Abstract
Cancer patients often have an activated clotting system and are at increased risk for venous thrombosis. In the present study, we analyzed tissue factor (TF) expression in 4 different human pancreatic tumor cell lines for the purpose of producing derivative tumors in vivo. We found that 2 of the lines expressed TF and released TF-positive microparticles (MPs) into the culture medium. The majority of TF protein in the culture medium was associated with MPs. Only TF-positive cell lines activated coagulation in nude mice, and this activation was abolished by an anti–human TF Ab. Of the 2 TF-positive lines, only one produced detectable levels of human MP TF activity in the plasma when grown orthotopically in nude mice. Surprisingly, < 5% of human TF protein in plasma from tumor-bearing mice was associated with MPs. Mice with TF-positive tumors and elevated levels of circulating TF-positive MPs had increased thrombosis in a saphenous vein model. In contrast, we observed no difference in thrombus weight between tumor-bearing and control mice in an inferior vena cava stenosis model. The results of the present study using a xenograft mouse model suggest that tumor TF activates coagulation, whereas TF on circulating MPs may trigger venous thrombosis.
Introduction
The link between cancer and venous thromboembolism (VTE) is referred to as Trousseau syndrome. Interestingly, different cancer types are associated with different rates of VTE, with pancreatic cancer having one of the highest rates.1,2 A VTE risk-scoring model has been developed that stratifies ambulatory cancer patients undergoing chemotherapy into 3 VTE risk categories based on 5 parameters: (1) the site of the primary tumor, (2) prechemotherapy leukocyte count, (3) platelet count, (4) hemoglobin level, and (5) body mass index.3 Recently, this model was expanded to include the biomarkers D-dimer and soluble P-selectin.4
Another potential circulating biomarker of VTE risk in pancreatic cancer patients is microparticle (MP) tissue factor (TF).5-9 Full-length TF (flTF) is a transmembrane protein that activates the coagulation cascade.10 In addition, an alternatively spliced form of TF (asTF) has been identified that lacks a membrane anchor and therefore can be released as a soluble protein.11 Increased TF expression is correlated with poor prognosis in pancreatic cancer.12-14 Cultured human pancreatic tumor lines express variable levels of both flTF and asTF and release TF-positive MPs containing flTF into the culture medium.14-19 In some patients with pancreatic cancer, high levels of TF-positive MPs are found in the circulation and, in a small pilot study, were predictive of VTE.5-7,9,20
In a mouse model of human colorectal tumors, human TF protein is released into the circulation.21 In nude mice bearing orthotopic human pancreatic tumors (L3.6pl) plasma levels of human TF protein were correlated with the levels of thrombin-antithrombin (TAT) complex, a marker of the activation of coagulation.22 Further, plasma from these tumor-bearing mice was found to enhance thrombin generation in vitro in a human TF-dependent manner.22 Another study found that human (SOJ-4) and mouse (PANC02) pancreatic cell lines expressed TF, and the investigators observed an accumulation of tumor-derived MPs at the site of thrombosis and increased thrombosis in a microvascular model.18
The objective of the present study was to determine the role of tumor-derived TF in the activation of coagulation and thrombosis in a xenograft mouse model of human pancreatic tumors. We found that only TF-positive tumors activated coagulation and that this activation was abolished by inhibition of human TF. Two TF-positive pancreatic tumor cell lines activated coagulation, but only one had detectable levels of circulating TF-positive MPs, which suggested that activation of coagulation was due to TF expression by the tumor itself rather than to TF on the MPs. Mice with elevated levels of TF-positive MPs exhibited increased thrombosis in a saphenous vein model, but not in an inferior vena cava (IVC) stenosis model.
Methods
Cell lines
Human pancreatic (MIAPaCa-2 [CRL-1420], PANC-1 [CRL-146]), Capan-2 [HTB 80], HPAF-II [CRL-1997] and HPAC [CRL-2119]) and colorectal cancer cell lines (HT-29 [HTB-38], SW620 [CCL-227], and HCT116 [CCL-247]) were obtained from ATCC and cultured in their recommended medium in a 5% CO2 incubator at 37°C.
Abs and proteins
PE-labeled mouse IgG control (#555574), mouse anti–human TF (#550312) and FITC-conjugated anti–human MUC-1 (CD227; #559774) Abs were obtained from BD Biosciences. FITC-conjugated goat anti–human IgG (AP113F) was obtained from Millipore. A goat polyclonal Ab against GAPDH (sc-48167) was obtained from Santa Cruz Biotechnology. A rat inhibitory anti–mouse TF mAb (1H1) was obtained from Genentech Inc.23 A mouse inhibitory anti–human TF mAb (HTF-1) was used to detect and inhibit human TF.24 Rabbit polyclonal anti-flTF and anti-asTF Abs were used to detect the different forms of TF.11 Human FVIIa and human FX were purchased from Enzyme Research Laboratories. Recombinant mouse FVIIa was kindly provided by Dr Lars Petersen (Novo Nordisk, Bagsvaerd, Denmark).25
RNA isolation and microarray hybridization
cDNA was labeled with Cy5-dUTP and a reference control (Stratagene) was labeled with Cy3-dUTP using a low-RNA input linear amplification kit (Agilent Technologies) and hybridized overnight at 65°C to Agilent 4 × 44K whole human genome arrays. Arrays were washed and scanned (Agilent Technologies) and images were uploaded to the University of North Carolina microarray database (https://genome.unc.edu). Data were analyzed using LOWESS normalization. Data were excluded for genes with poor spot quality or those that did not have a mean intensity greater than 10 for 1 of the 2 channels (green and red) in at least 70% of the experiments. The log2 ratio of the mean red intensity over mean green intensity was calculated for each gene and went through LOWESS normalization.26 Missing data were imputed using the k-nearest neighbors imputation (KNN) with k = 10.27 All microarray data are available at the Gene Expression Omnibus under accession number GSE37575.
Measurement of TF mRNA levels in pancreatic lines
PCR amplification was performed at 94°C for 3 minutes, 94°C for 45 seconds, 55°C for 30 seconds, 72°C for 45 seconds (29 cycles), and 72°C for 10 minutes. The TF primers were: forward, 5′-AAT GTG GAG AGC ACC GGT TC-3′ and reverse, 5′-CGT TCA TCT TCT ACG GTC ACA TTC-3′. The GAPDH primers were: forward, 5′-CAT GTT CGT CAT GGG TGT GAA CCA-3′ and reverse, 5′-AGT GAT GGC ATG GAC TGT GGT CAT-3′.
Western blotting
Western blotting was performed as described previously.28 Serum-free cell culture medium was centrifuged at 100 000g for 1 hour at 4°C to pellet MPs. The MP-free supernatant was collected and proteins precipitated using ammonium sulfate (final concentration, 0.315 g/mL). Samples were mixed with 6 × Laemmli sample buffer, boiled for 5 minutes, and then applied to Novex 4%-12% Tris-glycine gels to separate the proteins using electrophoresis before transferring to polyvinylidene difluoride membranes (Millipore). Membranes were blocked for 1 hour with Odyssey blocking buffer (LI-COR Biosciences). Primary Abs were incubated overnight at 4°C. After washing 3 times with PBS containing 0.1% Tween-20, membranes were incubated with fluorescence-labeled secondary Abs (1:15 000 dilution) for 1 hour. Membranes were then washed 3 times and analyzed using an Odyssey Infrared Imaging System (LI-COR Biosciences).
ELISA
The human TF antigen ELISA kit (no. 845) was obtained from American Diagnostica. The Enzygnost TAT micro ELISA kit (OWMG15) was obtained from Siemens Healthcare Diagnostics. The Asserachrom D-dimer kit (no. 00947) was obtained from Diagnostica Stago. For some experiments, the culture medium was centrifuged at 100 000g for 1 hour to pellet the MPs and generate MP-free medium.
One-stage clotting assay
The procoagulant activity of cell or tumor lysates was measured using a 1-stage clotting assay.29 The clotting times were determined using a Start4 clotting machine (Diagnostica Stago) and then converted into TF activity units based on a standard curve generated using human recombinant Innovin TF (Dade Behring) and human plasma. The TF activity of each sample was normalized to the total protein concentration.
Flow cytometric analysis on cells and MPs
Cells were detached using Versene Buffer (0.54mM EDTA, 140mM NaCl, 2.7mM KCl, 8.1mM Na2HPO4, 1.46mM KH2PO4, and 1mM glucose, pH 7.4), washed once, and resuspended in PBS supplemented with 1% BSA (PBS/BSA). Next, 5 × 105 cells were incubated with either PE-conjugated mouse IgG control or anti–human TF Ab (HTF-1) at room temperature for 1 hour with end-to-end rotation. Cells were centrifuged at 500g for 5 minutes, washed once, and resuspended in PBS/BSA buffer before samples were analyzed using a Cyan Flow Cytometer (Beckman-Coulter) with FlowJo Version 7.6.4 software (TreeStar). For analysis of MPs, serum-free culture medium was collected from serum-starved cells and centrifuged at 500g for 5 minutes, followed by 1500g for 15 minutes to remove cells. Then, 200 μL of MP-containing medium was incubated with either PE-conjugated mouse anti–human TF (HTF-1), FITC-conjugated anti–human MUC-1 (CD227), or an isotype control for 30 minutes at room temperature. MPs were pelleted at 20 000g for 15 minutes, resuspended in PBS/BSA buffer, and analyzed using a LSRII Flow Cytometer (BD Biosciences) with FlowJo Version 7.6.4 software (TreeStar).
Measurement of MP TF activity in cell culture medium or mouse plasma
MP TF activity was measured using a 2-stage clotting assay as described previously.30 Briefly, 100 μL of either culture medium or plasma was added to 1 mL of HBSA buffer (137mM NaCl, 5.38mM KCl, 5.55mM glucose, 10mM HEPES, and 0.1% BSA, pH 7.5) and centrifuged at 20 000g for 30 minutes at 4°C to pellet the MPs. MPs were then washed once with 1 mL of HBSA buffer and resuspended in 100 μL of HBSA buffer. MPs derived from mouse plasma were incubated with mouse FVIIa and FX in the presence of an anti–mouse TF Ab (1H1), anti–human TF Ab (HTF-1), or isotype IgG controls, followed by the addition of FXa substrate (FXa 8595) to measure the total FXa generation. Human FVIIa was used for MPs derived from culture medium.
Measurement of phosphatidylserine-positive microparticles
The level of phosphatidylserine (PS)–positive MPs was measured using a Zymuphen MP-activity kit (Hyphen BioMed).
Mouse pancreatic tumor models
All animal studies were approved by University of North Carolina at Chapel Hill Animal Care and Use Committee and comply with National Institute of Health guidelines. Nude mice (6-8 weeks of age) were purchased from Harlan Sprague Dawley. Human pancreatic cells (1 × 106 cells per mouse) were injected orthotopically into the pancreas of mice and tumors were grown for 8 weeks. For the subcutaneous tumor model, HPAF-II cells (1 × 106 cells per mouse) were injected into the right flank of mice and tumors were grown for 6 weeks. Tumor size was monitored every 3 days. Whole blood was collected from the IVC into sodium citrate (final concentration, 0.38%). Mouse plasma was prepared by centrifuging at 4000g for 15 minutes, followed by a clearance spin of 13 000g for 2 minutes. Plasma was divided into 50-μL aliquots and frozen at −80°C.
Thrombosis models
The IVC stenosis model was performed as described previously, reducing lumen size by approximately 90%.31 Side branches of the IVC were not ligated. Blood was collected from the IVC just above the ligation site 3 hours after stenosis. Mice were then euthanized and thrombi were weighed. The FeCl3-induced saphenous vein thrombosis model was performed as described previously.32 Blood flow was monitored using a 20-MHz Doppler flow probe (Indus Instruments). Occlusion was defined as the absence of blood flow for 60 seconds. Time to occlusion was defined as the time after injury at cessation of blood flow. The experiment was terminated at 40 minutes.
Statistical analysis
Data are shown as means ± SD. Differences between groups were analyzed using the Student t test or 1-way ANOVA with a Dunn multiple comparison test. P < .05 was considered statistically significant.
Results
Analysis of TF mRNA expression in human pancreatic and colorectal cancer cell lines
The goal of the present study was to evaluate the contribution of pancreatic tumor-derived TF to the activation of coagulation and thrombosis in a xenograft mouse model. To select appropriate cell lines, we analyzed levels of TF mRNA in 8 different human cancer cell lines (5 pancreatic and 3 colorectal lines) using an in-house gene array analysis and the Wooster Cell Line Database (Oncomine; Table 1). Interestingly, there was a wide variation in TF mRNA expression in the different cell lines. The pancreatic cell lines HPAF-II, HPAC, and Capan-2 expressed high levels of TF mRNA, whereas little or no expression was detected in the MIAPaCa-2 and PANC-1 pancreatic lines. The colorectal line HCT116 expressed lower levels of TF mRNA than the TF-positive pancreatic lines, but these levels were higher than those observed in the HT-29 and SW620 cell lines.
Characterization of TF expression in 4 human pancreatic tumor cell lines
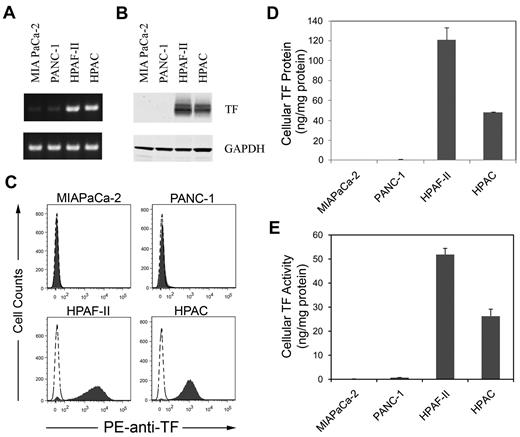
We selected 2 TF-positive (HPAF-II and HPAC) and 2 low-TF–expressing (MIAPaCa-2 and PANC-1) human pancreatic cancer lines for further studies. MIAPaCa-2, PANC-1, and HPAF-II cells contain mutations in K-ras and p53 characteristic of pancreatic ductal adenocarcinoma.33,34 Consistent with the gene array data, HPAF-II and HPAC cells expressed high levels of TF mRNA, protein, and activity, whereas MIAPaCa-2 and PANC-1 cells had undetectable levels of TF expression (Figure 1A-E). HPAF-II cells expressed more TF protein and activity than did HPAC cells (Figure 1D-E).
Levels of TF expression in 4 human pancreatic cell lines. TF mRNA (A) was measured using RT-PCR and TF protein (B) was measuring by Western blot. GAPDH was used as the loading control. TF protein expression on the cells was determined by flow cytometry using a PE-conjugated HTF-1 Ab (C). Levels of TF protein (D) was measured by ELISA and TF activity (E) was measured by a 1-stage clotting assay (n = 3).
Levels of TF expression in 4 human pancreatic cell lines. TF mRNA (A) was measured using RT-PCR and TF protein (B) was measuring by Western blot. GAPDH was used as the loading control. TF protein expression on the cells was determined by flow cytometry using a PE-conjugated HTF-1 Ab (C). Levels of TF protein (D) was measured by ELISA and TF activity (E) was measured by a 1-stage clotting assay (n = 3).
Measurement of TF protein and MP TF activity in the culture medium of human pancreatic tumor cell lines
Cancer cells in culture spontaneously release procoagulant MPs into the culture medium.34,35 We showed previously that all TF activity in the culture medium of HCT116 cells and the squamous cell carcinoma cell line A431 was associated with MPs.15 As expected, in the present study, HPAF-II and HPAC cells, but not MIAPaCa-2 and PANC-1 cells, expressed TF and released TF-positive MPs into the culture medium (Figure 2A-B). The amount of MP TF activity in the culture medium was similar for both HPAF-II and HPAC cells, but HPAC cells released more TF protein (Figure 2B). MIAPaCa-2 and PANC-1 cells released more PS-positive MPs than did HPAF-II and HPAC cells (Figure 2B). We also determined the amount of TF protein and activity associated with the MPs. Almost all of the TF activity and most of the TF protein in the culture medium of HPAFII cells was associated with the MPs (Figure 2C). However, a significant amount of human TF protein signal in the culture medium was also detected in the MP-free culture medium (Figure 2C). To further analyze the nature of the TF protein in the MPs and MP-free medium, we analyzed TF protein in the 2 culture medium fractions by Western blot. As expected, MPs contained flTF, whereas the MP-free culture medium from HPAF-II and HPAC cells contained degraded TF (Figure 2D). Although the anti-TF polyclonal Ab did not detect any intact asTF, we reprobed the membrane with an anti-asTF–specific Ab, but still did not detect any asTF in the culture medium or in MPs from HPAF-II and HPAC cells (Figure 2D).
Levels of TF expression in the culture medium of human pancreatic cell lines. Confluent cells were serum starved overnight and medium was prepared by centrifugation at 500g for 5 minutes and 1500g for 15 minutes. Levels of TF-positive MPs were determined by flow cytometry using a PE-labeled HTF-1 Ab (A). Levels of human TF protein were measured using a TF ELISA (B-C). Levels of TF activity on the MPs were measured using the MP TF activity assay (B-C). PS-positive MPs were measured using the Zymuphen MP-activity kit (B). All data are shown as means ± SD (n = 4). In panel C, the levels of TF protein and TF activity in the culture medium (CM), MPs, and MP-free supernatant (SN) were measured. The culture medium was centrifuged at 100 000g for 1 hour to pellet the MPs. Proteins in the SN were precipitated with ammonium sulfate. TF was detected by Western blot using specific Abs against flTF or asTF. Recombinant flTF (Innovin TF) and recombinant asTF were used as controls.
Levels of TF expression in the culture medium of human pancreatic cell lines. Confluent cells were serum starved overnight and medium was prepared by centrifugation at 500g for 5 minutes and 1500g for 15 minutes. Levels of TF-positive MPs were determined by flow cytometry using a PE-labeled HTF-1 Ab (A). Levels of human TF protein were measured using a TF ELISA (B-C). Levels of TF activity on the MPs were measured using the MP TF activity assay (B-C). PS-positive MPs were measured using the Zymuphen MP-activity kit (B). All data are shown as means ± SD (n = 4). In panel C, the levels of TF protein and TF activity in the culture medium (CM), MPs, and MP-free supernatant (SN) were measured. The culture medium was centrifuged at 100 000g for 1 hour to pellet the MPs. Proteins in the SN were precipitated with ammonium sulfate. TF was detected by Western blot using specific Abs against flTF or asTF. Recombinant flTF (Innovin TF) and recombinant asTF were used as controls.
Assessment of activation of coagulation in mice containing TF-positive tumors grown subcutaneously or orthotopically
The growth of subcutaneous tumors is much easier to monitor, but orthotopic tumors are a better model of human disease because tumor cells grow in the organ of origin. Both subcutaneous and orthotopic tumor mouse models have been used to study the release of tumor-derived TF protein and TF-positive MPs into the circulation.21,22 We chose to use HPAF-II cells in the present study because they expressed the highest levels of TF. Similarly sized orthotopic and subcutaneous HPAF-II tumors were grown in nude mice (orthotopic tumors 0.97 ± 0.33 g, n = 7; subcutaneous tumors 0.98 ± 0.13 g, n = 6) and TF protein in the plasma and activation of coagulation were measured. Mice bearing orthotopic tumors had significantly higher levels of human TF protein and MP TF activity compared with subcutaneous tumors (Figure 3A-B), and only mice with orthotopic tumors had elevated levels of TAT and D-dimer (Figure 3C-D).
Comparison of the levels of TF in the plasma and activation of coagulation in mice containing orthotopic and subcutaneous HPAF-II tumors. HPAF-II cells were injected into the pancreas (n = 7) or the right flank (n = 6) of nude mice and tumors were grown for 6-8 weeks. Blood was drawn from the IVC and plasma was prepared. Plasma levels of human TF protein (A), MP TF activity (B), TAT (C), and D-dimer (D) were measured and results are shown as means ± SD. Orthotopic HPAF-II pancreatic tumors were grown in nude mice for 8 weeks. Tumor-bearing mice were injected intraperitoneally twice with HTF-1 (n = 4) or mouse IgG (n = 4). Mice were euthanized after 24 hours and blood was collected from the IVC and plasma was prepared. Plasma levels of TAT (E) and D-dimer (F) were measured and are shown as means ± SD. *P < .05.
Comparison of the levels of TF in the plasma and activation of coagulation in mice containing orthotopic and subcutaneous HPAF-II tumors. HPAF-II cells were injected into the pancreas (n = 7) or the right flank (n = 6) of nude mice and tumors were grown for 6-8 weeks. Blood was drawn from the IVC and plasma was prepared. Plasma levels of human TF protein (A), MP TF activity (B), TAT (C), and D-dimer (D) were measured and results are shown as means ± SD. Orthotopic HPAF-II pancreatic tumors were grown in nude mice for 8 weeks. Tumor-bearing mice were injected intraperitoneally twice with HTF-1 (n = 4) or mouse IgG (n = 4). Mice were euthanized after 24 hours and blood was collected from the IVC and plasma was prepared. Plasma levels of TAT (E) and D-dimer (F) were measured and are shown as means ± SD. *P < .05.
Tumor-derived human TF activates coagulation in mice
A previous study showed that tumor-derived TF levels are correlated with TAT levels in tumor-bearing mice, and tumor-derived human TF present in mouse plasma increased thrombin generation in vitro.22 However, this result does not demonstrate that tumor-derived human TF is responsible for the activation of coagulation in mice. In the present study, we determined the role of tumor-derived human TF in the activation of coagulation directly by treating mice bearing orthotopic HPAF-II tumors with an inhibitory anti–human TF mAb (HTF-1). We have shown previously that HTF-1 specifically blocks human TF without affecting mouse TF.30 Mice bearing HPAF-II tumors received 2 IP injections of either HTF-1 (20 mg/kg) or mouse IgG (20 mg/kg) 2 days apart, and mice were euthanized 1 day later. HTF-1 treatment reduced plasma levels of TAT and D-dimer significantly (Figure 3E-F), demonstrating that tumor-derived human TF accounts for all of the activation of coagulation in this mouse xenograft model of pancreatic cancer.
Comparison of levels of MP TF activity and activation of coagulation in mice containing TF-negative and TF-positive human pancreatic tumors
We also measured the levels of MP TF activity and activation of coagulation in nude mice bearing tumors derived from the different human pancreatic cell lines. In a pilot experiment, we investigated whether the different cell lines (MIAPaCa-2, PANC-1, Capan-2, HPAF-II, HT-29, SW620, and HCT116) activated coagulation in nude mice when tumors were grown orthotopically to a size of approximately 1 g. Only mice containing HPAF-II tumors exhibited a significant increase in levels of TAT in the plasma (data not shown). Similarly, no elevation in plasma TAT levels was observed in mice containing approximately 1 g of HCT116 tumors grown subcutaneously (J.R., unpublished data, 2004). These studies and the in vitro studies described above led us to select 2 TF-positive (HPAF-II and HPAC) and 2 low TF expressing (MIAPaCa-2, PANC-1) cell lines for further studies.
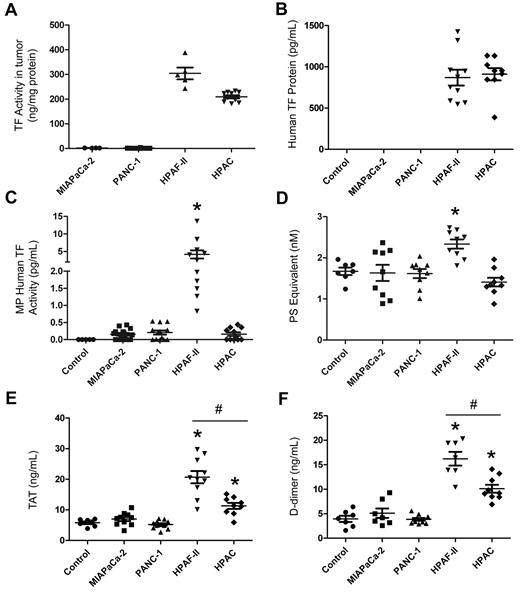
Tumors were grown orthotopically in nude mice and levels of human TF protein, MP TF activity, PS-positive MPs and activation of coagulation were measured. Tumors formed from the different lines were all approximately 1 g (MIAPaCa-2, 0.90 ± 0.21 g, n = 12; PANC-1: 0.85 ± 0.16 g, n = 13; HPAF-II: 0.98 ± 0.30 g, n = 11; HPAC, 1.03 ± 0.11 g, n = 11). As expected, we found that HPAF-II and HPAC tumors isolated from mice expressed high levels of TF activity, whereas no TF activity was detected in MIAPaCa-2 and PANC-1 tumors (Figure 4A). HPAF-II cell–derived tumors have slightly higher levels of TF expression than HPAC cell–derived tumors. Interestingly, the TF activity of the primary tumors isolated from tumor-bearing mice was approximately 6-fold higher than that on the parental cell in culture (300 ng/mg of protein on HPAF-II tumors vs 50 ng/mg of protein on HPAF-II cells). No human TF protein was detected in control mice (no tumor) or mice bearing MIAPaCa-2 or PANC-1 tumors, whereas human TF protein was present in plasma from mice bearing HPAF-II or HPAC tumors (Figure 4B). Ninety-five percent of the human TF protein signal observed in the plasma of mice bearing TF-positive tumors was present in MP-free plasma, with the remainder associated with MPs (data not shown).
Analysis of circulating levels of TF protein, MP TF activity, PS-positive MPs, and activation of coagulation in mice with different human pancreatic tumors. Four pancreatic cancer cell lines (MIAPaCa-2, PANC-1, HPAF-II, and HPAC) were injected into the pancreas of nude mice and tumors were grown for 8 weeks (n = 4-11 mice per group). Blood was collected from the IVC and plasma was prepared. TF activity of the tumor lysate (A) and levels of plasma human TF protein (B), human TF activity in MPs (C), PS-positive MPs (D), TAT (E), and D-dimer (F) were measured and are shown as dot plots with means ± SEM. Differences between groups were analyzed using 1-way ANOVA with the Dunn multiple comparison test. *P < .05 control versus HPAF-II (C-F) or HPAC (E-F); #P < .05 HPAF-II versus HPAC (E-F).
Analysis of circulating levels of TF protein, MP TF activity, PS-positive MPs, and activation of coagulation in mice with different human pancreatic tumors. Four pancreatic cancer cell lines (MIAPaCa-2, PANC-1, HPAF-II, and HPAC) were injected into the pancreas of nude mice and tumors were grown for 8 weeks (n = 4-11 mice per group). Blood was collected from the IVC and plasma was prepared. TF activity of the tumor lysate (A) and levels of plasma human TF protein (B), human TF activity in MPs (C), PS-positive MPs (D), TAT (E), and D-dimer (F) were measured and are shown as dot plots with means ± SEM. Differences between groups were analyzed using 1-way ANOVA with the Dunn multiple comparison test. *P < .05 control versus HPAF-II (C-F) or HPAC (E-F); #P < .05 HPAF-II versus HPAC (E-F).
We also measured human and mouse MP TF activity in plasma using the species-specific Abs 1H1 and HTF-1 to inhibit either mouse TF activity in MPs derived from the host or human TF activity in MPs derived from the tumor, respectively. A high level of tumor-derived human MP TF activity was observed in mice bearing HPAF-II tumors, whereas only background levels of human TF activity were observed in MPs isolated from plasma of HPAC tumors (Figure 4C). Mice containing MIAPaCa-2 or PANC-1 tumors had no detectable human MP TF activity (Figure 4C). A very low baseline of host-derived mouse MP TF activity (0.11 ± 0.13 pg/mL, n = 13) was observed in control mice, and the level was not significantly increased in the plasma of mice bearing tumors derived from any of the 4 cell lines (MIAPaCa-2, 0.08 ± 0.08 pg/mL, n = 12 tumors; PANC-1, 0.09 ± 0.10 pg/mL, n = 13 tumors; HPAF-II, 0.12 ± 0.21 pg/mL, n = 11 tumors; and HPAC, 0.13 ± 0.11 pg/mL, n = 11 tumors).
The levels of PS-positive MPs in mice bearing MIAPaCa-2, PANC-1, and HPAC tumors were similar to control mice, but levels were elevated in mice with HPAF-II tumors (Figure 4D). Finally, we measured levels of TAT and D-dimer in plasma from tumor-bearing mice and controls. We observed baseline levels of TAT and D-dimer in control mice (no tumor) and no increases in mice with the TF-negative tumors (Figure 4E-F). In contrast, mice with HPAF-II and HPAC tumors had significantly higher levels of both TAT and D-dimer compared with controls, indicating activation of coagulation (Figure 4E-F). Levels of TAT and D-dimer in the plasma of mice bearing HPAF-II tumors were significantly higher than those from mice bearing HPAC tumors. These results indicate that only TF-positive tumors activate coagulation, and that the levels of TF-positive MPs are not always correlated with the levels of TAT.
Analysis of MPs from HPAF-II and HPAC cells
The absence of detectable levels of TF-positive MPs in mice bearing HPAC tumors suggested that these MPs may be removed from the circulation more rapidly than are HPAF-II MPs. To examine this possibility, MPs were collected from the culture medium of HPAF-II and HPAC cells and injected into mice to examine the rate of clearance. We injected 1 ng of MP TF activity for each tumor line into nude mice. The MPs were cleared very rapidly from the circulation. No difference in clearance was observed between HPAF-II and HPAC MPs (Figure 5A). We also measured plasma TAT levels in the mice with the injected MPs. Both HPAF-II and HPAC MPs increased TAT levels significantly, but higher levels were observed with HPAF-II MPs (Figure 5B).
Analysis of MPs from HPAF-II and HPAC cells. (A) Nude mice were injected intravenously with 100 μL of HPAF-II MPs (1 ng of TF activity) or HPAC MPs (1 ng of TF activity), blood was collected immediately (n = 2) or after 2 minutes (n = 3), and human TF activity in isolated MPs was determined. (B) Nude mice were injected intravenously with 100 μL of PBS (n = 6), HPAF-II MPs (1 ng of TF activity, n = 6), or HPAC MPs (1 ng of TF activity, n = 6), and blood was collected 15 minutes later. Plasma TAT levels were measured and results are shown as dot plots with means ± SEM. *P < .05. (C) MUC-1 expression on MPs from HPAF-II and HPAC cells. MPs in serum-free culture medium from HPAF-II and HPAC cells were incubated with FITC labeled anti–human MUC-1 Ab or FITC-labeled isotype control. Stained MPs were analyzed with an LSRII flow cytometer. Results are shown as histograms of the log fluorescence intensity.
Analysis of MPs from HPAF-II and HPAC cells. (A) Nude mice were injected intravenously with 100 μL of HPAF-II MPs (1 ng of TF activity) or HPAC MPs (1 ng of TF activity), blood was collected immediately (n = 2) or after 2 minutes (n = 3), and human TF activity in isolated MPs was determined. (B) Nude mice were injected intravenously with 100 μL of PBS (n = 6), HPAF-II MPs (1 ng of TF activity, n = 6), or HPAC MPs (1 ng of TF activity, n = 6), and blood was collected 15 minutes later. Plasma TAT levels were measured and results are shown as dot plots with means ± SEM. *P < .05. (C) MUC-1 expression on MPs from HPAF-II and HPAC cells. MPs in serum-free culture medium from HPAF-II and HPAC cells were incubated with FITC labeled anti–human MUC-1 Ab or FITC-labeled isotype control. Stained MPs were analyzed with an LSRII flow cytometer. Results are shown as histograms of the log fluorescence intensity.
We speculated that HPAF-II and HPAC MPs express different proteins on their surface. Mucins are expressed by epithelial cells, and mucin-1 (MUC-1) in particular is often expressed by pancreatic adenocarcinomas.36 Indeed, MUC-1 was detected previously on MPs isolated from patients with pancreatic tumors.8 Interestingly, in the present study, we found high MUC-1 expression on the surface of HPAC MPs, but not HPAF-II MPs (Figure 5C).
Analysis of venous thrombosis in tumor-bearing mice
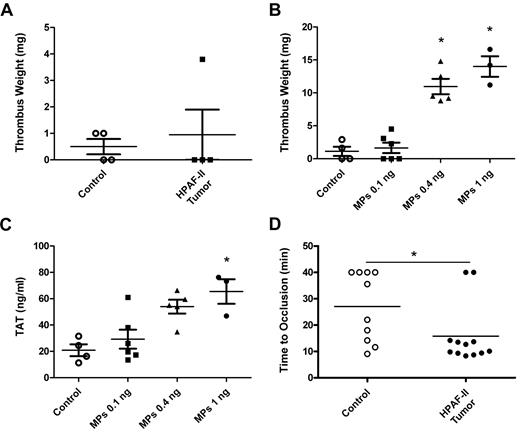
Previous studies have reported an increase in thrombosis in the microvasculature of the mesentery of tumor-bearing mice.18 We chose to examine thrombosis in larger vessels to more closely model venous thrombosis in cancer patients. First, we analyzed thrombosis in control mice and mice containing HPAF-II tumors using an IVC stenosis model. A previous study reported increased thrombosis at 3 hours in mice containing PANC02 tumors compared with controls.37 In contrast, in the present study, we observed similar-sized thrombi in mice bearing HPAF-II tumors and control mice (Figure 6A), which suggested that the levels of TF-positive MPs present in tumor-bearing mice were insufficient to enhance thrombosis in this model. We determined the level of exogenous TF-positive MPs that were required to increase thrombosis in this IVC stenosis model. Mice were injected with different amounts of HPAF-II MPs at 15 minutes and 1 hour and then thrombus weights were measured at 3 hours. A level of 0.4 or 1 ng (40 and 100 times the level of endogenous TF-positive MPs observed in mice bearing HPAF-II tumors, respectively) was required to increase thrombosis (Figure 6B). Similarly, high levels of exogenous TF-positive MPs were required to increase TAT levels (Figure 6C). Next, we analyzed thrombosis in a saphenous vein injured with ferric chloride and found that mice with HPAF-II tumors grown orthotopically had significantly shortened occlusion times in this venous thrombosis model compared with control mice (Figure 6D).
Thrombosis in mice bearing HPAF-II tumors. IVC stenosis in control mice (n = 4) or tumor-bearing mice (n = 4) was induced. Three hours after the ligation, all mice were euthanized and the weight of the thrombus (in milligrams) was determined (A). IVC stenosis in control nude mice was performed. These mice were injected with PBS or the indicated doses (0.1, 0.4, and 1 ng of TF activity) of exogenous HPAF-II MPs twice at 15 minutes and 1 hour after the ligation. Blood was collected from the IVC just above the ligation site 3 hours after the ligation. Thrombus weight (B) and TAT levels (C) were measured. Saphenous vein thrombosis was induced by 10% FeCl3 in control (n = 10) and HPAF-II tumor-bearing mice (n = 12; D). Occlusion times were measured and are shown as dot plots with means. *P < .05.
Thrombosis in mice bearing HPAF-II tumors. IVC stenosis in control mice (n = 4) or tumor-bearing mice (n = 4) was induced. Three hours after the ligation, all mice were euthanized and the weight of the thrombus (in milligrams) was determined (A). IVC stenosis in control nude mice was performed. These mice were injected with PBS or the indicated doses (0.1, 0.4, and 1 ng of TF activity) of exogenous HPAF-II MPs twice at 15 minutes and 1 hour after the ligation. Blood was collected from the IVC just above the ligation site 3 hours after the ligation. Thrombus weight (B) and TAT levels (C) were measured. Saphenous vein thrombosis was induced by 10% FeCl3 in control (n = 10) and HPAF-II tumor-bearing mice (n = 12; D). Occlusion times were measured and are shown as dot plots with means. *P < .05.
Discussion
In the present study, we found a significant increase in the activation of coagulation only in mice containing TF-expressing HPAF-II or HPAC human pancreatic tumors grown orthotopically. In contrast, no activation of coagulation was observed in mice containing the same size HPAF-II tumors grown subcutaneously. The activation of coagulation in HPAF-II tumor-bearing mice was inhibited by the administration of an anti–human TF mAb, which indicates that tumor-derived TF is responsible for the activation of coagulation in this model.
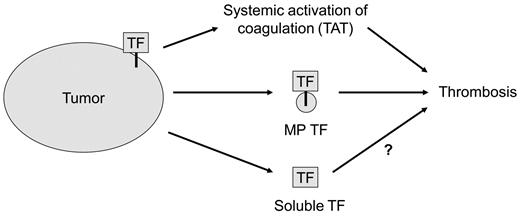
The 2 sources of human TF in the tumor-bearing mice were the tumor itself and the circulating TF-positive MPs. However, the levels of tumor TF were considerably higher than the levels of TF present on circulating MPs. Although most of the TF in solid tumors is separated from the blood, the tumor vasculature is inherently leaky and this allows circulating macromolecules such as clotting factors to enter the tumor.38 We propose that clotting factors enter the tumor and are activated in a TF-dependent manner, and then activated coagulation proteases, such as thrombin, re-enter the bloodstream. Interestingly, in the present study, 2 different TF-positive pancreatic lines activated coagulation in mice, but only 1 line had detectable levels of TF-positive MPs in the circulation. Furthermore, the level of exogenous TF-positive MPs derived from HPAF-II cells required to increase TAT levels was 100-fold higher than the level of endogenous TF-positive MPs present in tumor-bearing mice. These results suggest that TF expression in the tumor itself is the major source of TF that leads to systemic activation of coagulation (Figure 7).
Sources of tumor TF that activate coagulation and enhance thrombosis. We propose that tumor TF may cause the activation of coagulation that leads to increased levels of circulating TAT in the circulation, whereas TF-positive MPs may enhance venous thrombosis at a distal site. Tumors also release degraded TF protein (soluble TF) into the plasma.
Sources of tumor TF that activate coagulation and enhance thrombosis. We propose that tumor TF may cause the activation of coagulation that leads to increased levels of circulating TAT in the circulation, whereas TF-positive MPs may enhance venous thrombosis at a distal site. Tumors also release degraded TF protein (soluble TF) into the plasma.
The results of the present study highlight the importance of analyzing several different human pancreatic tumor cell lines before selecting one to use in a xenograft mouse model. Based on clinical studies, we selected 2 cell lines (HPAF-II and HPAC) that expressed TF and released TF-positive MPs into the culture medium. Surprisingly, only HPAF-II tumors produced detectable levels of TF-positive MPs in the plasma of nude mice. We observed a similar clearance of exogenous HPAF-II and HPAC MPs from the circulation, although the HPAF-II MPs were more procoagulant. Mice with HPAF-II tumors also had increased levels of PS-positive MPs, although the mechanism for this is not clear. These results indicate that HPAF-II cells are the best cell line with which to study the role of TF-positive MPs in venous thrombosis. However, MPs isolated from patients with pancreatic tumors also express the mucin MUC-1.8 We found that both HPAC and HPAF-II cells expressed MUC-1 (data not shown), but only MPs from HPAC cells had detectable levels of MUC-1. At present, it is unclear whether MUC-1 plays a role in enhancing venous thrombosis. Further studies are required to determine which proteins on tumor-derived MPs affect their thrombogenicity and binding properties.
In a previous study, we found that levels of human TF protein and MP TF activity were correlated in plasma samples from pancreatic cancer patients.6 Therefore, we were surprised that in the present study, mice containing HPAC tumors had elevated levels of human TF protein but not human MP TF activity. Furthermore, the majority of the human TF protein present in the plasma of mice bearing HPAF-II tumors was not associated with MPs. We found that the TF protein in the MP-free culture supernatant of HPAF-II and HPAC cells was degraded. We could not analyze the integrity of human TF in the plasma of tumor-bearing mice because of insufficient sample size; however, we speculate that this TF protein signal may represent degraded protein. A previous study indicated that the TF found in human plasma is not flTF because the isolated TF had a molecular weight of approximately 29 kDa by Western blotting rather than the expected size of 47 kDa.39 These results suggest that tumors release both functionally active flTF on MPs and inactive, degraded TF protein. Measurement of the level of human TF protein in plasma is easier than the measurement of MP TF activity, but has a lower sensitivity, and concerns have been raised about the specificity of some of the commercial ELISAs.6,40,41 The MP TF activity assay has greater sensitivity but more variability than an ELISA for detecting elevated levels of TF in the plasma of cancer patients. These results suggest that it would be best to measure plasma MP TF activity in addition to plasma TF protein levels to identify cancer patients who have an increased risk of thrombosis.
A recent study reported that tumor-derived MPs bind to a site of vascular injury and that mice with PANC02 tumors have increased mesenteric microvascular thrombosis compared with controls.18 Similarly, in the present study, we found that mice bearing HPAF-II tumors had enhanced thrombosis compared with control mice in a saphenous vein model of thrombosis. Mice containing subcutaneous PANC02 tumors were also found to have larger thrombi in a 3-hour IVC stenosis model.37 In contrast, in the present study, we did not observe an increase in thrombosis in mice bearing HPAF-II tumors. In fact, we found that the level of exogenous HPAF-II TF-positive MPs required to increase thrombosis in this model was 40 times greater than the level of endogenous TF-positive MPs in HPAF-II tumor-bearing mice. The reasons for different results between these 2 models are unclear, but may be due to differences in the IVC stenosis model. Interestingly, we showed recently that TF expression by leukocytes triggers thrombosis in an IVC stenosis model.42 Our mouse model of human pancreatic cancer used herein has the advantage of being able to differentiate tumor-derived TF from host-derived TF. Indeed, we did not observe an increase in host-derived MP TF activity in the plasma of mice containing HPAF-II tumors. A limitation of our model is that it is a xenograft model using immunocompromised mice that may alter the host response to the tumor.
In conclusion, several studies have shown that the levels of TF-positive MPs and MP TF activity are increased in pancreatic cancer patients.5,7,8 Moreover, levels of MP TF activity are predictive of VTE in pancreatic cancer patients, and VTE patients with elevated levels of MP TF activity have decreased survival, which may reflect the presence of more aggressive tumors.6,20,43 These results suggest that MP TF may be a good biomarker with which to identify those pancreatic cancer patients at risk for VTE. In our mouse model of human pancreatic cancer, TF expression by the tumor itself seems to be the major source of TF that activates coagulation. Mice bearing HPAF-II tumors and elevated levels of TF-positive MPs exhibited increased saphenous vein thrombosis but no increased thrombosis was seen in an IVC stenosis model. Future studies are required to understand the contribution of TF expression by leukocytes and MPs to venous thrombosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Charlene M. Ross at the animal core facility of Lineberger Comprehensive Cancer Center for technical assistance.
This work was supported by grants from the National Institutes of Health (HL-095096 to N.M. and N.S.K. and HL-094740 to A.S.W.) and the University Cancer Research Fund. M.M.A. was supported by a National Institutes of Health training grant (T32ES007017).
National Institutes of Health
Authorship
Contribution: J.-G.W. designed and performed the experiments, analyzed the results, and wrote the manuscript; J.E.G. performed the experiments and wrote the manuscript. M.M.A, J.C.C, P.C., and J.C.W. performed the experiments; D.K., V.Y.B., and R.R.B. provided important reagents and expertise; J.R., F.C.C., A.S.W., R.P., N.S.K., and J.J.Y. analyzed the data and provided scientific input; and N.M. supervised the experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nigel Mackman, Division of Hematology/Oncology, Department of Medicine, University of North Carolina at Chapel Hill, 98 Manning Dr, Chapel Hill, NC 27599-7035; e-mail: nmackman@med.unc.edu.