Abstract
Allogeneic hematopoietic stem cell transplantation (SCT) is well accepted as a curative treatment approach for younger patients with myelodysplastic syndrome (MDS) and has become one of the most frequent indications for allogeneic SCT as reported to the Center for International Blood and Marrow Transplant Research. However, MDS patients are usually elderly with a median age of approximately 75 years at diagnosis. Large register studies have confirmed the feasibility of the procedure in elderly MDS patients; and in the register of the European Group for Blood and Marrow Transplantation, one-third of the allogeneic transplant procedures for MDS were performed in 2010 in patients older than 60 years. Despite its curative potential, its role in the treatment of elderly MDS patients is less defined. Because of the inherent complications of the transplantation leading to treatment-related mortality and the risk of relapse, a careful calculation of the benefit for each patient is mandatory, taking into account comorbidities, disease status, donor selection, and effective nontransplant therapies. Prospective multicenter studies are needed to define optimal intensity of the conditioning regimen, timing of transplantation within a treatment algorithm, including drug-based therapies, and posttransplant strategies to reduce the risk of relapse.
Introduction
Myelodysplastic syndrome (MDS) summarizes a heterogeneous group of hematologic diseases, which are characterized by a clonal abnormality of hematopoietic stem cells resulting in cytopenias, abnormal blasts, and risk of transformation into acute myeloid leukemia (AML). The clinical course of the disease varies from an indolent course over several years to a more rapid progression within months.1,2 MDS is predominantly a malignant disease of an elderly person, with a median age at diagnosis of approximately 75 years,3 and more than 80% are reported to be older than 60 years.4 Allogeneic stem cell transplantation (SCT) is considered to be a curative treatment option in MDS patients, and its role in treatment of “younger” patients with MDS is well established,5-12 even if the data relating outcomes are obtained mainly from retrospective studies. For many years, because of therapy-related morbidity and mortality, allogeneic SCT has been performed only in younger patients with MDS, whereas elderly patients who represent the majority of MDS patients have been excluded. It has to be pointed out that, in the transplant setting, patients older than 50 years are considered to be “elderly patients.” Furthermore, even when transplantation is performed on patients up to the age of 75 years, the majority of transplanted patients are still younger than the median age of MDS patients at diagnosis (∼ 75 years).
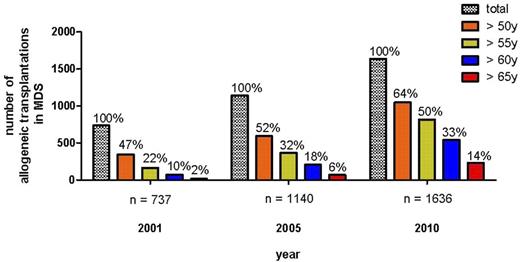
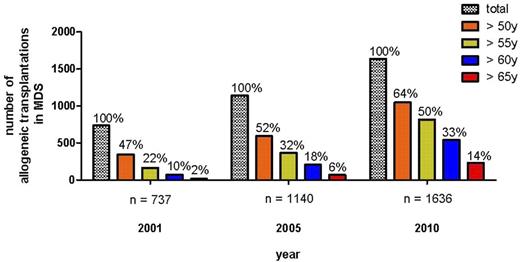
The introduction of toxicity-reduced, reduced-intensity conditioning (RIC) or nonmyeloablative conditioning regimens has resulted in a drastic reduction of transplant-related toxicity and mortality, leading to a rapidly growing number of transplantations in elderly patients with hematologic diseases.13-15 In the register of the European Group for Blood and Marrow Transplantation (EBMT), the numbers of allogeneic SCT for MDS/secondary acute myeloid leukemia have increased from 737 in 2001 to 1636 in 2010 (Figure 1). In parallel, the percentage of transplantation in MDS patients older than 50 years, older than 60 years, or older than 65 years increased from 47%, 10%, and 2%, respectively, in 2001 to 64%, 33%, and 14%, respectively, in 2010 (Figure 1). Despite the increase of literature reporting the feasibility of this approach in elderly MDS patients, prospective comparing trials are lacking; and despite the curative potential even in elderly patients, there is great need to define the population of elderly MDS patients who will benefit with a high probability of long-term freedom from disease versus the inherent complications of the transplantation procedure. It is also important to develop the timing of treatment sequence of allogeneic transplantation in the context of novel drugs, such as hypomethylating agents or immunomodulating drugs.
Development of allogeneic SCT for MDS/sAML in EBMT register according to age categories.
Development of allogeneic SCT for MDS/sAML in EBMT register according to age categories.
Does recipient age influence transplant outcome?
The majority of the large retrospective trials consider a patient's age as a major prognostic factor for therapy-related mortality.7,8,16 In one of these studies, patient age is also associated with a high risk of relapse.16 These results were obtained mainly after standard myeloablative conditioning. Two recent large register trials address the specific issue of elderly MDS patients and allogeneic SCT (Table 1).
The retrospective study of the of the EBMT reported on 1333 patients older than 50 years with MDS.17 Interestingly, despite advanced age, 38% of the patients received a standard myeloablative conditioning regimen. The patients were divided into age categories of 50 to 60 years and more than 60 years. The cumulative incidence of nonrelapse mortality (NRM) at 4 years was 36% for the 50- to 60-year group and 39% for the group 60 years or older (P = .39). The cumulative incidence of relapse at 4 years was 32% for the 50- to 60-year group and 41% for the group 60 years or older (P = .02), whereas overall survival (OS) at 4 years did not differ between the groups (34% vs 27%, P = .2). In a multivariate analysis for OS, only advanced stage of the disease at time of transplantation (hazard ratio [HR] = 1.55) was associated with inferior survival.
The Center for International Blood and Marrow Transplantation (CIBMTR) performed a similar study in 1080 elderly patients with MDS or AML in complete remission (CR) who received an RIC or nonmyeloablative conditioning regimen followed by allogeneic SCT. The patients were divided into 4 age categories: 40 to 54 years, 55 to 69 years, 60 to 65 years, and 65 years of age or older. There was a trend for a higher incidence of relapse and NRM in the group 60 to 65 years and 65 years of age or older compared with the 2 groups younger than 60 years (Table 1), but none of these differences reached statistical significance. However, a selection bias for the older transplant cohort has to be taken into account while interpreting the data. Disease-free survival (DFS) and OS did not differ between the age categories. In a multivariate analysis, age had no significant effect on OS, whereas low performance status, mismatched unrelated donors, and unfavorable cytogenetic were associated with inferior OS.18 It is controversial whether elderly MDS patients who undergo allogeneic SCT have a higher risk of GVHD. Whereas in some HLA-identical sibling transplantation studies recipient age was associated with an increased risk of graft versus host disease,19 in unrelated transplantation the National Marrow and Donor Program reported that only increased donor age was associated with a higher risk of severe acute GVHD.20 In sibling transplantation, donor age is closely linked to recipient age, and so it has been difficult to demonstrate an effect of age on GVHD incidence in matched sibling transplant. A more recent trial, including more than 1000 patients, did not show significant impact of age on occurrence of GVHD.18 However, if GVHD occurs, steroids are often less tolerated in older than in younger patients.
These 2 large retrospectives studies suggest that recipient age alone cannot be considered as contraindication for allogeneic SCT.
Impact of comorbidities
Because patient's age per se is not a major risk factor, other factors, such as comorbidities that by nature increase with increasing age, are taken into account. Comorbidity has a major impact on outcome of MDS patients in the nontransplant setting,21 and several scores have been developed for allogeneic SCT. The Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI), developed by Sorror et al, has been demonstrated to be useful in patients with MDS or AML who underwent allogeneic SCT after RIC or standard conditioning regimen.22 If patients were stratified according to HCT-CI score and disease status, the risk of NRM increased with higher HCT-CI score and high-risk disease status. Patients with the highest HCT-CI score and also high-risk disease had the worst survival rate (OS at 2 years: 29%) independent of the intensity of the conditioning regimen because the lower NRM in the RIC-treated patients was offset by a higher risk of relapse.22 Other reports confirmed inferior survival in MDS patients with higher comorbidity scores after allogeneic SCT independent of the chronologic age of the patients.23,24
Role of iron overload
Iron overload, which is not part of most comorbidity scores, may also influence toxicity and survival after transplantation and should be considered as comorbidity. Iron overload in MDS patients is caused by red blood cell transfusion and also by an increased gastrointestinal absorption of iron because of ineffective erythropoiesis.25 This iron is accumulated in macrophages in tissues, such as liver, spleen, and bone marrow.
Once the capacity of plasma transferrin to bind iron is exhausted, nontransferrin-bound iron (NTBI) appears in blood and labile plasma iron as redox-active component of NTBI and is considered the most likely mediation of tissue damage and higher risk of infectious complications.26
Iron overload is usually determined by serum ferritin in patients who undergo allogeneic SCT. An elevated serum ferritin of more than or equal to 1000 μg/L has been associated with a higher NRM and increased risk of infection after allogeneic SCT and myeloablative conditioning.27,28 The retrospective study by the Guppo Italiano Trapianto Midollo Osseo confirmed the impact of elevated serum ferritin on outcome of allogeneic transplantation but only after myeloablative conditioning and not after RIC.29 More appropriate methods for iron overload, such as liver or cardiac MRI, transfusion frequencies, and measurement of NTBI, and/or labile plasma iron, are needed to determine the impact of iron overload on outcome after SCT.30 Most of the transplant-related mortality resulting from elevated serum ferritin occurs within the first 3 months after SCT.27 It is very likely that the complications of iron overload, such as infections and organ toxicity, are the result of the release of NTBI and labile plasma iron induced by the cytotoxic conditioning regimen catalyzing reactive oxygen species. In our own study, we could show that, in patients who underwent allogeneic SCT after myeloablative conditioning regimen NTBI and transferring, saturation peaked early after starting conditioning therapy and remained elevated up to 2 weeks after initiation of the conditioning regimen.31 First attempts to use chelation, such as deferasirox, during conditioning regimen have shown dramatic reduction of labile plasma iron.32 Alternatively, administration of plasma apotransferrin has been shown to lower NTBI during SCT and restored the inhibitory effect on growth of Staphylococcus epidermidis in vitro.33
The available data suggest a major impact of comorbidity on outcome after transplantation, but the most valuable comorbidity score needs to be determined and validated in larger cohorts of patients. Iron overload can be considered as comorbidity and is associated with a higher NRM. Prospective allogeneic SCT trials in MDS should include measurement of overload and determination of comorbidity indices. However, it is currently unclear how to treat iron overload in patients who undergo allogeneic SCT.
Should all elderly patients receive only RIC?
The exploration of RIC regimens resulted in less toxicity and has broadened the application of allogeneic SCT, especially in the cohort of elderly patients with hematologic malignancies.13,14 The rationale for RIC is to promote graft-versus-leukemia effect without excessive toxicity of the conditioning regimen, therefore resulting in less treatment-related mortality. The EBMT performed a retrospective comparing RIC with standard myeloablative conditioning in 836 patients with MDS who received stem cell grafts from HLA-identical siblings. The EBMT found a lower risk for treatment-related mortality (P = .015) but a significantly higher risk for relapse (P = .001) for the RIC transplanted patients, resulting in similar survival rates in both groups.34 The CIBMTR compared transplant outcome in more than 3000 patients with AML or MDS who received allogeneic SCT either after RIC or nonmyeloablative conditioning or after standard myeloablative conditioning. Those patients who received a RIC regimen had a higher risk of relapse and less probability of disease control compared with those who received a conventional ablative regimen.35 Other single-center retrospective studies have shown that a lower intensity of the conditioning regimen results in higher relapse rate and inferior outcome, especially in patients with more advanced disease.36,37 A more recent retrospective analysis from EBMT also suggests that the very low-intensity approach (2 Gy total body irradiation) has the highest risk of relapse in MDS and AML patients, and the treatment-related mortality after RIC transplantation has not reached a plateau after 1 year and differs only slightly at 5 years compared with myeloablative conditioning (Rodrigo Martino, oral presentation at EBMT meeting, Geneva, April 2012). All these studies have been performed retrospectively, and the inherent bias in selecting patients for a specific intensity of conditioning regimen should be considered because patient assignment to either RIC or myeloablative conditioning was based on physician or patient preference. An ongoing prospective randomized trial comparing RIC versus myeloablative conditioning from the MDS Subcommittee of the EBMT is addressing this issue (#NCT00682396).
However, in discussing intensity of conditioning regimen, it has to be pointed out that there is no clear cut-off between RIC and the myeloablative conditioning regimen. The major achievement of the new conditioning regimens is the reduced organ toxicity that broadens the application of allogeneic SCT to elderly patients. However, reducing organ toxicity does not exclude myeloablation or reduced antileukemic activity. For instance, targeting drug level or using intravenous formulation as part of the conditioning regimen has substantially reduced toxicity of busulfan; both have been shown to reduce transplant-related complications and improve outcome for MDS patients,38,39 allowing the use of this drug at myeloablative doses safely. Furthermore, cyclophosphamide as a toxic drug with only limited antileukemic activity can be substituted by fludarabine, inducing less organ toxicity without obvious loss of antileukemic activity.40 Other alkylating drugs, such as treosulfan, can also be used safely as part of a myeloablative conditioning regimen with low toxicity and NRM in MDS patients.41,42
Even if results from prospective randomized trials are lacking, available literature suggests that patients up to 70 years of age can tolerate myeloablative conditioning regimen and that age per se should not be a criterion for selecting the intensity of the conditioning regimen rather than performance status/comorbidity and the status of the disease at time of transplantation. The current data also support that there is no “one-size-fits-all” conditioning regimen in elderly MDS patients, and the selection of regimen intensity has to become more individualized.
Role of cytogenetics and molecular genetics on outcome
Cytogenetic abnormalities have an important impact on prognosis of patients undergoing allogeneic SCT for MDS. The established risk groups regarding cytogenetic abnormalities within the International Prognostic Scoring System (IPSS) have also major impact on outcome after allogeneic SCT.43,44
The EBMT reported 692 patients with an OS of 47% for good-risk, 40% for intermediate-risk, and 31% for high-risk cytogenetic IPSS.44 The failure to transplant was mainly attributed to a higher risk of relapse, which was 34% for good-risk, 35% for intermediate-risk, and 57% for high-risk patients.44 Once patients with IPSS high-risk cytogenetics were analyzed in detail, more recent data from EBMT suggest that single abnormalities on chromosome 7 had significantly better survival than complex or monosomal karyotype.45 If outcome of transplantation was analyzed according to the new 5 cytogenetic risk groups proposed for MDS, a significant difference for the risk of relapse could be observed between the very-good-risk (8%), good-risk (17%), intermediate-risk (19%), poor-risk (26%), and the very-poor-risk cytogenetics (48%) groups.46 Improvement in outcome might be achieved using pretransplantation novel drugs, such as lenalidomide or hypomethylating agents, which have been shown to induce cytogenetic remission in patients with 5q− or monosomy 7 abnormalities.47,48
Besides cytogenetic abnormalities, modern genomic technology, such as next-generation sequencing and mass spectrometry-based genotyping, allow the detection of molecular abnormalities, even in cytogenetically normal patients, such as mutations in TP53, EHZ2, ETV6, RUNX1, TET2, and ASXL1, which influence survival in a nontransplantation setting.49 Furthermore, newly discovered mutations of the splicing machinery have become the most frequent detectable mutations in MDS patients, which might also have an impact on outcome in a nontransplantation setting.50,51
Reducing the number of blasts before transplantation: the issue of cytoreductive pretransplantation therapy
The EBMT study of allogeneic SCT in elderly MDS patients suggests that, in advanced stage, relapse is the most frequent cause of treatment failure.17 The number of blasts and not being in CR at time of transplantation are the most significant factors for inferior outcome, especially after RIC transplantation in MDS patients.7,16,34,37,52 Therefore, the issue of performing induction chemotherapy before transplantation has been a matter of debate since starting allogeneic transplantation in MDS patients. Unfortunately, the only randomized study from the EBMT had to be stopped because of slow recruitment, and as a result, no valid data are available. Smaller retrospective single-center studies showed no conclusive results,52-54 and it is probable that obtained results are biased by the selection of patients who received intensive chemotherapy before transplantation and those who did not achieve CR and never received a transplantation. Especially in the older populations, the intensive induction chemotherapy carries the risk of long-lasting myelosuppression and organ toxicities. To overcome the limitation of long-lasting myelosuppression but gain the antileukemic effect of pretransplantation induction chemotherapy, some centers have evaluated AML-like induction chemotherapies, such as anthracycline/cytosine, arabinoside/fludarabine, or clofarabine/cytosine arabinoside-based chemotherapy, followed by only 3 days' rest before performing a RIC and subsequent allogeneic SCT.55-57 With those conditioning regimens and matched related or unrelated donors, 2-year OS rates of 69% and 56% can be achieved.56,57
Newer agents, such as 5-azacytidine or decitabine, which have been shown to be active in MDS,58,59 may also be used as pretransplantation cytoreductive therapy. However, the CR rate of approximately 10% is much lower than after conventional induction chemotherapy, and the reported trials confirmed the feasibility without significant survival benefit.60,61 Larger studies from EBMT and CIBMTR are currently under investigation regarding this issue.
Timing of transplantation
The clinical course of MDS patients varies from several years to only a few months. Prognostic score systems, such as the IPSS.1 which includes number of blasts, cytopenias and cytogenetic, or the WHO prognostic scoring system, which includes also the transfusion dependency,62 may help to determine the prognosis of MDS patients at diagnosis or during the course of the disease.63 Although these scoring systems have been validated in a nontransplantation MDS cohort, both scoring systems have also been shown to be useful as a predictive model for outcome after allogeneic SCT.64,65 The arguments for early transplantation are based on low incidence of relapse. The Seattle group reported on outcome of unrelated SCT in MDS patients according to IPSS before transplantation. No relapse was seen in the IPSS low-risk group; whereas in the IPSS high-risk group, the relapse rate increases to 42%, resulting in an relapse-free survival of 80% in the low-risk group and only 29% among patients with an IPPS score higher than 2.16 A similar study was performed from EBMT in 374 patients with early disease defined as refractory anemia or refractory anemia with ring sideroblasts because IPSS scores were only available in half of the patients. The predictive factors for survival were recipient age, year of transplantation, and interval between diagnosis and transplantation. Earlier transplantation after diagnosis was associated with a 10% increase of survival rate at 4 years (57% vs 47%).66
Whether these data support early transplantation in elderly MDS patients is questionable because no comparisons with nontransplantation approaches were made, and IPSS low-risk patients often do not require treatment. Furthermore, the risk of NRM should be carefully balanced; and even if NRM has decreased in recent years, the NRM is still considerably high and should be carefully balanced with a nontransplantation approach.67 Despite its curative potential, in the face of treatment-related mortality and the risk of relapse, one might ask whether elderly patients should be offered allogeneic SCT at all and whether conservative, less toxic approaches would be more suitable.
So far, no prospective trial has compared allogeneic SCT with nontransplantation approaches. In the past, the nontransplantation options were supportive without obvious prolongation of life. Hypomethylating agents, such as 5-azacytidine, have shown prolongation of survival in MDS patients compared with other nontransplantation therapies.58,59 If younger patients with MDS who received an HLA-identical sibling transplantation after standard myeloablative conditioning were compared with a nontransplantation cohort within a multistate model (Markov model), the obtained results suggest that IPSS high and intermediate II patients benefit from immediate transplantation, whereas intermediate I or low-risk patients benefit more from a delayed (in case of progression) transplantation.67
The EBMT also recently used a multistate model to compare elderly (> 55 years) advanced MDS patients (RAEB or RAEB-t) who received allogeneic SCT and were reported to EBMT with a nontransplantation cohort from the Düsseldorf MDS registry. Here no benefit for allogeneic SCT could be found, but the analysis also showed the limitation of even sophisticated statistical analysis if patients from 2 different registries are compared and the selection of patients for transplant or nontransplantation is not clear.68 To address this important issue, the German MDS study group has started a prospective study in elderly (55-69 years) IPSS intermediate II or high-risk patients, comparing 5-azacytidine with a reduced-intensity allograft (#NCT01404741). Those studies should also include quality-of-life evaluation because any potential survival benefit should also be balanced with the gain or loss in quality of life.
From the clinical viewpoint, it is important to define optimal timing of allogeneic SCT in treatment algorithms, including hypomethylating and other effective drug-based agents, such as lenalidomide. For clinicians, it is reasonable to start with hypomethylating agents in elderly patients with intermediate II or high-risk IPSS while searching for a donor. But it remains unclear at which point of time the transplantation should be performed in responding patients: at time of best response or after progression? After failure to hypomethylating agents, the median survival is only 5.6 months. Long-term survival can then be achieved only by allogeneic SCT as salvage therapy (median survival, 19.5 months).69
Donor selection
SCTs from unrelated donors have been increasingly used in recent years. In the EBMT registry, unrelated SCTs accounted for 59% of all allogeneic transplants for MDS in 2009. Although early studies on unrelated SCT in MDS reported a NRM of more than 40%,70 the incidence of NRM has been considerably and steadily improved in recent years and is comparable to HLA-identical sibling transplantation after adjusting for other risk factors (N.K., unpublished EBMT data, March 2011). In addition, the National Marrow Donor Program reported a relative risk for DFS of 1.43 (P = .03) for unrelated stem cell transplantations for MDS performed between 1988 and 1993 versus more recent transplantations.71
In elderly patients with MDS, the available HLA-identical sibling is likely to have similar age as the recipient. Because in unrelated SCT increasing donor age is associated with inferior survival,20 the MDS subcommittee of EBMT investigated the influence of donor age in elderly MDS patients (> 50 years) who underwent allogeneic SCT. The question of the study was whether in elderly MDS patients a young unrelated donor would result in better outcome after allogeneic SCT than an HLA-identical sibling. A total of 719 patients with MDS and a median age of 58 years (range, 50-73 years) were included. The median age of the HLA-identical sibling donors was 56 years, and of the unrelated donors was 34 years. Although there was no influence of donor age in the HLA-identical sibling cohort, age of matched unrelated donor had significant influence on survival (HR = 1.03, P = .02), resulting in significantly improved survival in elderly MDS patients if the age of the unrelated donor was less than 30 years compared with HLA-identical sibling donors and matched unrelated donors older than 30 years: 40% versus 33% versus 24% (P = .04).
This retrospective study suggests that for elderly MDS patients, a young unrelated donor (< 30 years) is a more preferable donor than an HLA-identical sibling, but an HLA-identical donor should be preferred to an older matched unrelated donor.72
Preventing relapse
Relapse has become the main reason for treatment failure after allogeneic SCT in MDS patients. Although the cumulative incidence of NRM has substantially decreased in recent years, no major advancement in reducing the risk of relapse has been made.73
In the EBMT trial,17 the risk of relapse was significantly higher in patients 60 years of age or older compared with the age category 50 to 59 years (41% vs 32%, P = .02). This could be because those patients received more RIC regimen than the younger group but also because of biologic factors. Prevention of relapse after allogeneic SCT in MDS patients is therefore of even greater need in elderly patients. Because the treatment of relapse resulted in disappointing results, more effort has to be put into prevention of relapse. Limited experience of DLIs for relapse treatment or relapse prevention exists: in 2 studies, 14% to 22% achieved a CR, which lasted in 2 patients more than 5 years74,75 and prophylactic DLI improved relapse-free survival, but this has not been investigated in a randomized fashion and the results might be biased by patient selection.76
5-azacytidine in combination with DLI has shown activity with 23% CR after allogenic SCT in relapsed AML/MDS patients.77 The MD Anderson Cancer Center investigated 5-azacytidine in a dose-finding study as maintenance therapy after transplantation in high-risk AML and MDS patients. They observed no severe side effects and no impact of azacytidine on GVHD and chimerism. The recommended dose is 24 mg/m2 5 times and has currently been tested in a randomized trial.78,79 Potential effects of hypomethylating agents after transplantation are the increase expression on cancer-testis antigen, which may become the target for donor cells to induce a tumor-specific immune response.80,81 However, other in vitro studies show immunosuppressive properties of 5-azacytidine, which, in contrast, may abrogate effective immune response by increasing T-regulatory cells.82
To avoid overtreatment by prophylactic post-transplantation strategies, such as DLI or hypomethylating agents, a more optimized way would be therapeutic intervention based on detectable minimal molecular residual disease after transplantation, as it has been shown for CML, MPN, or AML.83
Monitoring CD34+ lineage-specific chimerism might be able to detect residual disease or early relapse and might allow successful intervention with 5-azacitidine.84 The more recent discovery of mutation in MDS patients,49,85 by modern sequencing techniques, allows for screening patients before transplantation for specific mutations and can be used after transplantation as a marker for residual disease and as a guide for adoptive immunotherapies to prevent clinical relapse.
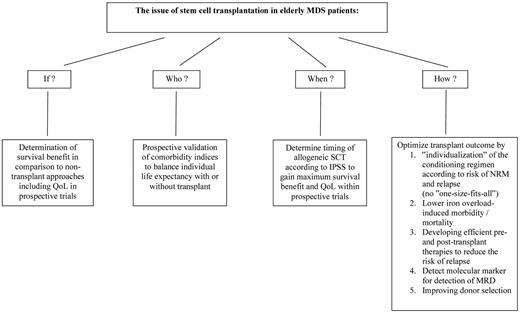
In conclusion, allogeneic SCT is well accepted as curative treatment approach in patients with MDS and has become the third most frequent indication for allogeneic SCT as reported to the CIBMTR.86 The majority of MDS patients are elderly, with a median age of 65 to 70 years at diagnosis. However, the role of SCT in elderly patients with MDS is not well defined. Large retrospective studies confirmed the feasibility of allogeneic SCT in elderly MDS patients as well as curative potential, but benefit in terms of survival has not been demonstrated yet in randomized trials. Chronologic age, which has been used for a long time as exclusion criteria for allogeneic SCT, is probably only a poor predictor for survival because of the lack of data on organ dysfunction and performance status. Besides chronologic age, physical function and organ comorbidities should be taken into account for selecting patients for transplantation. The intensity of the conditioning regimen is not well established, but retrospective studies suggest that there is no “one-size-fits-all” conditioning regimen for elderly MDS patients, and comorbidities and risk of relapse are the main determinants for selecting the intensity of the conditioning regimen in a more “individualized” fashion. Allogeneic SCT should early be included in the treatment plan for MDS patients, which includes novel agents, such as hypomethylating agents or immunomodulatory drugs to achieve cytogenetic response or/and reduction of the number of blasts prior transplantation. Molecular marker detectable with new sequencing methods should be investigated at the time of diagnosis and used as residual disease marker after transplantation to guide either drug-based or immunologic based therapies, such as donor lymphocyte infusion, alloreactive NK cells, or vaccination strategies to lower the risk of relapse and improve OS.
Acknowledgments
The author thanks the staff of the Department of Stem Cell Transplantation at the University Medical Center Hamburg for providing excellent care of the patients, Karen Klemt for editing the manuscript, and the members of the MDS subcommittee of the Chronic Leukemia Working Party of EBMT, especially Theo de Witte for fruitful discussion and study proposals to improve transplant results in MDS patients.
Authorship
Contribution: N.K. wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Nicolaus Kröger, Department of Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Martinistrasse 52, D-20246 Hamburg, Germany; e-mail: nkroeger@uke.uni-hamburg.de.