activity level was higher after transplantation of 10 × 106 BM cells than after 2 × 106 BM cells, 12 ± 5% and 6 ± 7% of normal FVIII activity levels, representing 0.12 ± 0.05 and 0.06 ± 0.07 IU/mL of human FVIII standard, respectively (P < .001; t test; Figure 2D). Compared with FVIII mRNA level by quantitative RT-PCR of 1.0 in WT BM cells, 1 year after BM transplantation FVIII mRNA levels were lower in recipients of 2 × 106 (n = 8) versus 10 × 106 BM cells (n = 7), 0.5 ± 0.4 and 0.8 ± 0.3 (P = NS; t test).
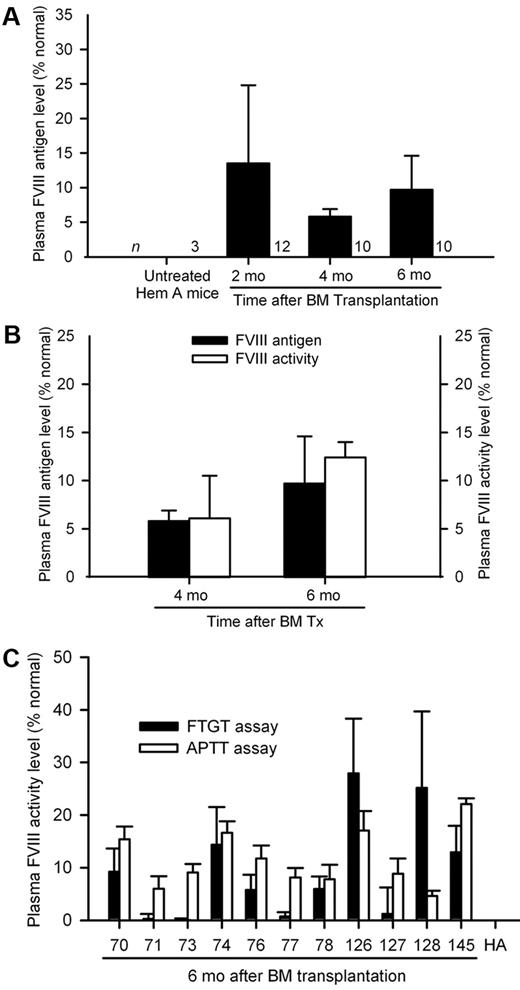
Transplantation of healthy BM and phenotypic correction in hemophilia A. (A) Flow cytometry of blood cells showing BM chimerism was similar 4 months after 2 × 106 to 10 × 106 GFP+ BM cells were transplanted in hemophilia mice. (B) FVIII mRNA expression in BM by single-step RT-PCR: lanes 1-13, BM-recipient hemophilia mice 3 to 6 months after transplantation of 2 × 106 whole BM cells; lane marked (+), GFP+ mouse BM; lane marked (−), hemophilia mouse liver; lane marked (W), PCR mix alone. β-actin RNA was loading control. (C) Percentage of therapeutic correction in 74 consecutive hemophilia mice with plasma FVIII activity (≥ 1% or 0.01 IU/mL of human FVIII standard) or without FVIII activity after BM transplants, including subgroups with 2 × 106 BM cells (n = 42) or 10 × 106 BM cells (n = 32). (D) Plasma FVIII activity levels with FTGT in hemophilia mice shown in preceding panel indicated less correction after 2 × 106 versus 10 × 106 BM cells (P < .001; t test; 100% mouse plasma FVIII activity corresponded to 1.02 IU/mL of human FVIII standard).
Transplantation of healthy BM and phenotypic correction in hemophilia A. (A) Flow cytometry of blood cells showing BM chimerism was similar 4 months after 2 × 106 to 10 × 106 GFP+ BM cells were transplanted in hemophilia mice. (B) FVIII mRNA expression in BM by single-step RT-PCR: lanes 1-13, BM-recipient hemophilia mice 3 to 6 months after transplantation of 2 × 106 whole BM cells; lane marked (+), GFP+ mouse BM; lane marked (−), hemophilia mouse liver; lane marked (W), PCR mix alone. β-actin RNA was loading control. (C) Percentage of therapeutic correction in 74 consecutive hemophilia mice with plasma FVIII activity (≥ 1% or 0.01 IU/mL of human FVIII standard) or without FVIII activity after BM transplants, including subgroups with 2 × 106 BM cells (n = 42) or 10 × 106 BM cells (n = 32). (D) Plasma FVIII activity levels with FTGT in hemophilia mice shown in preceding panel indicated less correction after 2 × 106 versus 10 × 106 BM cells (P < .001; t test; 100% mouse plasma FVIII activity corresponded to 1.02 IU/mL of human FVIII standard).
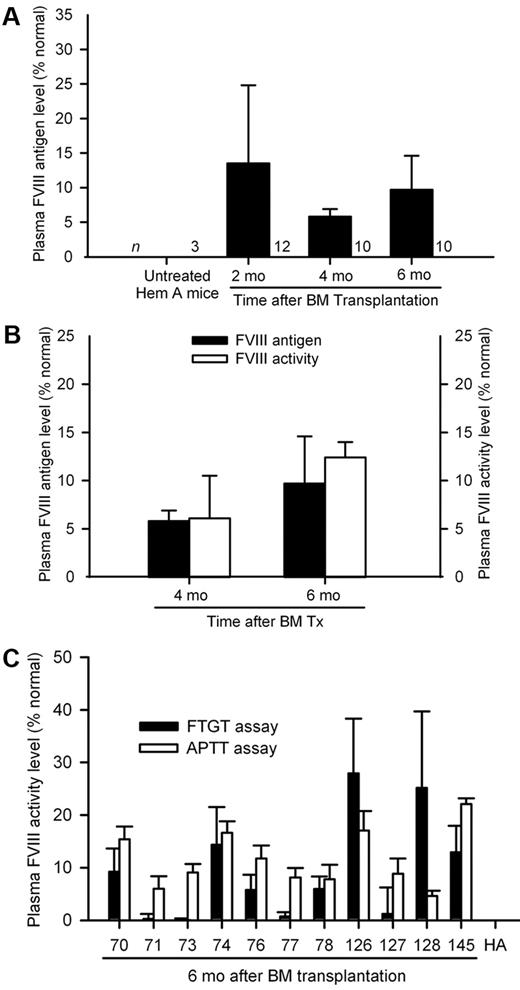
Because BM chimerism was comparable after transplantation of either 2 × 106 or 10 × 106 BM cells, the difference in plasma FVIII activity was unexplained. Therefore, we compared outcomes in another series of 30 hemophilia A mice with either 2 × 106 BM cells (n = 18) or 10 × 106 BM cells (n = 12). Serial blood samples were analyzed after 2, 4, and 6 months for FVIII activity. Once again, FTGT after 6 months showed lower plasma FVIII activity with 2 × 106 BM cells, 6 ± 4%, versus 10 × 106 BM cells, 11 ± 3%, representing 0.06 ± 0.04 and 0.11 ± 0.03 IU/mL of human FVIII standard, respectively (P < .001; t test; supplemental Figure 3). Presence of plasma FVIII antigen in mice was confirmed by immunoassay with Abcam ab53703 antibody monospecific for FVIII, which was nonreactive with other plasma or tissue proteins, including vWF, in hemophilia A mice (Figure 3A). We found plasma FVIII antigen (immunoassay) and activity (FTGT) levels were comparable in BM-treated mice (Figure 3B). Plasma FVIII antigen and activity levels remained in a steady range over several months, although we noted some rise in plasma FVIII activity and antigen levels 4 and 6 months after BM transplants, probably representing ongoing replacement of BM with donor cells. To confirm plasma FVIII activity measured by FTGT was detected by other assays, we reanalyzed 11 stored plasma samples, where FTGT showed FVIII activity and unthawed aliquots were available after various analyses. In these samples, FVIII activity was in similar range, FTGT, 9.3 ± 9.9%, and aPTT, 11.6 ± 5.4%, representing 0.10 ± 0.10 and 0.12 ± 0.06 IU/mL of human FVIII standard, respectively (P = .51; t test; Figure 3C).
FVIII replacement after BM transplantation. (A) Plasma FVIII antigen levels in groups of hemophilia A mice 2, 4, and 6 months after transplantation of 2 × 106 BM cells from GFP transgenic donors. (B) Correlation between plasma FVIII antigen and FVIII activity (FTGT) levels in hemophilia mice 4 and 6 months after transplantation of 10 × 106 BM cells, where both datasets were available for comparison. (C) Verification of plasma FVIII activity by aPTT in selected mouse plasma samples positive with FTGT assays 6 months after BM transplants. Mouse plasma FVIII activity and antigen level of 100% represented 1.02 IU/mL (FTGT), 1.04 IU/mL (aPTT), and 0.86 IU/mL (FVIII antigen assay) of human FVIII standard.
FVIII replacement after BM transplantation. (A) Plasma FVIII antigen levels in groups of hemophilia A mice 2, 4, and 6 months after transplantation of 2 × 106 BM cells from GFP transgenic donors. (B) Correlation between plasma FVIII antigen and FVIII activity (FTGT) levels in hemophilia mice 4 and 6 months after transplantation of 10 × 106 BM cells, where both datasets were available for comparison. (C) Verification of plasma FVIII activity by aPTT in selected mouse plasma samples positive with FTGT assays 6 months after BM transplants. Mouse plasma FVIII activity and antigen level of 100% represented 1.02 IU/mL (FTGT), 1.04 IU/mL (aPTT), and 0.86 IU/mL (FVIII antigen assay) of human FVIII standard.
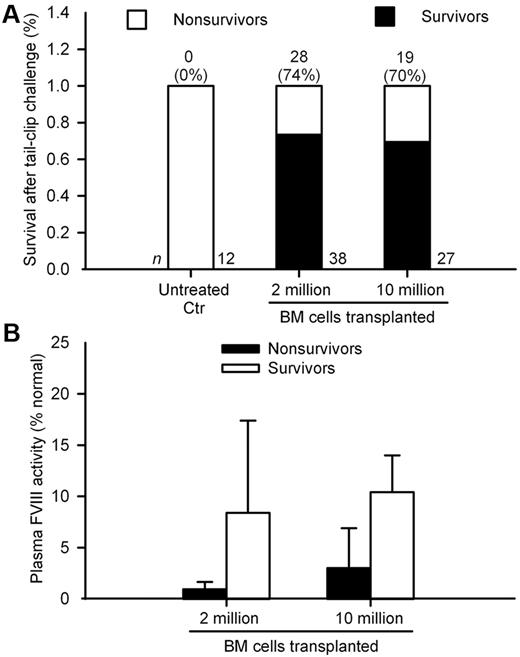
As restoration of plasma FVIII activity should have protected animals from trauma-induced bleeding, we challenged by tail-clip randomly selected mice 8, 12, 30, or 52 weeks after BM transplantation. In mice transplanted with 2 × 106 and 10 × 106 BM cells, survival was 74% and 70%, respectively, compared with 0% in untreated mice (P < .0001; Figure 4A). Plasma FVIII activity levels measured by FTGT were higher in mice surviving the tail-clip challenge compared with nonsurvivors (Figure 4B). However, outcomes after the tail-clip challenge were similar in mice with either 2 × 106 or 10 × 106 BM cells, which indicates only a threshold of FVIII activity was required for bleeding control, as expected.
Tail-clip assay in hemophilia A mice after BM transplantation. (A) Cumulative survival after tail-clip in control untreated mice and mice after transplantation of 2 × 106 or 10 × 106 BM cells. Animals were studied between 8 weeks to 52 weeks after BM transplantation. Total animal numbers are given at bottom and number of surviving mice with percentage survival is at top of bars. Only the difference in survival between untreated controls versus animals in the 2 treatment groups was significant (P < .0001; Fisher exact test). (B) Plasma FVIII activity in mice surviving was greater than mice not surviving after tail-clip assay in both groups (P < .001; t test). Mouse plasma FVIII activity of 100% represented 1.02 IU/mL of human FVIII standard.
Tail-clip assay in hemophilia A mice after BM transplantation. (A) Cumulative survival after tail-clip in control untreated mice and mice after transplantation of 2 × 106 or 10 × 106 BM cells. Animals were studied between 8 weeks to 52 weeks after BM transplantation. Total animal numbers are given at bottom and number of surviving mice with percentage survival is at top of bars. Only the difference in survival between untreated controls versus animals in the 2 treatment groups was significant (P < .0001; Fisher exact test). (B) Plasma FVIII activity in mice surviving was greater than mice not surviving after tail-clip assay in both groups (P < .001; t test). Mouse plasma FVIII activity of 100% represented 1.02 IU/mL of human FVIII standard.
We did not detect neutralizing antibodies against FVIII in hemophilia A mice after BM transplants, including in mice with or without plasma FVIII antigen and activity (supplemental Table 1). We noted that recipients were lethally irradiated, BM chimerism was near-total, and mice were naive for normal FVIII protein.
Identity of donor BM-derived cell type(s) imparting therapeutic benefits
Tissue surveys 4 to 6 months after GFP+ BM transplantation showed donor-derived cells in BM, liver, spleen, lungs, heart, and kidneys, as previously indicated (Figure 1, supplemental Figure 1). We studied liver further, because LSECs are known to produce FVIII and previous reports indicated that BM cells too could generate hepatocytes.35 However, we found only rare GFP+ hepatocytes, including in mice given J0-2 or MCT plus CCl4. No proliferation of donor-derived hepatocytes was observed, which is consistent with lack of hepatocyte selection.32,35 GFP+ donor-derived cells in vascular spaces were CD11b+ KC or MNC (supplemental Figure 4). Despite diligent searches for LSECs with GFP plus CD31, an endothelial marker, we found no BM-derived LSECs in liver, spleen, heart, or kidney, etc (supplemental Figure 5).
To identify FVIII in BM-derived cells, we isolated liver cells from mice 3 to 6 months after transplanting GFP+ BM cells. BM-derived CD11b+ KC or MNC were most abundant (supplemental Figure 6). Demonstration of FVIII mRNA in cell fractions required nested RT-PCR, which indicates that low levels of FVIII were expressed in BM-derived cells. This was different from only one PCR round needed to show FVIII mRNA in LSECs. To exclude significant contamination with LSECs of CD11b+ KCs, we isolated cells from Tie2-GFP transgenic donor mice. This showed 95% cells in our isolates were KCs and 4% were GFP+ LSECs (supplemental Figure 7). Quantitative RT-PCR confirmed that FVIII mRNA was expressed in healthy CD11b+ KCs and that LSECs produced FVIII far more efficiently (supplemental Figure 8).
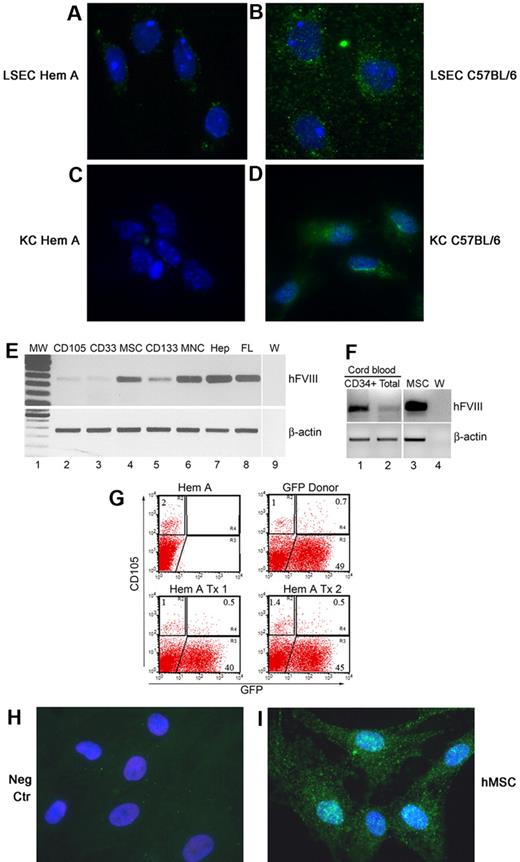
We localized FVIII protein in healthy LSECs and KCs by ab53703 antibody (Figure 5A-D). Essentially all KCs were stained with FVIII antibody. As cells from hemophilia A mice were negative, this verified antibody-reactivity only with FVIII. To determine what BM cell fractions contributed in FVIII synthesis, we obtained RNAs from total nucleated human BM cells along with the following BM cell fractions: CD133 (hematopoietic progenitor), CD33 (myeloid), CD105 (mesenchymal and other lineages), and MSCs. We found FVIII mRNA in hMSCs and CD133 cells (Figure 5E). In addition, FVIII mRNA was expressed in human cord blood (CB) cells (Figure 5F). This prompted us to examine whether donor BM generated MSCs in mouse BM. We found significant numbers of donor-derived CD105+ cells in animals (Figure 5G). Moreover, human BM-derived MSCs expressed FVIII protein, which was observed in all cultured cells (Figure 5H-I).
FVIII immunostaining with ab53703 in primary mouse liver cells after culture for 48 hours. (A) LSECs from hemophilia A mouse with no FVIII staining. (B) LSECs from healthy C57BL/6 mouse with FVIII as punctate green cytoplasmic dots. (C) CD11b+ KCs from hemophilia A mouse with no staining. (D) CD11b+ KCs from healthy C57BL/6 mouse with FVIII in cytoplasm. The abundance of FVIII in KCs was less than in LSECs. (E) FVIII mRNA in fractionated human BM by single-step RT-PCR: lane 1, molecular weight marker; lane 2, CD105 (mesenchymal and vasculogenic endothelial) cells; lane 3, CD33 (myeloid) cells; lane 4, MSCs; lane 5, CD133 (hematopoietic precursor) cells; lane 6, total BM MNCs; lane 7, adult human hepatocytes (Hep); lane 8, fetal human liver (FL); and lane 9, PCR mix (W). β-actin was amplified to verify RNA integrity. (F) Single-step RT-PCR for FVIII in CD34+ human CB cells (lane 1), total human CB cells (lane 2), BM-derived hMSCs used for transplantation studies in NOD/SCID hemophilia A mice (lane 3), and PCR mix (W; lane 4). (G) Flow cytometry of BM for abundance of CD105 cells from hemophilia A mouse, GFP transgenic donor mouse, and 2 hemophilia mice 9 months after transplantation of 10 × 106 BM cells from GFP donor mice. Note CD105+ cell fraction in BM of hemophilia mouse and GFP+ donor mouse was 2% and 1.7%, respectively (R2+R4 gates), with GFP expression in 70% of CD105 cells in donor. After BM transplantation, GFP+ donor BM cells constituted 33% and 26% of CD105 cells in hemophilia A mice. (H-I) FVIII staining with ab53703 in BM-derived hMSCs in culture with no signals when FVIII antibody was omitted (H) and staining of FVIII in cytoplasm (green dots) after inclusion of FVIII antibody (I). Panels A through D and H and I, DAPI counterstain (blue); original magnification ×630.
FVIII immunostaining with ab53703 in primary mouse liver cells after culture for 48 hours. (A) LSECs from hemophilia A mouse with no FVIII staining. (B) LSECs from healthy C57BL/6 mouse with FVIII as punctate green cytoplasmic dots. (C) CD11b+ KCs from hemophilia A mouse with no staining. (D) CD11b+ KCs from healthy C57BL/6 mouse with FVIII in cytoplasm. The abundance of FVIII in KCs was less than in LSECs. (E) FVIII mRNA in fractionated human BM by single-step RT-PCR: lane 1, molecular weight marker; lane 2, CD105 (mesenchymal and vasculogenic endothelial) cells; lane 3, CD33 (myeloid) cells; lane 4, MSCs; lane 5, CD133 (hematopoietic precursor) cells; lane 6, total BM MNCs; lane 7, adult human hepatocytes (Hep); lane 8, fetal human liver (FL); and lane 9, PCR mix (W). β-actin was amplified to verify RNA integrity. (F) Single-step RT-PCR for FVIII in CD34+ human CB cells (lane 1), total human CB cells (lane 2), BM-derived hMSCs used for transplantation studies in NOD/SCID hemophilia A mice (lane 3), and PCR mix (W; lane 4). (G) Flow cytometry of BM for abundance of CD105 cells from hemophilia A mouse, GFP transgenic donor mouse, and 2 hemophilia mice 9 months after transplantation of 10 × 106 BM cells from GFP donor mice. Note CD105+ cell fraction in BM of hemophilia mouse and GFP+ donor mouse was 2% and 1.7%, respectively (R2+R4 gates), with GFP expression in 70% of CD105 cells in donor. After BM transplantation, GFP+ donor BM cells constituted 33% and 26% of CD105 cells in hemophilia A mice. (H-I) FVIII staining with ab53703 in BM-derived hMSCs in culture with no signals when FVIII antibody was omitted (H) and staining of FVIII in cytoplasm (green dots) after inclusion of FVIII antibody (I). Panels A through D and H and I, DAPI counterstain (blue); original magnification ×630.
Compact bone in mice contained MSCs with nestin expression, which was shown to be a marker of MSCs.36 These nestin-positive MSCs in bone expressed FVIII. This indicated MSC capable of expressing FVIII normally resided in bones (supplemental Figure 9).
Short-term transplantation assays with BM-derived KCs and MSCs
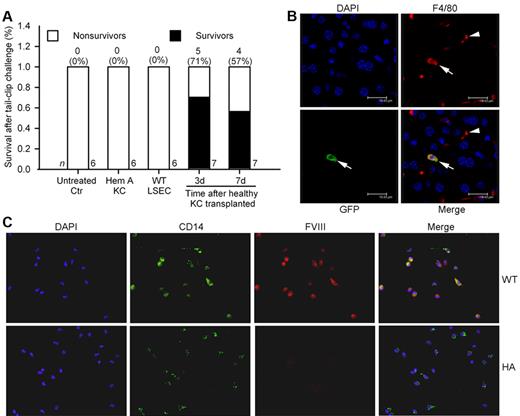
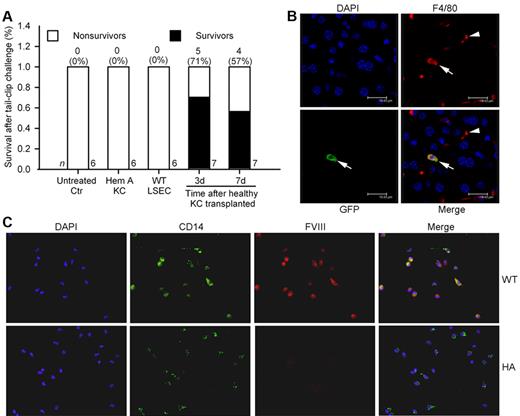
To demonstrate whether FVIII expression in BM-derived cells conferred therapeutic benefits, we isolated healthy CD11b+ KCs from GFP+ C57BL/6 mice. Cell phenotype was verified by flow cytometry for F4/80 antigen and assays of phagocytosis. Biodistribution and fate of transplanted KCs was established. After intravenous (IV) injection, KCs entered lungs and liver, although transplanted cell numbers started to decline beyond day 1. Therefore, this method permitted short-term analysis of FVIII replacement. We injected 2.5 to 3 × 106 healthy KCs from C57BL/6 mice into hemophilia mice intravenously followed by tail-clip after 3 days (n = 7) or 7 days (n = 7). Additional hemophilia mice received KCs from hemophilia A mice donors. All untreated hemophilia mice (n = 6) and hemophilia mice 3 days after KC transplantation from hemophilia A donors (n = 6) died after tail-clip. By contrast, several recipients of healthy KCs survived the tail-clip challenge (5 of 7 mice after 3 days; 4 of 7 mice after 7 days; Figure 6A). Costaining for GFP and F4/80 showed transplanted KCs in liver (Figure 6B). Peripheral blood MNCs isolated by CD11b marker, followed by CD14+ staining to verify their phenotype, showed FVIII expression in cells from WT mice (Figure 6C). The identity of CD11b+ MNCs was further verified by CD14 and CD115 staining, along with colocalization of FVIII by staining (supplemental Figure 10). Manual counting showed 84 ± 14% of CD14+ MNCs (1000 cells; n = 3 mice) expressed FVIII.
Intravenous transplantation of CD11b+ KC in C57Bl/6 hemophilia mice. (A) Percent survival in mice after tail-clip bleeding. All untreated control mice died, as did mice 3 days after transplantation of 2.5 to 3 × 106 KCs from hemophilia A donors, and mice 3 days after transplantation of 1 × 105 LSECs from C57Bl/6 mice (n = 6 ea). In recipients of 2.5 to 3 × 106 KCs from healthy C57Bl/6 GFP donors, tail-clip after 3 days and 7 days produced survival (P = .02 and 0.07, respectively; Fisher exact test). (B) Localization of transplanted KCs in liver by immunostaining for F4/80 (KC marker, red) and GFP (transplanted cell marker, green). F4/80+ KCs in liver sinusoids 3 days after KC transplantation in hemophilia mouse (arrowhead). A transplanted KC is shown with GFP plus F4/80 (arrow). (C) Shows CD11b+ peripheral blood MNCs from WT and hemophilia A mice with staining for CD14 and FVIII. Original magnification (B) ×630, (C) ×400.
Intravenous transplantation of CD11b+ KC in C57Bl/6 hemophilia mice. (A) Percent survival in mice after tail-clip bleeding. All untreated control mice died, as did mice 3 days after transplantation of 2.5 to 3 × 106 KCs from hemophilia A donors, and mice 3 days after transplantation of 1 × 105 LSECs from C57Bl/6 mice (n = 6 ea). In recipients of 2.5 to 3 × 106 KCs from healthy C57Bl/6 GFP donors, tail-clip after 3 days and 7 days produced survival (P = .02 and 0.07, respectively; Fisher exact test). (B) Localization of transplanted KCs in liver by immunostaining for F4/80 (KC marker, red) and GFP (transplanted cell marker, green). F4/80+ KCs in liver sinusoids 3 days after KC transplantation in hemophilia mouse (arrowhead). A transplanted KC is shown with GFP plus F4/80 (arrow). (C) Shows CD11b+ peripheral blood MNCs from WT and hemophilia A mice with staining for CD14 and FVIII. Original magnification (B) ×630, (C) ×400.
To exclude confounding from contaminating LSECs in KCs, we isolated LSECs from healthy C57BL/6 donors and injected 1 × 105 LSECs intravenously, because this cell number represented possible contamination by LSECs of KCs. After 3 days, the tail-clip caused death in all hemophilia mice (n = 6), which verifies that therapeutic benefits were from transplanted KCs. Available plasma from mice with KC transplants showed low levels of FVIII activity in 2 of 5 mice 3 days after transplant (2%, 5%, representing 0.02 and 0.05 IU/mL of human FVIII standard) and in 2 of 4 mice 7 days after transplant (3% each, representing 0.03 IU/mL of human FVIII standard).
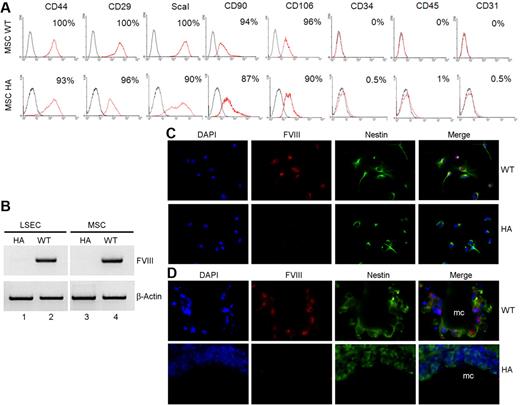
For hMSCs, we transplanted 5 × 106 cells intraperitoneally into nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) hemophilia A mice. After tail-clip, 3 of 3 hMSC-treated mice survived after 3 days, and 1 of 3 hMSC-treated mice survived after 7 days. Tail-clip caused mortality in all control untreated hemophilia A mice after 3 days and 7 days (n = 6 each). To verify FVIII was expressed in mouse BM-derived MSCs, we isolated MSCs from healthy C57BL/6 mice and hemophilia A mice. These displayed markers typical of MSCs (ie, CD29, CD44, ScaI, CD90, and CD106), whereas hematopoietic or endothelial markers (ie, CD34, CD45, or CD31) were absent (Figure 7A).24 WT mouse MSCs expressed FVIII mRNA (Figure 7B). MSCs expanded in culture were not contaminated with either hematopoietic or endothelial cells, because these expressed the mesenchymal marker, nestin (Figure 7C).36 Moreover, FVIII was again colocalized in nestin-positive MSCs. Similarly, after transplantation, MSCs coexpressed nestin and FVIII (Figure 7D). To ensure absence of hematopoietic and endothelial contaminants in transplanted MSCs, we performed flow cytometry (supplemental Figure 11). This showed mesenchymal markers (ie, CD105 and ScaI), whereas hematopoietic markers, CD11b, F4/80, and MHC-II were absent. Similarly, the endothelial marker, Tie2, was absent. Cultured MSCs stained for vimentin. Furthermore, cultured MSCs expressed FVIII. Therefore, endothelial or hematopoietic cells were absent from MSCs that were transplanted.
Analysis of mouse MSC from WT and hemophilia A C57Bl/6 mice. (A) FACS (fluorescence-activated cell sorter) characterization of MSCs isolated from bones of 3-week-old mice followed by 4 passages in culture. Cells were stained with antibodies shown. Markers typically associated with MSCs (ie, CD44, CD29, ScaI, CD90, and CD106) were present, whereas hematopoietic markers, CD34 and CD45, or endothelial marker, CD31, were absent. (B) RT-PCR showing FVIII mRNA in LSECs and MSCs from WT mice and its absence in cells from hemophilia A mice, along with β-actin mRNA to indicate equivalent cDNA loading. (C) Colocalization of nestin and FVIII by ab61390 FVIII antibody in MSCs from WT and hemophilia mice. (D) Identification of transplanted MSCs in hemophilia mice after 7 days with staining for nestin along with FVIII by ab61390 FVIII antibody; mc = microcarriers.
Analysis of mouse MSC from WT and hemophilia A C57Bl/6 mice. (A) FACS (fluorescence-activated cell sorter) characterization of MSCs isolated from bones of 3-week-old mice followed by 4 passages in culture. Cells were stained with antibodies shown. Markers typically associated with MSCs (ie, CD44, CD29, ScaI, CD90, and CD106) were present, whereas hematopoietic markers, CD34 and CD45, or endothelial marker, CD31, were absent. (B) RT-PCR showing FVIII mRNA in LSECs and MSCs from WT mice and its absence in cells from hemophilia A mice, along with β-actin mRNA to indicate equivalent cDNA loading. (C) Colocalization of nestin and FVIII by ab61390 FVIII antibody in MSCs from WT and hemophilia mice. (D) Identification of transplanted MSCs in hemophilia mice after 7 days with staining for nestin along with FVIII by ab61390 FVIII antibody; mc = microcarriers.
We transplanted 5 × 106 MSCs intraperitoneally into hemophilia mice (n = 6). After the tail-clip challenge, 3 days later all mice given MSCs from C57BL/6 hemophilia A mice (n = 6) and all untreated mice (n = 6) died. By contrast, 5 of 6 mice given MSCs from healthy C57BL/6 mice survived (P = .01; Fisher exact test). After 7 days, 4 of 7 mice given healthy MSCs survived. Thus, BM-derived MSCs could replace FVIII.
Discussion
This study established that BM transplantation corrected hemophilia in mice, and that donor BM-derived MNCs (KCs) and MSCs produced FVIII in this setting. Appearance of FVIII in treated mice was confirmed by multiple assays in plasma, tissues, and isolated cells. As donor BM generated neither endothelial cells nor hepatocytes, these cell types did not account for FVIII replacement after BM transplantation.
Previously, BM transplantation in hemophilia dogs was considered unsuccessful.16,17 The conclusion was based on studies in 3 dogs, although one of these died 34 days after BM transplantation with allograft-related complications, suggesting rejection. Up to 5% plasma FVIII activity was detected after BM transplantation in the first dog but one-stage clotting assay detected FVIII activity in untreated dogs as well.16 In 2 subsequent dogs, myeloid and lymphoid lineages were replaced by donor BM, and the animals survived for 7 months and 2 years, the former dying after bleeding from iliac crest BM aspiration.17 Despite plasma FVIII activity up to 8% normal, definitive information regarding plasma FVIII by one-stage clotting assay was unavailable. Whether donor BM-derived cells, (eg, MNCs, KCs, or other cells) expressed FVIII was unknown. The final extent of BM chimerism achieved was also unknown. Other uncertainties were introduced by unspecified composition of donor BM and whether additional donor BM-derived cell populations, (eg, MSCs) had been produced. This should be pertinent because after BM transplantation even in mouse models, which are far better controlled than dog models, long-term BM replacement is not invariant and is affected by the composition of donor BM cell subpopulations transplanted.37 Given that therapeutic correction was not observed in every mouse in our BM transplantation studies, repeating canine studies under better experimental conditions should be appropriate.
Similarly, BM transplantation in hemophilia has apparently been limited to only one child with aplastic anemia, where 3.5 × 108 total allogeneic BM cells/kg were given, along with FVIII, to maintain a plasma activity of 50%-100%.17 Despite BM engraftment in this child, FVIII activity levels did not increase above baseline more than 4 months after BM transplantation. However, lower levels of FVIII replacement after BM transplantation could not be identified in this situation. Another patient with high FVIII inhibitor titers received 6.8 × 108 allogeneic BM cells/kg, although high doses of recombinant FVIII were continued, and correction of hemophilia could not be demonstrated.18,19 This person survived for only 46 days. Thus the clinical experience of BM transplantation in hemophilia A is essentially noninformative.
Furthermore, studies of plasma FVIII levels in healthy individuals with splenectomy, aplastic anemia, immunodeficiency, etc, and selective depletion of hematopoietic cell types, are noninformative because of confounding by FVIII expression in the healthy liver.
Previously, studies in hemophilia mice included transplantation of WT hematopoietic stem cells (HSCs) transduced with various FVIII constructs.31,38,39 These reports did not provide outcomes in animals treated with nontransduced healthy BM cells. However, in some animal groups transplantation of WT HSCs did not lead to detectable FVIII activity.38 We do not know precise reasons for such negative outcomes, although it should be noteworthy that several studies used highly selected HSC subpopulations (eg, ScaI+ cells), which had been transduced with viral vectors, cultured in methylcellulose, and followed by transplantation of at most 7 × 105 cells. By contrast, in our studies, BM cells were transplanted in considerably larger numbers immediately after isolation without selection, genetic manipulation, or culture. In gene therapy studies with HSCs, it was unknown as to whether nonhematopoietic lineages had been reconstituted (eg, MSC in BM), which was also different from our studies. Whether expression of transgenes in transplanted HSCs affected cell survival and/or proliferation was unknown. Similarly, whether transgene expression induced immune response with clearance of transplanted HSCs in some instances was also unknown.
By contrast, our studies offer an explanation for the observation of how plasma FVIII was preserved after replacement of the liver with hemophilia A donor liver in healthy dogs, as well as one person,9,10 because FVIII would now be produced in only extrahepatic sites.
Despite extensive BM chimerism, all hemophilia mice did not survive the bleeding challenge. Whether this was because of less BM chimerism or lower functionality of donor-derived cells in some mice was unknown. In addition, although the number of donor BM-derived KCs or MNCs was large, whether every donor-derived cell produced equal amounts of FVIII was not known. Donor BM-derived cells did produce FVIII, as shown by FVIII mRNA and protein in KCs and MSCs, along with cessation of bleeding in hemophilia mice given healthy MSCs or KCs. This tail-clip bleeding assay in hemophilia mice was previously validated, and was sensitive to low levels of FVIII replacement,5,6,31 although other methods for testing bleeding control could be useful in hemophilia mouse studies.40 We considered control of bleeding in hemophilia mice after transplantation of 2.5 to 3 × 106 healthy KCs, representing < 20% of all KCs, was significant because BM transplantation reconstituted MNCs throughout the body. Therefore, it was reasonable that the extent to which MNCs and KCs were replaced after BM transplantation should have produced greater plasma FVIII activity levels. This contrasts with transplantation of 2.5 to 3 × 106 KCs, along with engraftment of only some cells, producing lower plasma FVIII levels. After BM transplantation, therapeutic effects lasted indefinitely (at least 1 year), whereas hemostasis after transplantation of healthy KCs or MSCs lasted only a few days.
The extent of hematopoietic replacement was similar after transplantation of either 2 × 106 or 10 × 106 BM cells, whereas FVIII activity was greater in the latter situation. This led us to search for other BM-derived cell types and identified MSCs with the capacity to express FVIII mRNA and protein, as well as to correct bleeding in hemophilia mice after transplantation. We confirmed the absence of hematopoietic and endothelial contaminants in our studies with MSCs. Given that MSC expressed FVIII before transplantation, it was unnecessary for MSCs to have undergone lineage conversion after transplantation (eg, to endothelial cells) for FVIII replacement.
The absence of donor BM-derived endothelial cells in our studies differed from IV injection of mouse BM-derived cells after toxic doses of acetaminophen or monocrotaline to hemophilia mice.41,42 In those studies, injection of BM-derived cells was thought to produce efficient engraftment of transplanted cells in liver, including generation of hepatocytes and LSECs in sufficient numbers to indefinitely correct FVIII deficiency. However, we observed neither hepatocytes nor LSECs to any significance in BM transplantation studies, despite extensive BM chimerism in lethally irradiated mice, which argued against the possibility of permanent correction of hemophilia by peripheral injection of BM-derived cells. Absence of Tie2-GFP+ or CD31+ donor BM-derived endothelial cells in our study supported this conclusion, as was also reached by others.43-45 Similarly, as donor BM-derived parenchymal cells (eg, hepatocytes) were very rare after BM transplantation, it was unlikely these had roles in FVIII replacement. In another study, despite transplantation of hepatocytes in large numbers, FVIII deficiency was not corrected in hemophilia mice,6 although in another study, transplanted hepatocytes corrected hemophilia in mice.46 Nonetheless, lower levels of FVIII mRNA and protein in KCs (MNCs) and MSCs, relative to LSECs, confirmed that endothelial cells will be more potent sources of FVIII. Whether simultaneous expression and proximity of FVIII to vWF in LSECs or other endothelial cells would be advantageous to KCs, MNCs, or MSCs is unknown.
Our findings should account for maintenance of FVIII activity levels in clinical situations. The ability of endothelial cells in liver and other organs, as well as of nonhepatic cell types, to produce FVIII offer frameworks for understanding alterations in FVIII levels during diseases.47 Similarly, transplantation studies showing efficacy of dissociated spleen cells or splenic tissue in people with hemophilia should be accounted for by FVIII production in monocytes, besides endothelial cells,7,48,49 because the spleen is a major reservoir of MNCs and macrophages.50 After splenic cell transplants, plasma FVIII activity levels peaked in 4 to 7 days,48 which was in agreement with our studies of KCs and MSCs transplants, which showed control of bleeding after 3 days.
Identification of FVIII expression in human BM cells and CB cells emphasizes the significance of our findings. However, our efforts to study transplanted human BM cells were limited by difficulties in xenografting of NOD/SCID hemophilia A mice, with < 2% BM chimerism (not shown). Therefore, alternative experimental models of hemophilia A for testing human cells (eg, nonhuman primates), which do not yet exist, could be helpful for translating our findings.
From a biologic perspective, as FVIII is a critical requirement for clotting, one could view maintenance of plasma FVIII activity at required levels through high capacity “central” systems, that is, LSEC or endothelia in major organs would be efficient for that purpose, with lower capacity “auxiliary” systems in cells capable of avoiding diseases affecting endothelia, that is, MSCs in BM or elsewhere. Expression of FVIII in MNC may serve as a “peripheral” system for delivering FVIII to bleeding sites. This model should help reconcile plasma FVIII levels in disease-specific contexts.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank C. Zhang, G. Ukpong, G. Ranaldo, and D. Zanolini for provided assistance.
This work was supported in part by National Institutes of Health grants R01 DK071111, P30 DK41296, and P30 CA13330. A.F. was a recipient of Liver Scholar Award from the American Liver Foundation/American Association for Studies of Liver Diseases, and was supported in part by Ricerca Sanitaria Finalizzata della Regione Piemonte, Italy (2008 and 2009), and by grant GGP09280, Telethon Foundation, Italy.
National Institutes of Health
Authorship
Contribution: A.F. designed and performed experiments, analyzed data, and contributed to generating funding, and writing the paper; S.R. and R.S. designed experiments, analyzed data, and contributed to the writing of paper; S.M. performed experiments and analyzed data; and S.G. conceived and designed research, generated funding, analyzed data, and contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sanjeev Gupta, Albert Einstein College of Medicine, Ullmann Bldg, Rm 625, 1300 Morris Park Ave, Bronx, NY, 10461; e-mail: sanjvgupta@pol.net or sanjeev.gupta@einstein.yu.edu.